Integrin-Targeting Strategies for Adenovirus Gene Therapy
Abstract
1. Introduction
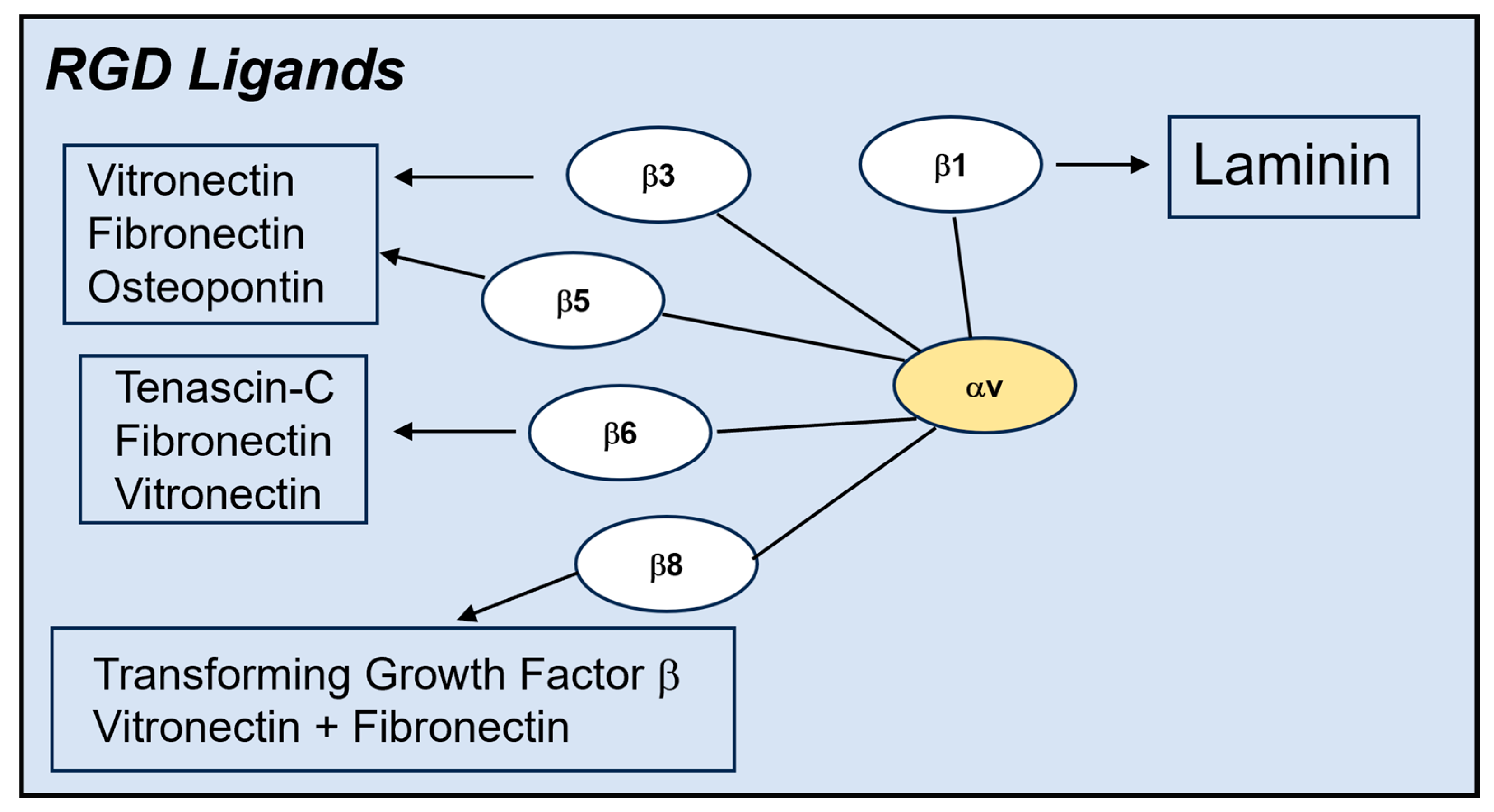
1.1. Vitronectin-Binding Integrins αvβ3 and αvβ5 Promote AdV Uptake into Cells
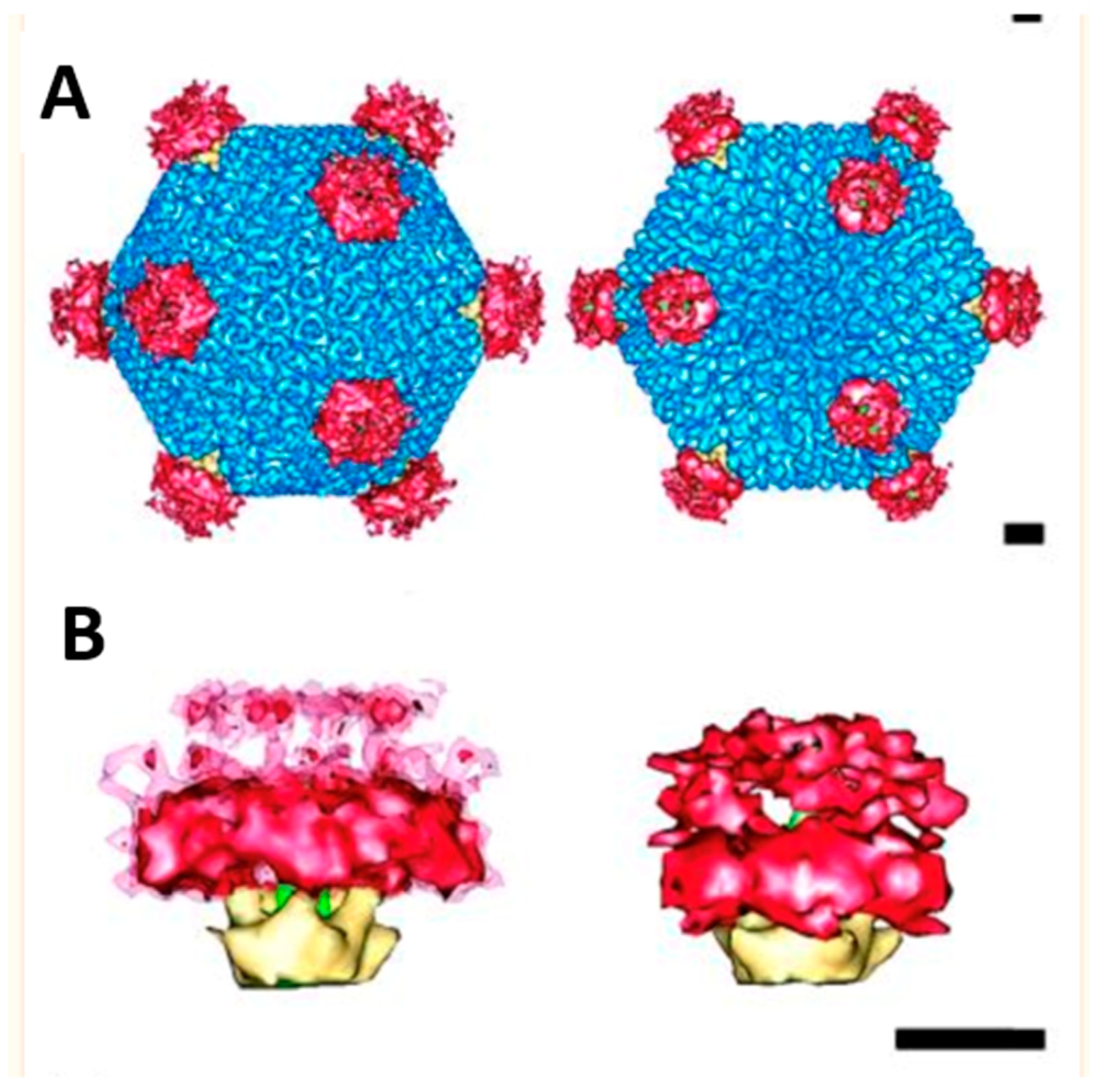
1.2. Cryo-EM Structure Analyses of AdV in Complex with Integrins
2. Consequences of Integrin Binding to Adenovirus Particles
The Role of Different αv Integrins and Other Integrin Types in Human AdV Cell Entry
3. Modifications of AdV to Alter Cell Tropism
3.1. Integrin αvβ6 as a Novel Target for AdV Cancer Therapy
3.2. Equipping Other Viruses and Non-Viral Nanoparticles with RGD Sequences
4. Conclusions and Future Endeavors
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, G.; Wang, W.; Luo, B. Overview: Structural biology of integrins. Methods Mol. Biol. 2012, 757, 81–99. [Google Scholar] [PubMed]
- Cheresh, D.A.; Spiro, R.C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronection, fibronectin, and von Willebrand factor. J. Biol. Chem. 1987, 262, 17703–17711. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Springer, T.A. Integrin activation and structural rearrangement. Immunol. Rev. 2002, 186, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin trafficking in cells and tissues. J. Nat Cell Biol. 2019, 2, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Delon, I.; Brown, N.H. Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 2007, 19, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Isberg, R.R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science 1991, 252, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Relman, D.; Tuomanen, E.; Falkow, S.; Golenbock, D.T.; Saukkonen, K.; Wright, S.D. Recognition of a bacterial adhesion by an integrin: Macrophage CR3 (aMb2, CD11b/CD18) binds filamentous hemagglutinin of Bordatella pertussis. Cell 1990, 61, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.; Shepley, M.P.; Chan, B.M.C.; Hemler, M.E.; Finberg, R.W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science 1992, 255, 1718–1720. [Google Scholar] [CrossRef]
- Fox, G.; Parry, N.R.; Barnett, P.V.; McGinn, B.; Rowlands, D.J.; Brown, F. The cell attachment of foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J. Gen. Virol. 1989, 70, 625–637. [Google Scholar] [CrossRef]
- Everett, S.F.; Ginsberg, H.S. A toxinlike material separatable from type 5 adenovirus particles. Virology 1958, 6, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Peirera, H.G. A protein factor responsible for the early cytopathic effect of adenoviruses. Virology 1958, 6, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Boudin, M.L.; Boulanger, P. Assembly of adenovirus penton base and fiber. Virology 1982, 126, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.; Chroboczek, J.; Jocrot, B. Determination of the nucleotide sequence for the penton base gene of human adenovirus type 5. Gene 1988, 69, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Harfe, B.; Freimuth, P. Mutations that alter an ArG-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolishes its cell-rounding activity and delay virus reproduction in flat cells. J. Virol. 1993, 67, 5198–5205. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins avb3 and avB5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Bieri, M.; Hendrickx, R.; Bauer, M.; Yu, B.; Jetzer, T.; Dreier, B.; Mittl, P.; Sobek, J.; Plulckthun, A.; Greber, U.F.; et al. The RGD-binding integrins avb6 and avb8 are receptors for mouse adenovirus1 and -3 infection. PLoS Pathog. 2021, 17, e1010083. [Google Scholar] [CrossRef]
- Mathias, M.; Galleno, M.; Nemerow, G.R. Interactions of soluble recombinant integrin avb5 with human adenoviruses. J. Virol. 1998, 72, 8669–8675. [Google Scholar] [CrossRef]
- Stewart, P.L.; Burnett, R.M.; Cyrklaff, M.; Fuller, S.D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell 1991, 67, 145–154. [Google Scholar] [CrossRef]
- Athappilly, F.K.; Murali, R.; Rux, J.J.; Cai, Z.; Burnett, R.M. The refined crystal structure of hexon, the major coat protein of adenovirus type 2 at 2.9 Å resolution. J. Mol. Biol. 1994, 242, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.L.; Chiu, C.Y.; Huang, S.; Muir, T.; Zhao, Y.; Chait, B.; Mathias, P.; Nemerow, G.R. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. 1997. EMBO J. 1997, 16, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Mathias, P.; Nemerow, G.R.; Stewart, P.L. Structure of adenovirus complexed with its internalization receptor, avb5 integrin. J. Virol. 1999, 73, 6759–6768. [Google Scholar] [CrossRef] [PubMed]
- Veesler, D.; Cupelli, K.; Burger, M.; Graber, P.; Stehle, T.; Johnson, J.E. Single-particle EM reveals plasticity of interactions between the adenovirus penton base and integrin avb3. Proc. Natl. Acad. Sci. USA 2014, 111, 8815–8819. [Google Scholar] [CrossRef] [PubMed]
- Stupack, D.G.; Li, E.; Silletti, S.A.; Kehler, J.A.; Geahlen, R.L.; Hahn, K.; Nemerow, G.R.; Cheresh, D.A. Matrix valency regulated integrin-mediated lymphoid adhesion via Syk Kinase. J. Cell Biol. 1999, 144, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Stupack, D.; Klemke, R.; Cheresh, D.A.; Nemerow, G.R. Adenovirus endocytosis via av integrins requires phosphoinositide-3-OH Kinase. J. Virol. 1998, 72, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Stupack, D.; Bokoch, G.M.; Nemerow, G.R. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho Family GTPases. J. Virol. 1998, 72, 8806–8812. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Gomez-Gonzalez, A. Adenovirus-a blueprint for gene delivery. Curr. Opin. Virol. 2021, 48, 49–56. [Google Scholar] [CrossRef]
- Greber, U.F.; Willetts, M.; Webster, P.; Helenius, A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 1993, 75, 477–486. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus protei VI mediates membrane disruption following capsid disassembly. J. Virol. 2005, 79, 1992–2000. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Nemerow, G.R. Adenovirus membrane penetration: Tickling the tail of a sleeping dragon. Virology 2015, 479–480, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Saban, S.; Silvestri, M.; Nemerow, G.R.; Stewart, P.L. Visualization of a-helices in a 6 Å resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 2006, 80, 12049–12059. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, L.; Zhou, Z.H. Model of the trimeric fiber and its interactions with the pentameric penton base of human adenovirus by cryo-electron microscopy. J. Mol. Biol. 2011, 406, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Yu, X.; Barry, M.A. Refined capsid structure of human adenovirus D26 at 3.4 Å resolution. Viruses 2022, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Lindert, S.; Silvestry, M.; Mullen, T.M.; Nemerow, G.R.; Stewart, P.L. Cryo-electron microscopy structure of an adenovirus-integrin complex indicates conformation changes in both penton base and integrin. J. Virol. 2009, 83, 11491–11501. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Flatt, J.W. Adenovirus entry: From infection to immunity. Annu. Rev. Virol. 2019, 6, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Panetti, T.S.; McKeown-Longo, P.J. The alpha v beta 5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J. Biol. Chem. 1993, 268, 11492–11495. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Filardo, E.J.; Cheresh, D.A.; Nemerow, G.R. Integrin avb5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 1994, 127, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Brown, S.L.; Stupack, D.G.; Puente, X.S.; Cheresh, D.A.; Nemerow, G.R. Integrin avb1 is an adenovirus coreceptor. J. Virol. 2001, 75, 5405–5409. [Google Scholar] [CrossRef]
- Huang, S.; Kamata, T.; Takada, Y.; Ruggeri, Z.M.; Nemerow, G.R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 1996, 70, 4502–4508. [Google Scholar] [CrossRef]
- Storm, R.J.; Persson, B.D.; Skalman, L.N.; Frangsmyr, L.; Lindstrom, M.; Rankin, G.; Lundmark, R.; Domellof, F.P.; Arnberg, N. Human adenovirus type 37 uses ab1 and a3b1 integrins for infection of human corneal cells. J. Virol. 2017, 91, e02019-16. [Google Scholar] [CrossRef]
- Rajan, A.; Persson, B.D.; Frangsmyr, L.; Olofsson, A.; Sandblad, L.; Heino, J.; Takada, Y.; Mould, A.P.; Schnapp, L.M.; Gall, J.; et al. Enteric species F human adenoviruses use laminin-binding integrins as co-receptors for infection of Ht-29 cells. Sci. Rep. 2018, 8, 10019. [Google Scholar] [CrossRef]
- Soudais, C.; Laplace-Buihe, C.; Kissa, K.; Kremer, E.J. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 2001, 15, 2283–2285. [Google Scholar] [CrossRef]
- Simao, D.; Pinto, C.; Fernandes, P.; Peddie, C.J.; Piersanti, S.; Collinson, L.M.; Salinas, S.; Saggio, I.; Schiavo, G.; Kremer, E.J.; et al. Evaluation of helper-dependent canine adenovirus vectors in a 3D CNS model. Gene Ther. 2016, 23, 86–94. [Google Scholar] [CrossRef]
- Huang, X.; Griffiths, M.; Wu, J.; Farese, R.V., Jr.; Sheppard, D. Normal development, would healing, and adenovirus susceptibility in beta5-deficient mice. Mol. Cell Biol. 2000, 20, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Hautala, T.; Grunst, T.; Fabrega, A.; Freimuth, P.; Welsh, M.J. An interaction between penton base and av integrins plays a minimal role in adenovirus-mediated gen transfer to hepatocytes in vitro and in vivo. Gene Ther. 1998, 5, 1259–1264. [Google Scholar] [CrossRef]
- Parker, A.L.; Waddinton, S.N.; Nicol, C.G.; Shayakhmetov, D.M.; Buckley, S.M.; Denby, L.; Kemball-Cook, G.; Ni, S.; Lieber, A.; McVey, J.H.; et al. Multiple vitamin-K dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006, 108, 2554–2561. [Google Scholar] [CrossRef]
- Chesnokova, L.S.; Nishimura, S.L.; Hutt-Fletcher, L.M. Fusion of epithelial cells by Epstein-Barr Virus proteins is triggered by binding of vial glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc. Natl. Acad. Sci. USA 2009, 106, 20464–20469. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Shang, Y.; Zhan, Z.; Liu, X. How foot-and-mouth disease virus receptor mediates foot-and-mouth disease virus infection. Virol. J. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Brown, E.J.; Ginsberg, M.H.; Mackow, E.R. Cellular entry of Hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by b3 integrins. J. Virol. 1999, 73, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Roelvink, P.W.; Lee, G.M.; Einfeld, D.A.; Kovesdi, I.; Wickham, T.J. Identification of a conserved receptor-binding site on the fiber proteins of CAR recognizing adenoviridae. Science 1999, 286, 1568–1571. [Google Scholar] [CrossRef]
- Einfeld, D.A.; Schroeder, R.; Roelvink, P.W.; Lizonova, A.; King, C.R.; Wickham, T.J. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 2001, 75, 11284–11291. [Google Scholar] [CrossRef]
- Akiyama, M.; Thorne, S.; Kirn, D.; Roelvink, P.W.; Einfeld, D.A.; King, C.R.; Wickham, T.J. Ablating CAR and Integrin Binding in adenovirus vectors reduces nontarget organ transduction and permits sustained bloodstream persistence following intraperitoneal administration. Mol. Ther. 2004, 9, 218–230. [Google Scholar] [CrossRef]
- Bilbao, G.; Contreras, J.L.; Dmitriev, I.; Smyth, C.A.; Jenkins, S.; Eckhoff, D.; Thomas, F.; Curiel, D.T. Genetically modified adenovirus vector containing an RGD peptide in the HI loop of the fiber knob improves gene transfer to nonhuman primate isolated pancreatic islets. Am. J. Transpl. 2002, 2, 237–243. [Google Scholar] [CrossRef]
- Wu, H.; Seki, T.; Dmitriev, I.; Uil, T.; Kashentseva, E.; Han, T.; Curiel, D.T. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackie-adenovirus receptor-independent gene transfer efficiency. Hum. Gene Ther. 2002, 13, 1647–1653. [Google Scholar] [CrossRef]
- Van Putten, E.H.P.; Kleijn, A.; van Beusechem, V.W.; Noske, D.; Lamers, C.H.J.; de Goede, A.L.; Idema, S.; Hoefnagel, D.; Klozeman, J.J.; Fueyo, J.; et al. Convection enhanced delivery of the oncolytic adenovirus delta24-RGD in patients with recurrent GBM: A phase 1 clinical trial including correlative studies. Clin. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef]
- Robertson, S.; Parker, A.L.; Clarke, C.; Duffy, M.R.; Alba, R.; Nicklin, S.A.; Baker, A.H. Retargeting FX-binding-ablated HAdV-5 to vascular cells by inclusion of the RGD-4C peptide in hexon hypervariable region 7 and the HI loop. J. Gen. Virol. 2016, 97, 1911–1916. [Google Scholar] [CrossRef]
- Nestic, D.; Hozic, A.; Brkljaca, Z.; Butorac, A.; Pazur, K.; Jullienne, B.; Cindric, M.; Amriovic-Ristov, A.; Benihoud, K.; Majhen, D. Integrin avb3 and disulfide bonds play important roles in NGR-retargeted adenovirus transduction efficiency. Life Sci. 2022, 291, 120116. [Google Scholar] [CrossRef]
- Corti, A.; Curnis, F. Tumor vasculature targeting through NGR peptide-based drug delivery systems. Curr. Pharm. Biotechnol. 2011, 12, 1128–1134. [Google Scholar] [CrossRef]
- Sengupta, S.; Ulasov, I.V.; Thaci, B.; Ahmed, A.U.; Lesniak, M.S. Enhanced transduction and Replication of RGD-fiber modified adenovirus in primary T cells. PLoS ONE 2011, 6, e18091. [Google Scholar] [CrossRef]
- Balvers, R.K.; Belcaid, Z.; den Hengel, S.K.V.; Kloezeman, J.; de Vrij, J.; Wakimoto, H.; Hoeben, R.C.; Debets, R.; Leenstra, S.; Dirven, C.; et al. Locally-delivered T-cell-derived cellular vehicles efficiently track and deliver adenovirus delta24-RGD to infiltrating glioma. Viruses 2014, 6, 3080–3096. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Chekhonin, V.P. A new insight into aggregation of oncolytic adenovirus Ad5-delta-24- RGD during CsCl gradient ultracentrifugation. Sci. Rep. 2021, 11, 16088. [Google Scholar] [CrossRef]
- Koivisto, L.; Bi, J.; Hakkinen, L.; Larjava, H. Integrin avb6: Structure, function and role in health and disease. Int. J. Biochem. Cell Biol. 2018, 99, 186–196. [Google Scholar] [CrossRef]
- Haapasalmi, K.; Zhang, K.; Tonnesen, M.; Olerud, J.; Sheppard, D.; Salo, T.; Kramer, R.; Clark, R.A.F.; Uitto, V.-J.; Larjava, H. Keratinocytes in human wounds express avb6 integrin. J. Investig. Dermat. 1996, 106, 42–48. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Raghavan, S. Defining the role of integrin alphavbeta6 in cancer. Curr. Drug Targets 2009, 10, 645–652. [Google Scholar] [CrossRef]
- Guida, J.D.; Fejer, G.; Pirofski, L.A.; Brosnun, C.F.; Horwitz, M.S. Mouse adenovirus type 1 causes a fatal hemorrhagic encephalomyelitis in adult C57Bl/6 but not Balb/C mice. J. Virol. 1995, 69, 7674–7681. [Google Scholar] [CrossRef]
- Coughlan, L.; Vallath, S.; Saha, A.; Flak, M.; McNeishm, I.A.; Vassaux, G. In vivo retargeting of adenovirus type 5 to alphavbeta6 integrin results in reduced hepatocytotoxicity and improved tumor uptake following systemic delivery. J. Virol. 2009, 83, 6416–6428. [Google Scholar] [CrossRef]
- Uusi-Kerttula, H.; Davies, J.; Coughlan, L.; Hulin-Curtis, S.; Jones, R.; Hanna, L.; Chester, J.D.; Parker, A.L. Pseudotyped alphavbeta6 integrin-targeted adenovirus vectors for ovarian cancer therapies. Oncotarget 2016, 7, 27926–27937. [Google Scholar] [CrossRef]
- Davies, J.A.; Marlow, G.; Uusi-Kerttula, H.K.; Seaton, G.; Piggott, L.; Badder, L.M.; Clarkson, R.W.E.; Chester, J.D.; Parker, A.L. Efficient intravenous tumor targeting using the avb6 integrin-selective precision virotherapy Ad5null-A20. Viruses 2021, 13, 864. [Google Scholar] [CrossRef]
- Uusi-Kerttula, H.; Davies, J.A.; Thompson, J.; Wongthida, P.; Evgin, L.; Shim, K.G.; Bradshaw, A.; Baker, A.T.; Rizkallah, P.J.; Jones, R.; et al. Ad5Null-A20: A tropism modified, avb6 integrin-selective oncolytic adenovirus for epithelial ovarian cancer therapies. Clin. Cancer. Res. 2018, 24, 4215–4224. [Google Scholar] [CrossRef]
- Man, Y.K.S.; Davies, J.A.; Coughlan, L.; Pantelidou, C.; Blazquez-Moreno, A.; Marshall, J.F.; Hallden, G. The novel oncolytic adenoviral mutant Ad5-3delta-A20T retargeted to avb6 integrins efficiently eliminates pancreatic cancer cells. Mol. Cancer Ther. 2018, 17, 575–587. [Google Scholar] [CrossRef]
- Man, Y.K.S.; Foster, J.; Carapuca, E.; Davies, J.A.; Parker, A.L.; Sosabowski, J.; Hallden, G. Systemic delivery and SPECT/CT in vivo imaging of 125I-labelled oncolytic adenoviral mutants in models of pancreatic cancer. Sci. Rep. 2019, 9, 12840. [Google Scholar]
- Matilainen, H.; Makela, A.R.; Riikonen, R.; Saloniemi, R.; Korhonen, E.; Hyypia, T.; Heino, J.; Grabherr, R.; Oker-Blom, C. RGD motifs on the surface of baculovirus enhance transduction of human lung carcinoma cells. J. Biotechnol. 2006, 125, 114–126. [Google Scholar] [CrossRef]
- Qui, W.; Chandra, J.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Kesharwani, P.; Cao, H.L. New opportunities for RGD-engineered metal nanoparticles in cancer. Mol. Cancer 2023, 22, 87. [Google Scholar]
- Tang, R.; Xue, J.; Xu, B.; Shen, D.; Sudlow, G.P.; Achilefu, S. Tunable ultrasmall visible-to-extended near-infrared emitting silver sulfide quantum dots for integrin-targeted cancer imaging. ACS Nano 2015, 9, 220–230. [Google Scholar] [CrossRef]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Preat, V. Comparison of active, passive, and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent progress of RGD modified liposomes as multistage rocket against cancer. Front. Pharmacol. 2022, 12, 803304. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil- the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Koudelka, S.; Turanek, J. Liposomal paclitaxel formulations. J. Control. Release 2012, 163, 322–334. [Google Scholar] [CrossRef]
- Amin, M.; Badiee, A.; Jaafari, M.R. Improvement of pharmacokinetic and antitumor activity of PEGylated doxorubicin by targeting with N-methylated cyclic RGD peptide in mice bearing C-26 carcinomas. Int. J. Pharm. 2013, 458, 324–333. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, R.; Chen, J.; Kuang, Q.; Tang, J.; Mei, L.; Zhang, Q.; Gao, H.; Zhang, Z.; He, Q. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials 2014, 35, 4835–4847. [Google Scholar] [CrossRef]
- Man, Y.K.S.; Aguirre-Hernandez, C.; Fernandez, A.; Martin-Duque, P.; Gonzalez, R.; Hallden, G. Complexing the oncolytic Ad∆∆ and Ad-3∆∆-A-20T with cationic nanoparticles enhances viral infection and spread in prostate and pancreatic cancer models. Int. J. Mol. Sci. 2022, 23, 8884. [Google Scholar] [CrossRef]
- Vigne, E.; Mahfouz, I.; Dedieu, J.F.; Brie, A.; Perricaudet, M.; Yeh, P. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J. Virol. 1999, 73, 5156–5161. [Google Scholar] [CrossRef]
- Besson, S.; Vragniau, C.; Vassal-Stermann, E.; Dagher, M.C.; Fender, P. The adenovirus dodecahedron: Beyond the platonic story. Viruses 2020, 12, 718. [Google Scholar] [CrossRef]
- Rome, L.H.; Kickhoefer, V.A. Development of the vault particle as a platform technology. ACS Nano 2013, 7, 889–902. [Google Scholar] [CrossRef]
- Mikyas, Y.; Makabi, M.; Raval-Fernandes, S.; Harrington, L.; Kickhoefer, V.A.; Rome, L.H. Cryoelectron microscopy imaging of recombinant and tissue derived vaults: Localization of the MVP N termini and VPARP. J. Mol. Biol. 2004, 344, 91–105. [Google Scholar] [CrossRef]
- Han, M.; Kickhoefer, V.A.; Nemerow, G.R.; Rome, L.H. Targeted vault nanoparticles engineered with and endosomalytic peptide deliver biomolecules to the cytoplasm. ACS Nano 2011, 5, 6128–6137. [Google Scholar] [CrossRef][Green Version]
- Lai, C.Y.; Wiethoff, C.M.; Kickhoefer, V.A.; Rome, L.H.; Nemerow, G.R. Vault nanoparticles containing an adenovirus-derived membrane lytic protein facilitate toxin and gene transfer. ACS Nano 2009, 3, 691–699. [Google Scholar] [CrossRef]
- Xia, Y.; Ramgopal, Y.; Li, H.; Shang, L.; Srinivas, P.; Kickhoefer, V.A.; Rome, L.H.; Preiser, P.R.; Boey, F.; Zhang, H.; et al. Immobilization of recombinant vault nanoparticles on solid substrates. ACS Nano 2010, 4, 1417–1424. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemerow, G.R. Integrin-Targeting Strategies for Adenovirus Gene Therapy. Viruses 2024, 16, 770. https://doi.org/10.3390/v16050770
Nemerow GR. Integrin-Targeting Strategies for Adenovirus Gene Therapy. Viruses. 2024; 16(5):770. https://doi.org/10.3390/v16050770
Chicago/Turabian StyleNemerow, Glen R. 2024. "Integrin-Targeting Strategies for Adenovirus Gene Therapy" Viruses 16, no. 5: 770. https://doi.org/10.3390/v16050770
APA StyleNemerow, G. R. (2024). Integrin-Targeting Strategies for Adenovirus Gene Therapy. Viruses, 16(5), 770. https://doi.org/10.3390/v16050770




