Impact of COVID-19 Pandemic Restrictions on Respiratory Virus Patterns: Insights from RSV Surveillance in Gwangju, South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Surveillance and Sample Collection
2.2. RNA Extraction and Real-Time PCR
2.3. Library Preparation and Sequencing
2.4. Sequence Data and Analysis
2.5. Phylogenetic Analysis
3. Results
3.1. Epidemiology of RSV
3.2. Demographic Distribution of RSV
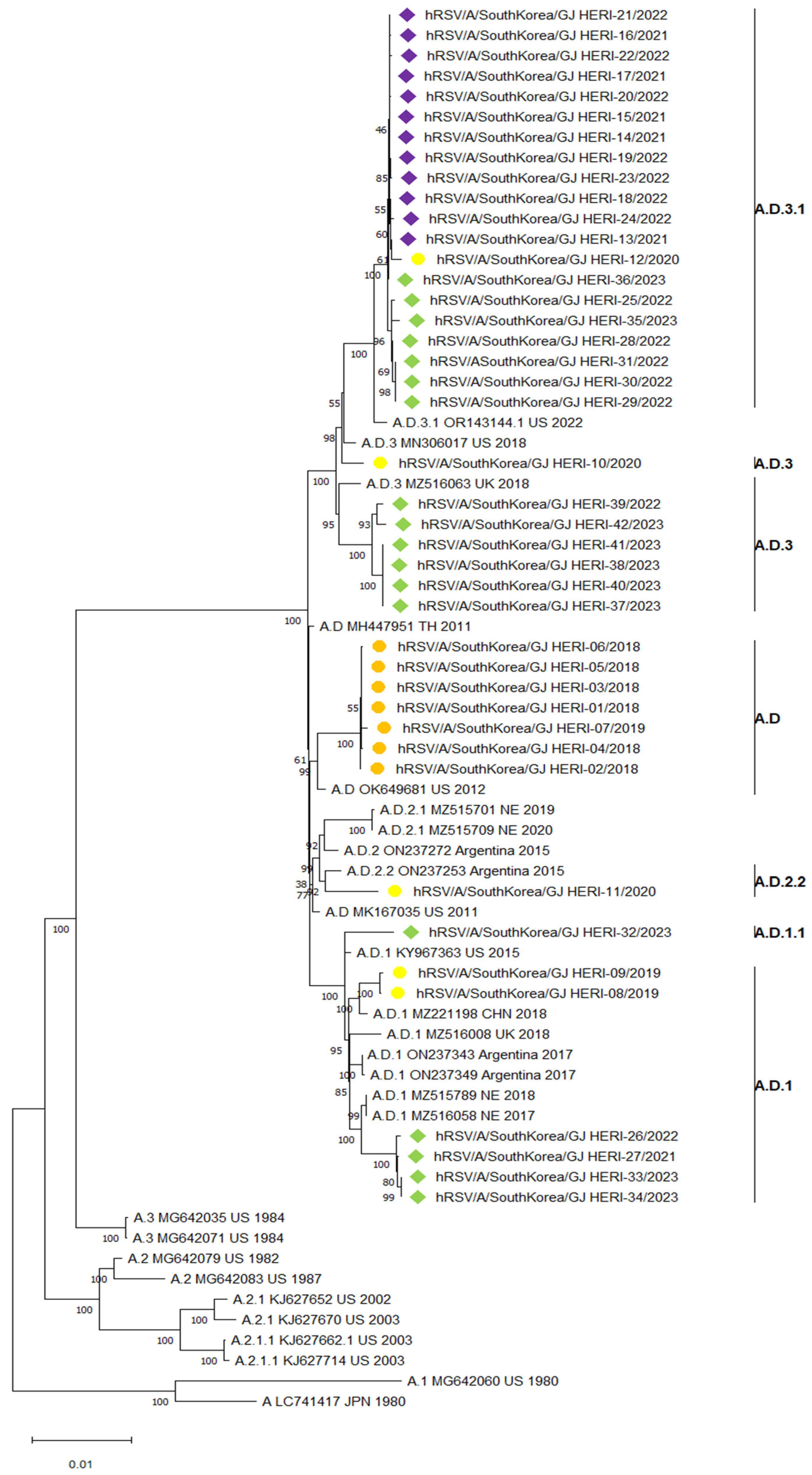
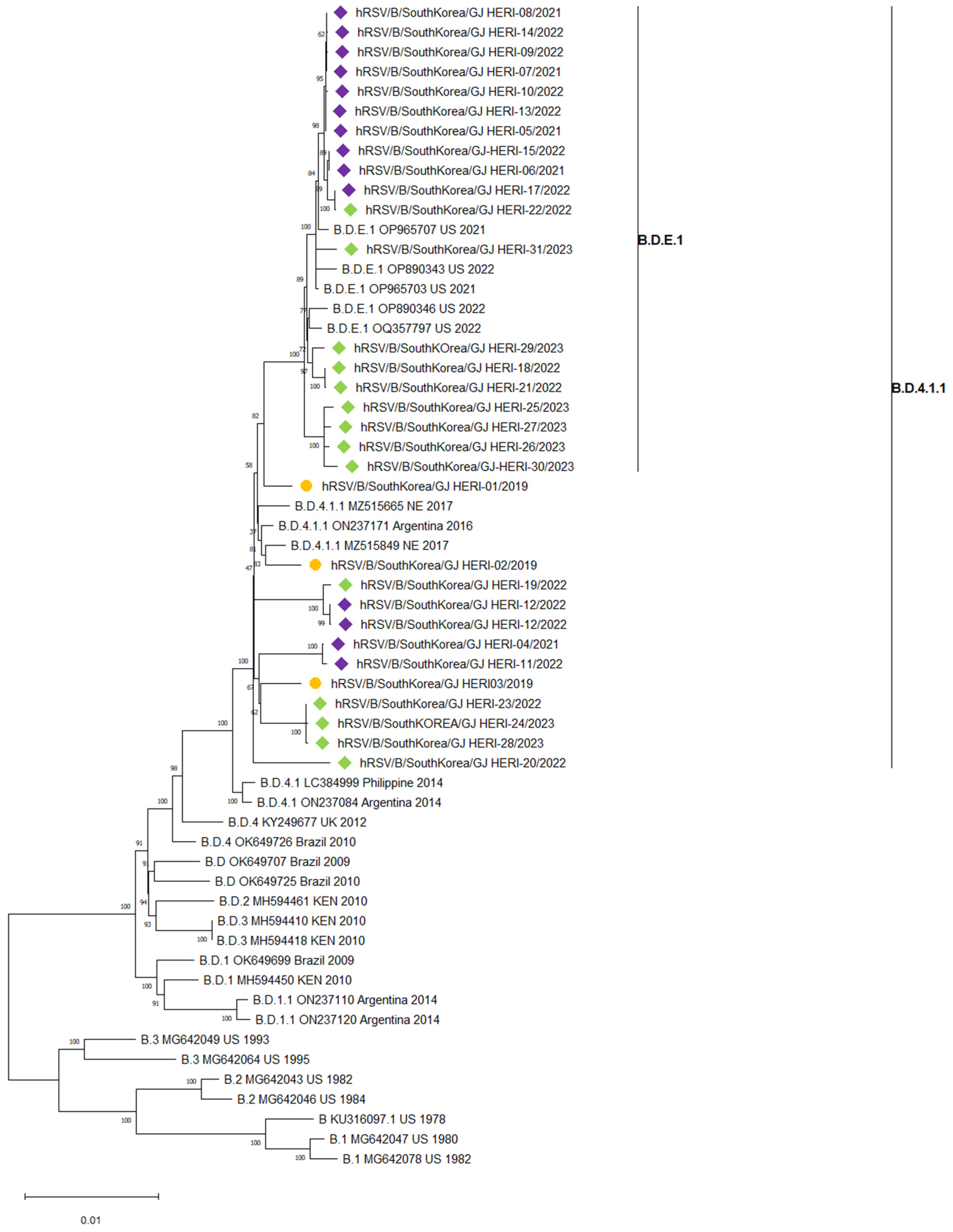
3.3. Phylogenetic Analysis of RSV Whole Genome Sequences
3.4. Deduced Amino Acid Sequence of the RSV F Protein
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, M.L.; Scott, E.; Kuypers, J.; Nalla, A.K.; Roychoudury, P.; Chu, H.Y. Epidemiology of Respiratory Syncytial Virus Across Five Influenza Seasons Among Adults and Children One Year of Age and Older—Washington State, 2011/2012–2015/2016. J. Infect. Dis. 2021, 223, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-acquired pneumonia requiring hospitalization among US children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory syncytial virus infection in adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, C.; Abidi, S.H.; Harker, J.; Stevenson, N.J. Revisiting respiratory syncytial virus’s interaction with host immunity, towards novel therapeutics. Cell. Mol. Life Sci. 2020, 77, 5045–5058. [Google Scholar] [CrossRef] [PubMed]
- Eiland, L.S. Respiratory syncytial virus: Diagnosis, treatment and prevention. J. Pediatr. Pharmacol. Ther. 2009, 14, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. FDA Approves First Respiratory Syncytial Virus (RSV) Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine (accessed on 5 September 2023).
- Pfizer. U.S. FDA Approves ABRYSVO™, Pfizer’s Vaccine for the Prevention of Respiratory Syncytial Virus (RSV) in Older Adults. Available online: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-abrysvotm-pfizers-vaccine-prevention (accessed on 5 September 2023).
- Japan National Broadcasting Company. Japan’s First Vaccine for Respiratory Syncytial Virus Infections Approved for People 60 Years and Older. Available online: https://www3.nhk.or.jp/news/html/20230828/k10014177051000.html (accessed on 5 September 2023).
- GSK. European Commission Authorises GSK’s Arexvy, the First Respiratory Syncytial Virus (RSV) Vaccine for Older Adults. Available online: https://www.gsk.com/en-gb/media/press-releases/european-commission-authorises-gsk-s-arexvy-the-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults/ (accessed on 7 June 2023).
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. In Challenges and Opportunities for Respiratory Syncytial Virus Vaccines; Anderson, L.A., Graham, B.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 372, pp. 3–38. [Google Scholar]
- Lee, C.Y.; Fang, Y.P.; Wang, L.C.; Chou, T.Y.; Liu, H.F. Genetic diversity and molecular epidemiology of circulating respiratory syncytial virus in central Taiwan, 2008–2017. Viruses 2021, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Gilman, M.S.; Furmanova-Hollenstein, P.; Pascual, G.; van ‘t Wout, A.B.; Langedijk, J.P.; McLellan, J.S. Transient opening of trimeric prefusion RSV F proteins. Nat. Commun. 2019, 10, 2105. [Google Scholar] [CrossRef]
- Peret, T.C.; Hall, C.B.; Schnabel, K.C.; Golub, J.A.; Anderson, L.J. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 1998, 79, 2221–2229. [Google Scholar] [CrossRef]
- Eshaghi, A.; Duvvuri, V.R.; Lai, R.; Nadarajah, J.T.; Li, A.; Patel, S.N.; Low, D.E.; Gubbay, J.B. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: A novel genotype with a 72 nucleotide G gene duplication. PLoS ONE 2012, 7, e32807. [Google Scholar] [CrossRef] [PubMed]
- Venter, M.; Madhi, S.A.; Tiemessen, C.T.; Schoub, B.D. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: Identification of new subgroup A and B genotypes. J. Gen.Virol. 2001, 82, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Dapat, I.C.; Shobugawa, Y.; Sano, Y.; Saito, R.; Sasaki, A.; Suzuki, Y.; Kumaki, A.; Zaraket, H.; Dapat, C.; Oguma, T.; et al. New genotypes within respiratory syncytial virus group B genotype BA in Niigata, Japan. J. Clin. Microbiol. 2010, 48, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Streng, A.; Goettler, D.; Haerlein, M.; Lehmann, L.; Ulrich, K.; Prifert, C.; Krempl, C.; Weißbrich, B.; Liese, J.G. Spread and clinical severity of respiratory syncytial virus A genotype ON1 in Germany, 2011–2017. BMC Infect. Dis. 2019, 19, 613. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Lucion, M.F.; Shilts, M.H.; Juárez, M.D.V.; Gentile, A.; Mistchenko, A.S.; Viegas, M.; Das, S.R. Evolutionary dynamics of respiratory syncytial virus in Buenos Aires: Viral diversity, migration, and subgroup replacement. Virus Evol. 2023, 9, vead006. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, D.H.; Chen, D.; Guo, L.; Yang, H.; Shi, Y.S.; Wang, Y.J.; Wang, W.K.; Xie, Z.P.; Zhang, R.F. Gradual replacement of all previously circulating respiratory syncytial virus A strain with the novel ON1 genotype in Lanzhou from 2010 to 2017. Medicine 2019, 98, e15542. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, S.; Venter, M. Replacement of previously circulating respiratory syncytial virus subtype B strains with the BA genotype in South Africa. J. Virol. 2011, 85, 8789–8797. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Ruis, C.; Neher, R.; Meijer, A.; Aziz, A.; Hinrichs, A.; Gottberg, A.V.; Roemer, C.; Amoako, D.G.; Acuña, D.; et al. The unified proposal for classification of human respiratory syncytial virus below the subgroup level. medRxiv 2024. [Google Scholar] [CrossRef]
- Ramaekers, K.; Rector, A.; Cuypers, L.; Lemey, P.; Keyaerts, E.; Van Ranst, M. Towards a unified classification for human respiratory syncytial virus genotypes. Virus Evol. 2020, 6, veaa052. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, X.; Avadhanula, V.; Shepard, S.S.; Kim, D.K.; Hixson, J.; Piedra, P.A.; Bahl, J. Novel and extendable genotyping system for human respiratory syncytial virus based on whole-genome sequence analysis. Influenza Other Respir. Viruses 2022, 16, 492–500. [Google Scholar] [CrossRef]
- Pangesti, K.N.; Ansari, H.R.; Bayoumi, A.; Kesson, A.M.; Hill-Cawthorne, G.A.; Abd El Ghany, M. Genomic characterization of respiratory syncytial virus genotypes circulating in the paediatric population of Sydney, NSW, Australia. Microb. Genom. 2023, 9, 001095. [Google Scholar] [CrossRef] [PubMed]
- Fourgeaud, J.; Toubiana, J.; Chappuy, H.; Delacourt, C.; Moulin, F.; Parize, P.; Scemla, A.; Abid, H.; Leruez-Ville, M.; Frange, P. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S. Seasonality of respiratory syncytial virus—United States, 2017–2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Redlberger-Fritz, M.; Springer, D.N.; Aberle, S.W.; Camp, J.V.; Aberle, J.H. Respiratory syncytial virus surge in 2022 caused by lineages already present before the COVID-19 pandemic. J. Med. Virol. 2023, 95, e28830. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Wu, T.H.; Fang, Y.P.; Chang, J.C.; Wang, H.C.; Lin, S.J.; Mai, C.H.; Chang, Y.C.; Chou, T.Y. Delayed respiratory syncytial virus outbreak in 2020 in Taiwan was correlated with two novel RSV-A genotype ON1 variants. Influenza Other Respir. Viruses 2022, 16, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, D.; Piubelli, C.; Formenti, F.; Silva, R.; Perandin, F. Resurgence of respiratory virus after relaxation of COVID-19 containment measures: A real-world data study from a regional hospital of Italy. Int. J. Microbiol. 2022, 2022, 4915678. [Google Scholar] [CrossRef] [PubMed]
- Eden, J.S.; Sikazwe, C.; Xie, R.; Deng, Y.M.; Sullivan, S.G.; Michie, A.; Levy, A.; Cutmore, E.; Blyth, C.C.; Britton, P.P.; et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat. Commun. 2022, 13, 2884. [Google Scholar] [CrossRef]
- Dolores, A.; Stephanie, G.; Mercedes, N.J.; Érica, G.; Mistchenko, A.S.; Mariana, V. RSV reemergence in Argentina since the SARS-CoV-2 pandemic. J. Clin. Virol. 2022, 149, 105126. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.H.; Cho, S.J.; Lee, Y.U.; Lee, K.; Lee, Y.P.; Seo, J.; Chung, Y.S. Genetic analysis of HPIV3 that emerged during the SARS-CoV-2 pandemic in Gwangju, South Korea. Viruses 2022, 14, 1446. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, S.H.; Lee, H.; Lee, Y.U.; Mun, J.; Park, S.; Park, J.; Park, J.S.; Lee, K.; Lee, C.M.; et al. Re-Emergence of HMPV in Gwangju, South Korea, after the COVID-19 Pandemic. Pathogens 2023, 12, 1218. [Google Scholar] [CrossRef]
- Muñoz-Escalante, J.C.; Comas-García, A.; Bernal-Silva, S.; Robles-Espinoza, C.D.; Gómez-Leal, G.; Noyola, D.E. Respiratory syncytial virus A genotype classification based on systematic intergenotypic and intragenotypic sequence analysis. Sci. Rep. 2019, 9, 20097. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Hwang, J.; Yoon, S.Y.; Lim, C.S.; Cho, Y.; Lee, C.K.; Nam, M.H. Molecular characterization of human respiratory syncytial virus in Seoul, South Korea, during 10 consecutive years, 2010–2019. PLoS ONE 2023, 18, e0283873. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Galiano, M.; Nauwelaers, I.; Trento, A.; Openshaw, P.J.; Mistchenko, A.S.; Zambon, M.; Viegas, M. Toward unified molecular surveillance of RSV: A proposal for genotype definition. Influenza Other Respir. Viruses 2020, 14, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Phyu, W.W.; Wagatsuma, K.; Nagai, T.; Sano, Y.; Taniguchi, K.; Nagata, N.; Tomimoto, K.; Sato, I.; Kaji, H.; et al. Molecular Epidemiology of Respiratory Syncytial Virus during 2019–2022 and Surviving Genotypes after the COVID-19 Pandemic in Japan. Viruses 2023, 15, 2382. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Sereewit, J.; Pfalmer, D.; Nguyen, T.V.; Bakhash, S.A.M.; Sobolik, E.B.; Greninger, A.L. Genomic characterization of respiratory syncytial virus during 2022–23 outbreak, Washington, USA. Emerg. Infect. Dis. 2023, 29, 865. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.A.; Holland, S.C.; Smith, M.F.; Leonard, V.R.; Murugan, V.; Nordstrom, L.; Mulrow, M.; Salgado, R.; White, M.; Lim, E.S. Genomic Sequencing Surveillance to Identify Respiratory Syncytial Virus Mutations, Arizona, USA. Emerg. Infect. Dis. 2023, 29, 2380. [Google Scholar] [CrossRef] [PubMed]
- Korsun, N.; Trifonova, I.; Madzharova, I.; Alexiev, I.; Uzunova, I.; Ivanov, I.; Velikov, P.; Tcherveniakova, T.; Christova, I. Resurgence of respiratory syncytial virus with dominance of RSV-B during the 2022–2023 season. Front. Microbiol. 2024, 15, 1376389. [Google Scholar] [CrossRef]
- den Hartog, G.; van Kasteren, P.B.; Schepp, R.M.; Teirlinck, A.C.; van der Klis, F.R.; van Binnendijk, R.S. Decline of RSV-specific antibodies during the COVID-19 pandemic. Lancet Infect. Dis. 2023, 23, 23–25. [Google Scholar] [CrossRef]
- Reicherz, F.; Xu, R.Y.; Abu-Raya, B.; Majdoubi, A.; Michalski, C.; Golding, L.; Stojic, A.; Vineta, M.; Granoski, A.; Cieslask, Z.; et al. Waning immunity against respiratory syncytial virus during the coronavirus disease 2019 pandemic. J. Infect. Dis. 2022, 226, 2064–2068. [Google Scholar] [CrossRef]
- Casalegno, J.S.; Ploin, D.; Cantais, A.; Masson, E.; Bard, E.; Valette, M.; Fanget, R.; Targe, S.C.; Myar-dury, A.; Doret-Dion, M.; et al. Characteristics of the delayed respiratory syncytial virus epidemic, 2020/2021, Rhône Loire, France. Eurosurveillance 2021, 26, 2100630. [Google Scholar] [CrossRef]

| Season | 2018/2019 | 2019/2020 | 2020/2021 | 2021/2022 | 2022/2023 | p-Value 1 |
|---|---|---|---|---|---|---|
| Total no. of samples analyzed | 1248 | 1518 | 1194 | 1487 | 3470 | |
| No. of RSV positive samples | 49 (3.9%) | 54 (3.6%) | 151(10.2%) | 220(6.3%) | <0.01 * | |
| Gender | ||||||
| Male | 21 (42.9%) | 28 (51.8%) | 0 | 71 (47.0%) | 124 (56.4%) | |
| Female | 28 (57.1%) | 26 (48.1%) | 0 | 80 (53.0%) | 96 (43.6%) | |
| Age | ||||||
| 0–2 years | 15 (30.6%) | 24 (44.4%) | 0 | 65 (43.0%) | 116 (52.7%) | <0.01 * |
| 3–5 years | 17 (34.7%) | 17 (31.4%) | 0 | 65 (43.0%) | 66 (30.0%) | <0.01 * |
| 6–10 years | 6 (12.2%) | 2 (3.7%) | 0 | 7 (4.6%) | 15 (6.8%) | |
| 11–20 years | 2 (4.1%) | 1 (1.9%) | 0 | 4 (2.6%) | 7 (3.2%) | |
| 21–40 years | 2 (4.1%) | 2 (3.7%) | 0 | 4 (2.6%) | 4 (1.8%) | |
| 41–60 years | 3 (6.1%) | 3 (5.6%) | 0 | 1 (0.7%) | 4 (1.8%) | |
| 60–90 years | 4 (8.2%) | 5 (9.3%) | 0 | 5 (3.3%) | 8 (3.6%) |
| Antigen Site | Amino Acid Positions | RSV A Frequency No. (%) | RSV B Frequency No. (%) | ||||
|---|---|---|---|---|---|---|---|
| Change | Pre COVID-19 (n = 12) | Post COVID-19 (n = 30) | Change | Pre COVID-19 (n = 3) | Post COVID-19 (n = 28) | ||
| Φ | 62–96; 195–227 | S211N | 19 (67.8) | ||||
| I | 27–45; 312–318; 378–389 | K42R | 4 (13.3) | R42K | 12 (42.8) | ||
| S389P | 18 (64.2) | ||||||
| S389T | 1 (3.5) | ||||||
| II | 254–277 | S276N | 3 (25.0) | 19 (63.3) | S276N | 3 (10.7) | |
| III | 46–54; 301–311; 345–352; 367–378 | - | - | - | - | ||
| IV | 422–471 | E463D | 3 (10.7) | ||||
| V | 55–61; 146–194; 287–300 | S190N | 22 (78.5) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-J.; Kim, S.-H.; Mun, J.; Yun, J.-e.; Park, S.; Park, J.; Lee, Y.-U.; Park, J.-s.; Yun, H.; Lee, C.-m.; et al. Impact of COVID-19 Pandemic Restrictions on Respiratory Virus Patterns: Insights from RSV Surveillance in Gwangju, South Korea. Viruses 2024, 16, 850. https://doi.org/10.3390/v16060850
Cho S-J, Kim S-H, Mun J, Yun J-e, Park S, Park J, Lee Y-U, Park J-s, Yun H, Lee C-m, et al. Impact of COVID-19 Pandemic Restrictions on Respiratory Virus Patterns: Insights from RSV Surveillance in Gwangju, South Korea. Viruses. 2024; 16(6):850. https://doi.org/10.3390/v16060850
Chicago/Turabian StyleCho, Sun-Ju, Sun-Hee Kim, Jeongeun Mun, Ji-eun Yun, Sujung Park, Jungwook Park, Yeong-Un Lee, Ji-su Park, Haebi Yun, Cheong-mi Lee, and et al. 2024. "Impact of COVID-19 Pandemic Restrictions on Respiratory Virus Patterns: Insights from RSV Surveillance in Gwangju, South Korea" Viruses 16, no. 6: 850. https://doi.org/10.3390/v16060850
APA StyleCho, S.-J., Kim, S.-H., Mun, J., Yun, J.-e., Park, S., Park, J., Lee, Y.-U., Park, J.-s., Yun, H., Lee, C.-m., Kim, J.-P., & Seo, J.-M. (2024). Impact of COVID-19 Pandemic Restrictions on Respiratory Virus Patterns: Insights from RSV Surveillance in Gwangju, South Korea. Viruses, 16(6), 850. https://doi.org/10.3390/v16060850





