Efficient Production of Enterovirus 71 (EV71) Virus-like Particles by Controlling Promoter Strength in Insect Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Strategy for HFMD-VLP Production
2.2. Cells and Viruses
2.3. Virus-Inducible Transient Expression
2.4. Fluorescence Intensity Measurement
2.5. Generation of Recombinant Viruses
2.6. SDS–PAGE and Western Blot Analysis
2.7. Purification of VLPs
2.8. Transmission Electron Microscopy
3. Results and Discussion
3.1. Determination of 3CD Expression Promoters
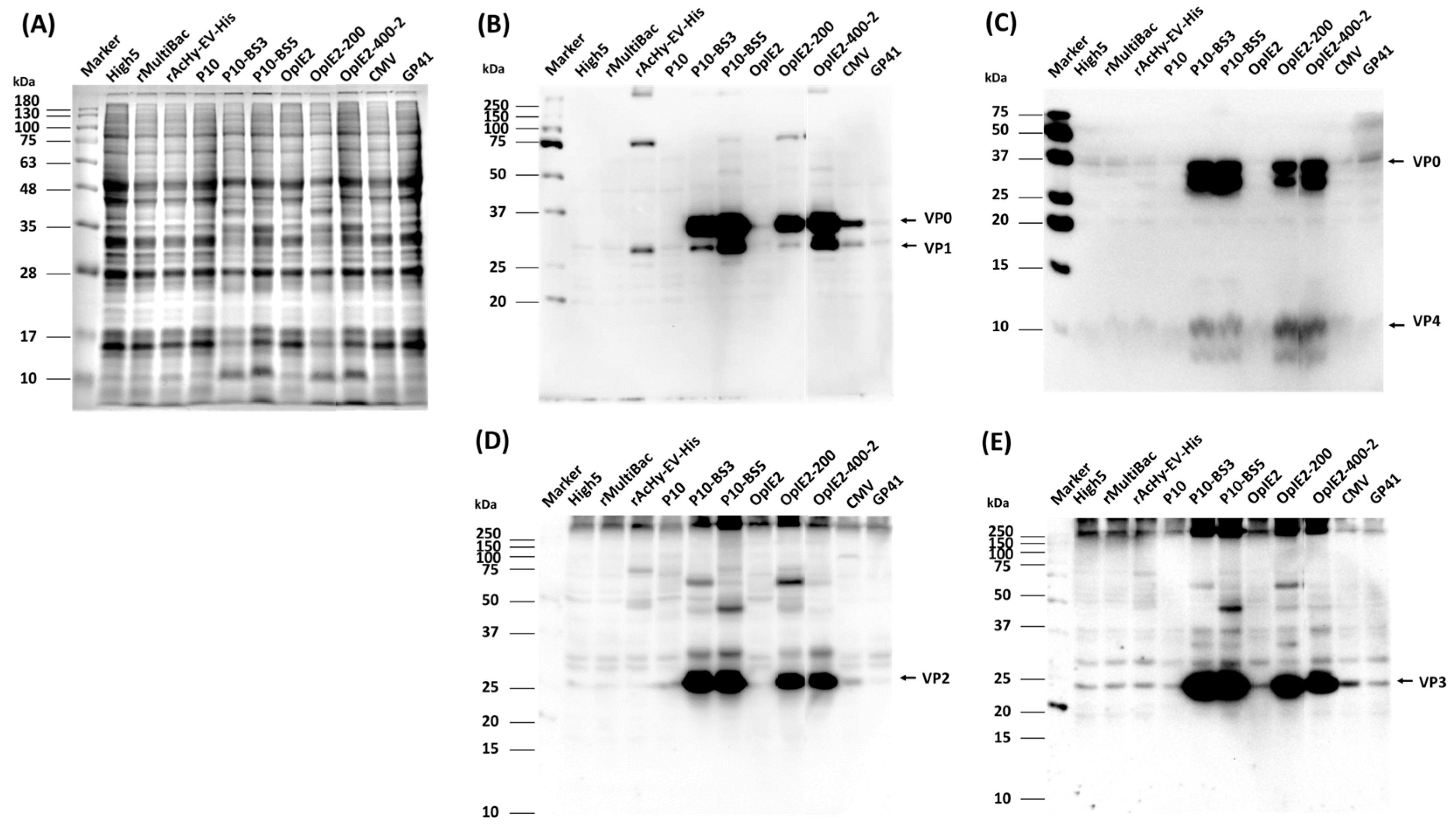
3.2. Expression of HFMDV Structural Proteins
3.3. HFMD-VLP Production
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, P.C.S.; Klein, M. Enterovirus 71. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 288–294.e3. [Google Scholar] [CrossRef]
- Cai, K.; Wang, Y.; Guo, Z.; Yu, H.; Li, H.; Zhang, L.; Xu, S.; Zhang, Q. Clinical characteristics and managements of severe hand, foot and mouth disease caused by enterovirus A71 and coxsackievirus A16 in Shanghai, China. BMC Infect. Dis. 2019, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, B.; Xie, T. Children with severe enterovirus A71 infection. BMC Pediatr. 2023, 23, 172. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Shih, S.R.; Tolbert, B.S.; Brewer, G. Enterovirus A71 Vaccines. Vaccines 2021, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, J.; Lu, J. Enterovirus 71 vaccine: Close but still far. Int. J. Infect. Dis. 2010, 14, e739–e743. [Google Scholar] [CrossRef]
- Ding, X.; Liu, D.; Booth, G.; Gao, W.; Lu, Y. Virus-like particle engineering: From rational design to versatile applications. Biotechnol. J. 2018, 13, e1700324. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Vicente, T.; Roldão, A.; Peixoto, C.; Carrondo, M.J.T.; Alves, P.M. Large-scale production and purification of VLP-based vaccines. J. Invertebr. Pathol. 2011, 107, S42–S48. [Google Scholar] [CrossRef]
- Gopal, R.; Schneemann, A. Production and application of insect virus-based VLPs. Methods Mol. Biol. 2018, 1776, 125–141. [Google Scholar] [CrossRef]
- Yamaji, H. Suitability and perspectives on using recombinant insect cells for the production of virus-like particles. Appl. Microbiol. Biotechnol. 2014, 98, 1963–1970. [Google Scholar] [CrossRef]
- Somasundaram, B.; Lua, L.H.L. Development of picornavirus-like particle vaccines. Pharm. Bioprocess. 2015, 3, 45–59. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.; Qu, M.; Hou, A.; Zhou, Y.; Sun, B.; Cai, L.; Gao, F.; Su, W.; Jiang, C. Determination of the cleavage site of enterovirus 71 VP0 and the effect of this cleavage on viral infectivity and assembly. Microb. Pathog. 2019, 134, 103568. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Huang, J.H.; Lai, C.W.; Sheng, H.C.; Shih, S.R.; Ho, M.S.; Hu, Y.C. Expression, purification and characterization of enterovirus-71 virus-like particles. World J. Gastroenterol. 2006, 12, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Hsu, J.T.; Huang, J.H.; Ho, M.S.; Ho, Y.C. Formation of enterovirus-like particle aggregates by recombinant baculoviruses co-expressing P1 and 3CD in insect cells. Biotechnol. Lett. 2003, 25, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Yeh, C.T.; Li, W.H.; Yu, C.P.; Lin, W.C.; Yang, J.Y.; Wu, H.L.; Hu, Y.C. Enhanced enterovirus 71 virus-like particle yield from a new baculovirus design. Biotechnol. Bioeng. 2015, 112, 2005–2015. [Google Scholar] [CrossRef]
- Chung, C.Y.; Chen, C.Y.; Lin, S.Y.; Chung, Y.C.; Chiu, H.Y.; Chi, W.K.; Lin, Y.L.; Chiang, B.L.; Chen, W.J.; Hu, Y.C. Enterovirus 71 virus-like particle vaccine: Improved production conditions for enhanced yield. Vaccine 2010, 28, 6951–6957. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Son, H.S.; Lee, S.W.; Yoon, Y.; Hyeon, J.Y.; Chung, G.T.; Lee, J.W.; Yoo, J.S. Efficient expression of enterovirus 71 based on virus-like particles vaccine. PLoS ONE 2019, 14, e0210477. [Google Scholar] [CrossRef] [PubMed]
- Bruder, M.R.; Aucoin, M.G. Utility of aternative promoters for foreign gene expression using the baculovirus expression vector system. Viruses 2022, 14, 2670. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gwak, W.S.; Bae, S.M.; Choi, J.B.; Han, B.K.; Woo, S.D. Increased productivity of the baculovirus expression vector system by combining enhancing factors. J. Asia-Pac. Entomol. 2018, 21, 1079–1084. [Google Scholar] [CrossRef]
- Gwak, W.S.; Kim, H.S.; Bae, J.S.; Kim, T.H.; Bae, S.M.; Woo, S.D. Development of a novel enhanced baculovirus expression vector via promoter combination. J. Asia-Pac. Entomol. 2020, 23, 909–914. [Google Scholar] [CrossRef]
- Kim, H.S.; Gwak, W.S.; Bae, J.S.; Kim, T.H.; Choi, J.B.; Han, B.K.; Woo, S.D. Effects of repeated burst sequences on the p10 promoter activity of Bombyx mori nucleopolyhedrovirus. J. Asia-Pac. Entomol. 2021, 24, 7–13. [Google Scholar] [CrossRef]
- Hitchman, R.B.; Locanto, E.; Possee, R.D.; King, L.A. Optimizing the baculovirus expression vector system. Methods 2011, 55, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.L.; Kanamasa, S.; Nishina, T.; Kato, T.; Park, E.Y. Enhanced gene expression in insect cells and silkworm larva by modified polyhedrin promoter using repeated burst sequence and very late transcriptional factor-1. Biotechnol. Bioeng. 2010, 107, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Surowy, H.M.; Tonevitsky, A.G.; Burwinkel, B. Interference in transcription of overexpressed genes by promoter-proximal downstream sequences. Sci. Rep. 2016, 6, 30735. [Google Scholar] [CrossRef]
- Wang, X.; Ku, Z.; Zhang, X.; Ye, X.; Chen, J.; Liu, Q.; Zhang, W.; Zhang, C.; Fu, Z.; Jin, X.; et al. Structure, immunogenicity, and protective mechanism of an engineered Enterovirus 71-like particle vaccine mimicking 80S empty capsid. J. Virol. 2017, 92, e01330-17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-S.; Moon, H.-J.; Choi, J.-B.; Han, B.-K.; Woo, S.D. Efficient Production of Enterovirus 71 (EV71) Virus-like Particles by Controlling Promoter Strength in Insect Cells. Viruses 2024, 16, 834. https://doi.org/10.3390/v16060834
Kim H-S, Moon H-J, Choi J-B, Han B-K, Woo SD. Efficient Production of Enterovirus 71 (EV71) Virus-like Particles by Controlling Promoter Strength in Insect Cells. Viruses. 2024; 16(6):834. https://doi.org/10.3390/v16060834
Chicago/Turabian StyleKim, Hyun-Soo, Hyuk-Jin Moon, Jae-Bang Choi, Beom-Ku Han, and Soo Dong Woo. 2024. "Efficient Production of Enterovirus 71 (EV71) Virus-like Particles by Controlling Promoter Strength in Insect Cells" Viruses 16, no. 6: 834. https://doi.org/10.3390/v16060834
APA StyleKim, H.-S., Moon, H.-J., Choi, J.-B., Han, B.-K., & Woo, S. D. (2024). Efficient Production of Enterovirus 71 (EV71) Virus-like Particles by Controlling Promoter Strength in Insect Cells. Viruses, 16(6), 834. https://doi.org/10.3390/v16060834





