DMSO and Its Role in Differentiation Impact Efficacy of Human Adenovirus (HAdV) Infection in HepaRG Cells
Abstract
1. Introduction
2. Materials and Methods
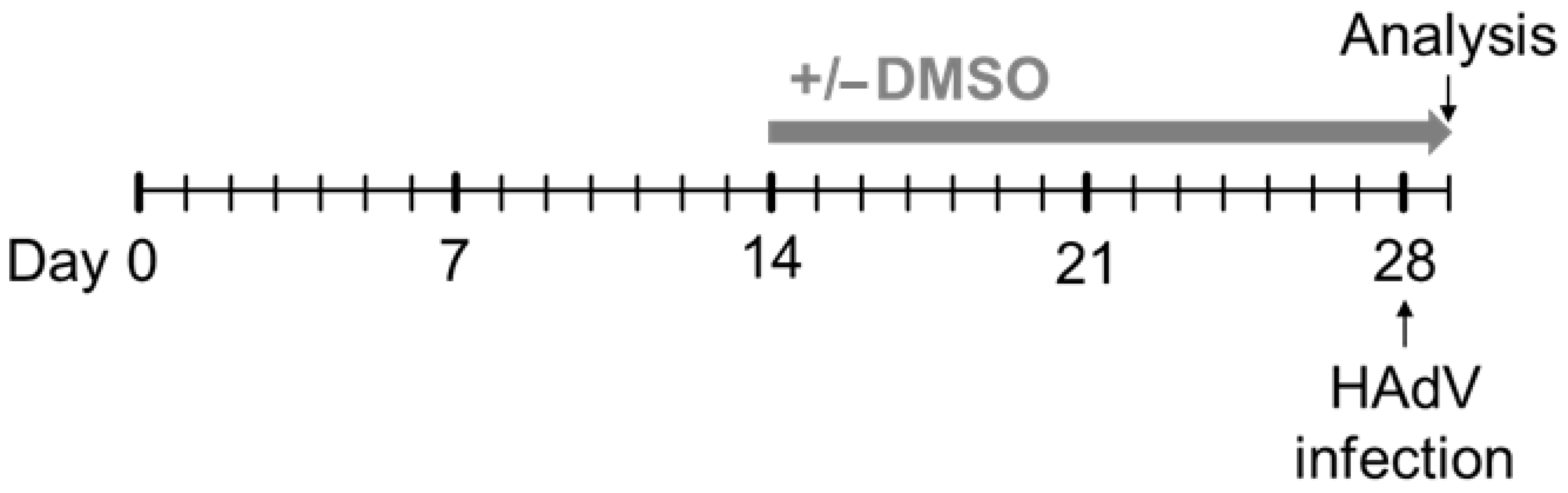
2.1. Cell Culture
2.2. Viruses and Viral Infection
2.3. Cytotoxicity and Cell Viability Assays
2.4. Antibodies and Protein Analysis
2.5. Quantification of Viral DNA
2.6. Transfection of HepaRG Cells
2.7. Statistical Analyses
3. Results
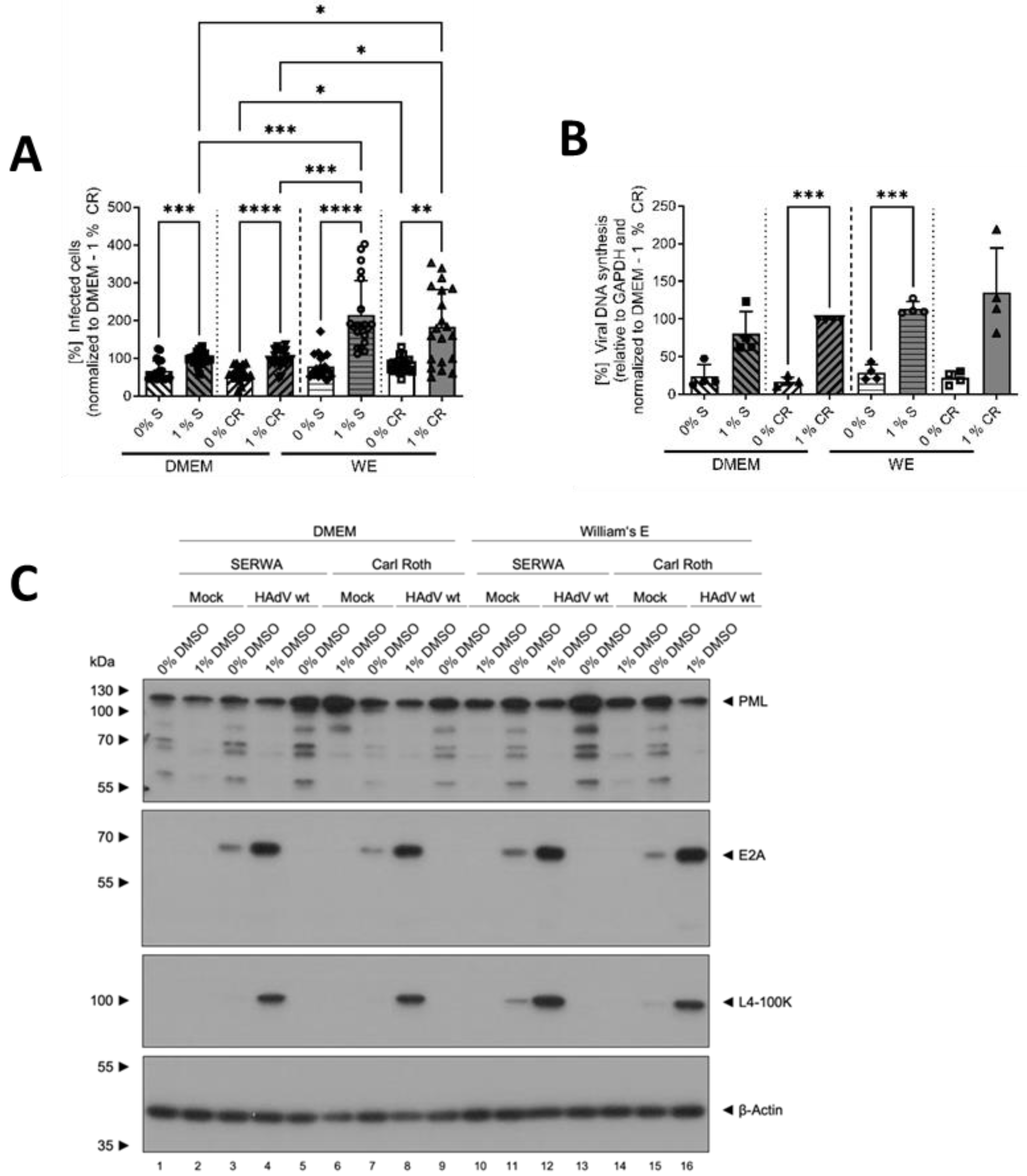
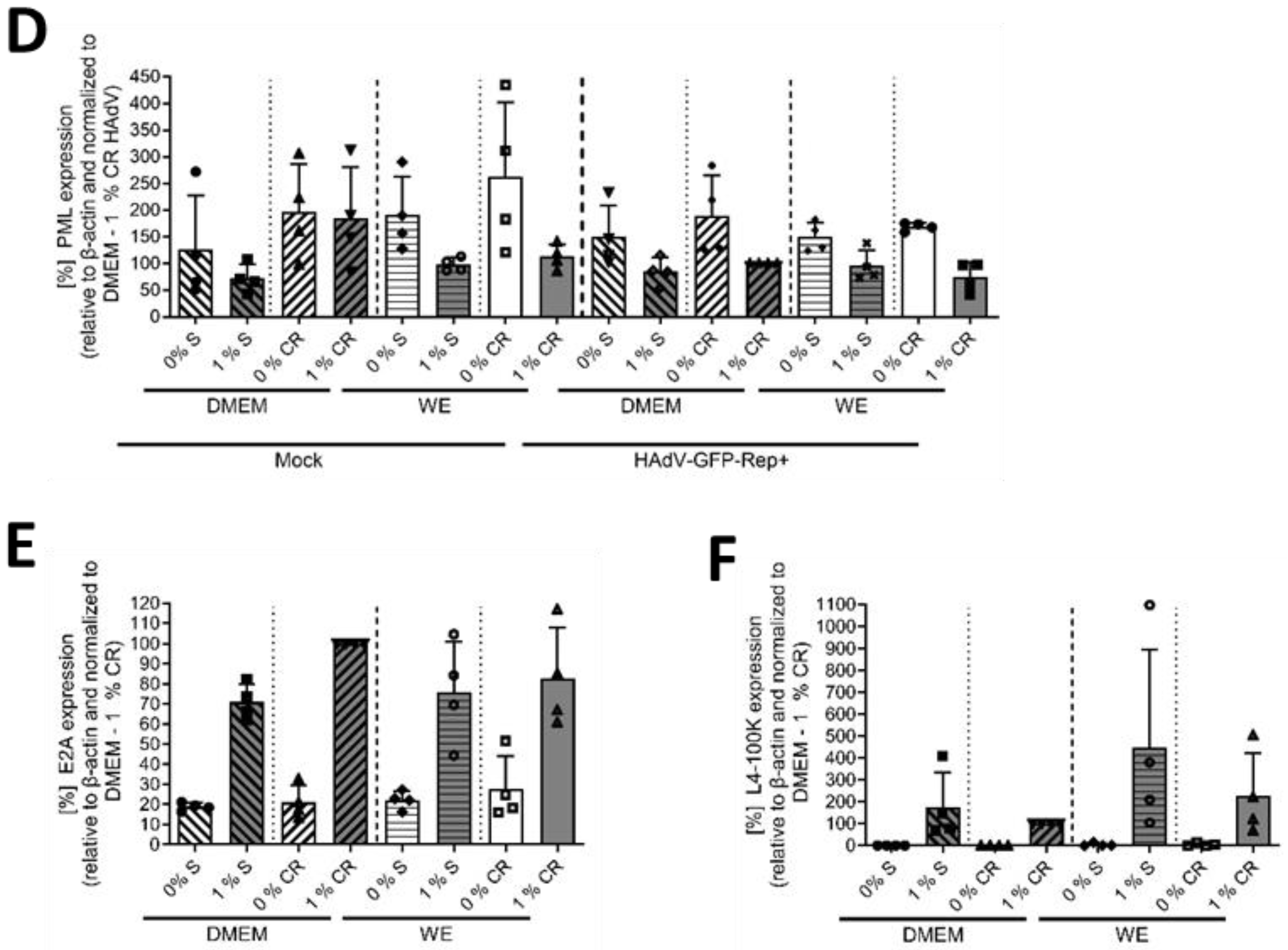
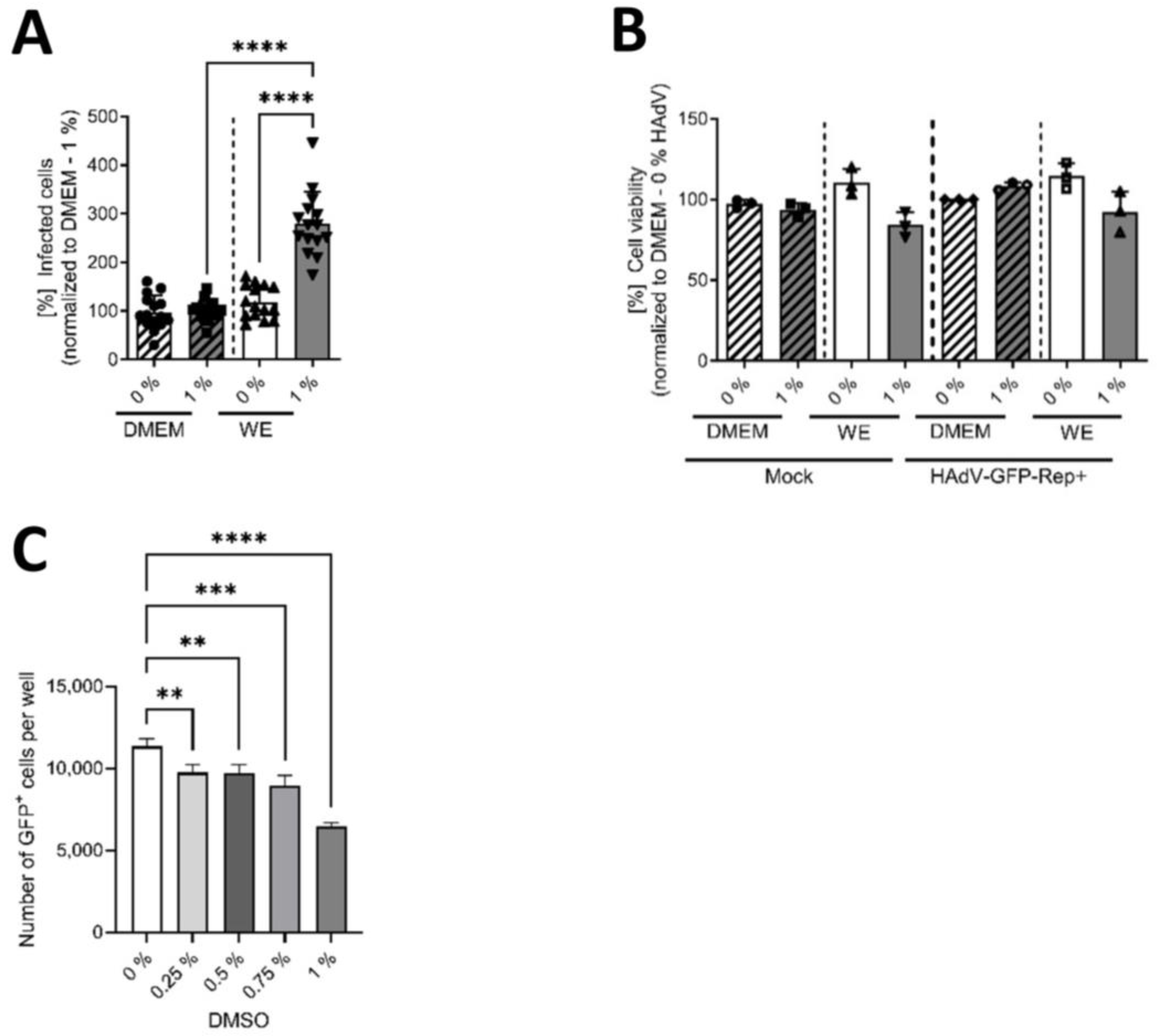
3.1. DMSO Increased the Infectability of HepaRG Cells Depending on the Type of Cell-Culture Medium
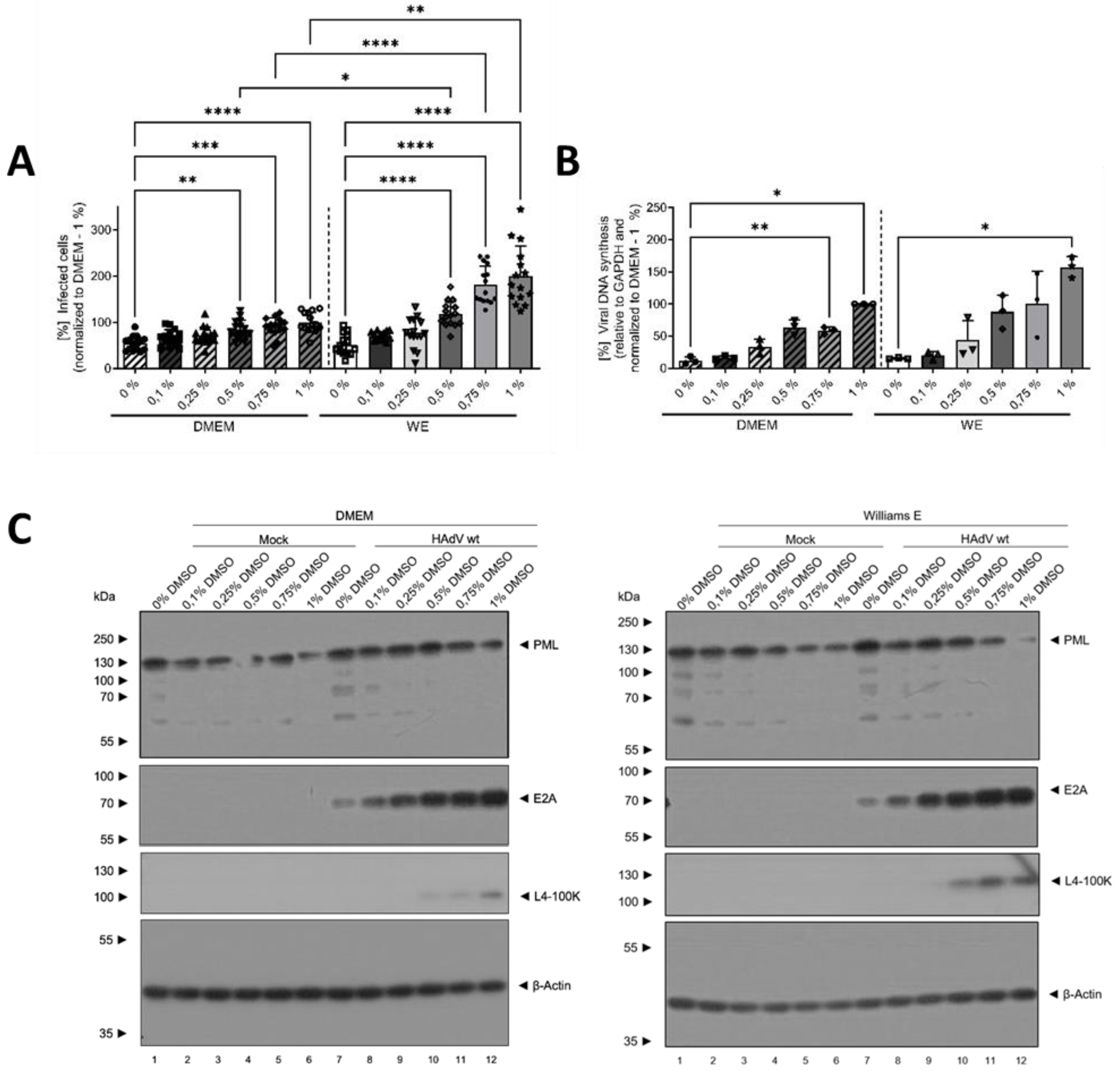
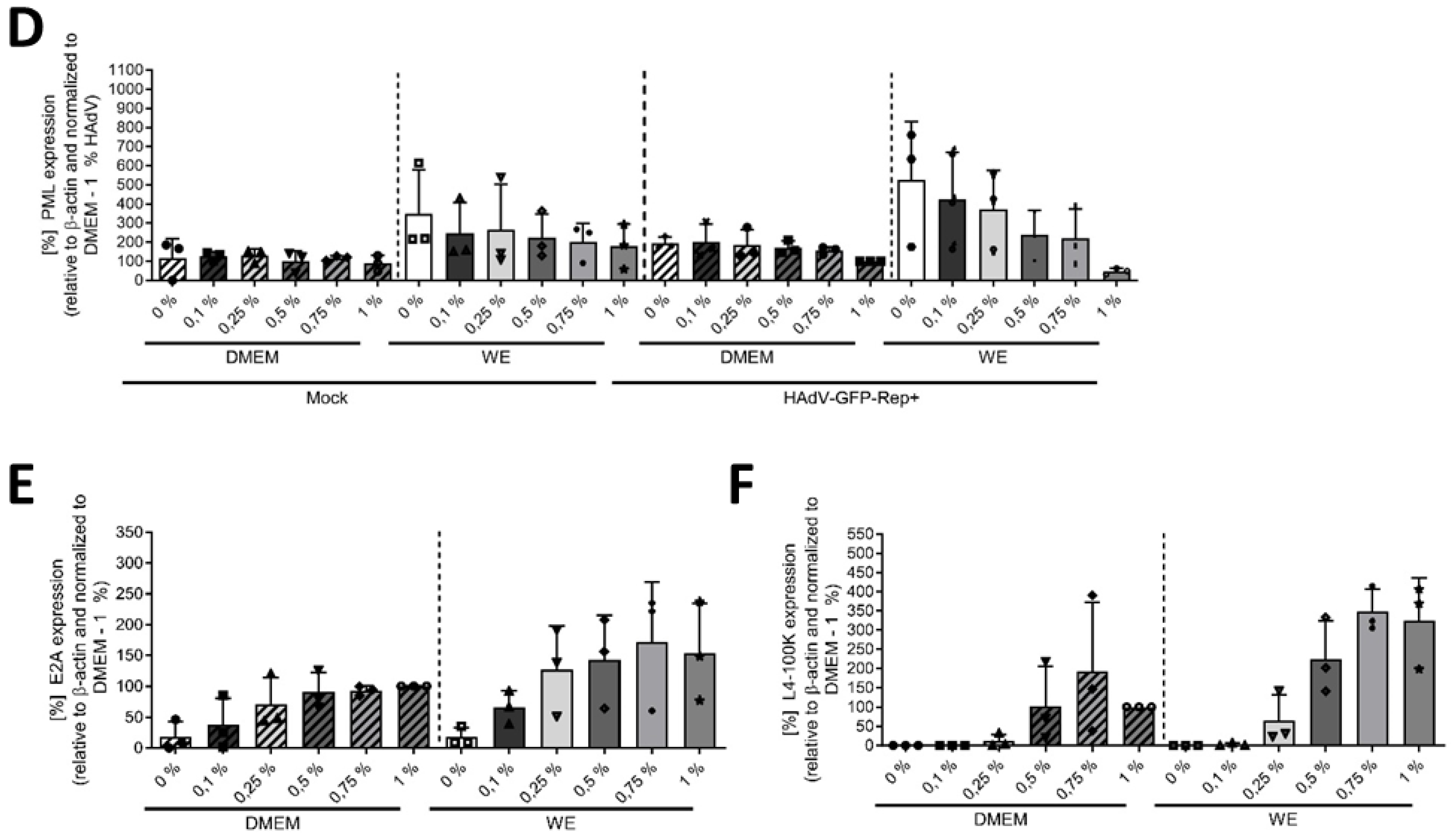
3.2. DMSO Dose-Dependently Increased HAdV Infection in HepaRG Cells
3.3. The Mechanism of Action of DMSO Was Based on Viral Entry and Not on Viral Replication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillouzo, A. Liver cell models in in vitro toxicology. Environ. Health Perspect. 1998, 106 (Suppl. 2), 511–532. [Google Scholar] [CrossRef] [PubMed]
- Guillouzo, A.; Morel, F.; Fardel, O.; Meunier, B. Use of human hepatocyte cultures for drug metabolism studies. Toxicology 1993, 82, 209–219. [Google Scholar] [CrossRef] [PubMed]
- LeCluyse, E.L. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur. J. Pharm. Sci. 2001, 13, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Pot-Schneider, H.; Aninat, C.; Kattler, K.; Fekir, K.; Jarnouen, K.; Cerec, V.; Glaise, D.; Salhab, A.; Gasparoni, G.; Takashi, K.; et al. Transcriptional and Epigenetic Consequences of DMSO Treatment on HepaRG Cells. Cells 2022, 11, 2298. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, C.; Hendriks, D.F.G.; Heyd, B.; Bachellier, P.; Ingelman-Sundberg, M.; Richert, L. Inter-individual differences in the susceptibility of primary human hepatocytes towards drug-induced cholestasis are compound and time dependent. Toxicol. Lett. 2018, 295, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Verrier, E.R.; Colpitts, C.C.; Schuster, C.; Zeisel, M.B.; Baumert, T.F. Cell Culture Models for the Investigation of Hepatitis B and D Virus Infection. Viruses 2016, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Gripon, P.; Diot, C.; Thézé, N.; Fourel, I.; Loreal, O.; Brechot, C.; Guguen-Guillouzo, C. Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J. Virol. 1988, 62, 4136–4143. [Google Scholar] [CrossRef] [PubMed]
- Guillouzo, A.; Corlu, A.; Aninat, C.; Glaise, D.; Morel, F.; Guguen-Guillouzo, C. The human hepatoma HepaRG cells: A highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem. Biol. Interact. 2007, 168, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Ma, X.; Zou, W.; Wang, C.; Bahbahan, I.S.; Ahuja, T.P.; Tolstikov, V.; Zern, M.A. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells 2010, 28, 674–686. [Google Scholar] [CrossRef]
- Anthérieu, S.; Chesné, C.; Li, R.; Guguen-Guillouzo, C.; Guillouzo, A. Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol. In Vitro 2012, 26, 1278–1285. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed]
- Isom, H.C.; Secott, T.; Georgoff, I.; Woodworth, C.; Mummaw, J. Maintenance of differentiated rat hepatocytes in primary culture. Proc. Natl. Acad. Sci. USA 1985, 82, 3252–3256. [Google Scholar] [CrossRef] [PubMed]
- Isom, I.; Georgoff, I.; Salditt-Georgieff, M.; Darnell, J.E., Jr. Persistence of liver-specific messenger RNA in cultured hepatocytes: Different regulatory events for different genes. J. Cell Biol. 1987, 105, 2877–2885. [Google Scholar] [CrossRef]
- Andersson, T.B.; Kanebratt, K.P.; Kenna, J.G. The HepaRG cell line: A unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin. Drug Metab. Toxicol. 2012, 8, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Anthérieu, S.; Chesné, C.; Li, R.; Camus, S.; Lahoz, A.; Picazo, L.; Turpeinen, M.; Tolonen, A.; Uusitalo, J.; Guguen-Guillouzo, C.; et al. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab. Dispos. 2010, 38, 516–525. [Google Scholar] [CrossRef]
- Gripon, P.; Rumin, S.; Urban, S.; Le Seyec, J.; Glaise, D.; Cannie, I.; Guyomard, C.; Lucas, J.; Trepo, C.; Guguen-Guillouzo, C. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 2002, 99, 15655–15660. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, M.; Ikegami, K.; Kremenska, Y.; Hattori, N.; Tanaka, S.; Yagi, S.; Shiota, K. Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells 2006, 24, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, L.; De Clercq, E.; Naesens, L. Clinical features and treatment of adenovirus infections. Rev. Med. Virol. 2008, 18, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Seto, D. “Human Adenovirus Working Group”, Retrieved 15 September 2023. Available online: http://hadvwg.gmu.edu/ (accessed on 15 September 2023).
- Dodge, M.J.; MacNeil, K.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Emerging antiviral therapeutics for human adenovirus infection: Recent developments and novel strategies. Antiviral Res. 2021, 188, 105034. [Google Scholar] [CrossRef]
- Saint-Pierre Contreras, G.; Conei Valencia, D.; Lizama, L.; Vargas Zuniga, D.; Avendano Carvajal, L.F.; Ampuero Llanos, S. An Old Acquaintance: Could Adenoviruses Be Our Next Pandemic Threat? Viruses 2023, 15, 330. [Google Scholar] [CrossRef]
- Rocholl, C.; Gerber, K.; Daly, J.; Pavia, A.T.; Byington, C.L. Adenoviral infections in children: The impact of rapid diagnosis. Pediatrics 2004, 113, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Miyamura, K.; Oba, T.; Terakura, S.; Kasai, M.; Kitaori, K.; Sasaki, T.; Kodera, Y. Oral ribavirin for severe adenovirus infection after allogeneic marrow transplantation. Bone Marrow Transpl. 2003, 32, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R.; Eid, A.J. Viral infections in transplant recipients. Minerva Med. 2009, 100, 479–501. [Google Scholar] [PubMed]
- Martínez-Aguado, P.; Serna-Gallego, A.; Marrugal-Lorenzo, J.A.; Gómez-Marín, I.; Sánchez-Céspedes, J. Antiadenovirus drug discovery: Potential targets and evaluation methodologies. Drug Discov. Today 2015, 20, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, S.; Wimmer, P.; Sirma, H.; Everett, R.D.; Blanchette, P.; Groitl, P.; Dobner, T. Proteasome-dependent degradation of Daxx by the viral E1B-55K protein in human adenovirus-infected cells. J. Virol. 2010, 84, 7029–7038. [Google Scholar] [CrossRef] [PubMed]
- Marion, M.J.; Hantz, O.; Durantel, D. The HepaRG cell line: Biological properties and relevance as a tool for cell biology, drug metabolism, and virology studies. Methods Mol. Biol. 2010, 640, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Mai, J.; Masser, S.; Groitl, P.; Herrmann, A.; Sternsdorf, T.; Brack-Werner, R.; Schreiner, S. ATO (Arsenic Trioxide) Effects on Promyelocytic Leukemia Nuclear Bodies Reveals Antiviral Intervention Capacity. Adv. Sci. 2020, 7, 1902130. [Google Scholar] [CrossRef]
- Reich, N.C.; Sarnow, P.; Duprey, E.; Levine, A.J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology 1983, 128, 480–484. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Kremmer, E.; Hofmann, M.; Wolf, H.; Dobner, T. Protein arginine methylation during lytic adenovirus infection. Biochem. J. 2004, 383, 259–265. [Google Scholar] [CrossRef][Green Version]
- Hofmann, S.; Plank, V.; Groitl, P.; Skvorc, N.; Hofmann, K.; Luther, J.; Ko, C.; Zimmerman, P.; Bruss, V.; Stadler, D.; et al. SUMO Modification of Hepatitis B Virus Core Mediates Nuclear Entry, Promyelocytic Leukemia Nuclear Body Association, and Efficient Formation of Covalently Closed Circular DNA. Microbiol. Spectr. 2023, 11, e0044623. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Stubbe, M.; Mai, J.; Schreiner, S. Double-edged role of PML nuclear bodies during human adenovirus infection. Virus Res. 2021, 295, 198280. [Google Scholar] [CrossRef] [PubMed]
- Mork, L.M.; Isaksson, B.; Boran, N.; Ericzon, B.G.; Strom, S.; Fischler, B.; Ellis, E. Comparison of culture media for bile Acid transport studies in primary human hepatocytes. J. Clin. Exp. Hepatol. 2012, 2, 315–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chandra, P.; Lecluyse, E.L.; Brouwer, K.L. Optimization of culture conditions for determining hepatobiliary disposition of taurocholate in sandwich-cultured rat hepatocytes. Vitr. Cell. Dev. Biol. Anim. 2001, 37, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Noya, F.; Balague, C.; Banerjee, N.S.; Curiel, D.T.; Broker, T.R.; Chow, L.T. Activation of adenovirus early promoters and lytic phase in differentiated strata of organotypic cultures of human keratinocytes. J. Virol. 2003, 77, 6533–6540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herbst, R.S.; Pelletier, M.; Boczko, E.M.; Babiss, L.E. The state of cellular differentiation determines the activity of the adenovirus E1A enhancer element: Evidence for negative regulation of enhancer function. J. Virol. 1990, 64, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Laporta, R.F.; Taichman, L.B. Adenovirus type-2 infection of human keratinocytes: Viral expression dependent upon the state of cellular maturation. Virology 1981, 110, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.C.; Braithwaite, A.W.; Silvestro, M.; Bellett, A.J. E1a-dependent expression of adenovirus genes in OTF963 embryonal carcinoma cells: Role of E1a-induced differentiation. Proc. Natl. Acad. Sci. USA 1990, 87, 8041–8045. [Google Scholar] [CrossRef] [PubMed]
- Taichman, L.B.; Reilly, S.S.; LaPorta, R.F. The role of keratinocyte differentiation in the expression of epitheliotropic viruses. J. Investig. Dermatol. 1983, 81, 137s–140s. [Google Scholar] [CrossRef][Green Version]
- Tiainen, M.; Spitkovsky, D.; Jansen-Durr, P.; Sacchi, A.; Crescenzi, M. Expression of E1A in terminally differentiated muscle cells reactivates the cell cycle and suppresses tissue-specific genes by separable mechanisms. Mol. Cell. Biol. 1996, 16, 5302–5312. [Google Scholar] [CrossRef]
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.D.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Wu, C.-H.; Chen, S.-J.; Sytwu, H.-K.; Lin, G.-J. Immunomodulatory effects and potential clinical applications of dimethyl sulfoxide. Immunobiology 2020, 225, 151906. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Nakamura, H.; Lam, V.; Hofs, E.; Cederberg, R.; Cait, J.; Hughes, M.R.; Lee, L.; Jia, W.; Adomat, H.H.; et al. DMSO Represses Inflammatory Cytokine Production from Human Blood Cells and Reduces Autoimmune Arthritis. PLoS ONE 2016, 11, e0152538. [Google Scholar] [CrossRef] [PubMed]
- Hollebeeck, S.; Raas, T.; Piront, N.; Schneider, Y.J.; Toussaint, O.; Larondelle, Y.; During, A. Dimethyl sulfoxide (DMSO) attenuates the inflammatory response in the in vitro intestinal Caco-2 cell model. Toxicol. Lett. 2011, 206, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Shayakhmetov, D.M. Cytokine Responses to Adenovirus and Adenovirus Vectors. Viruses 2022, 14, 888. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Stubbe, M.; Hofmann, S.; Masser, S.; Dobner, T.; Boutell, C.; Groitl, P.; Schreiner, S. PML Alternative Splice Products Differentially Regulate HAdV Productive Infection. Microbiol. Spectr. 2022, 10, e0078522. [Google Scholar] [CrossRef]
- Geigert, J.; DeWitt, S.K.; Neidleman, S.L.; Lee, G.; Dalietos, D.J.; Moreland, M. DMSO is a substrate for chloroperoxidase. Biochem. Biophys. Res. Commun. 1983, 116, 82–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, K.; Hofmann, S.; Weigl, F.; Mai, J.; Schreiner, S. DMSO and Its Role in Differentiation Impact Efficacy of Human Adenovirus (HAdV) Infection in HepaRG Cells. Viruses 2024, 16, 633. https://doi.org/10.3390/v16040633
Hofmann K, Hofmann S, Weigl F, Mai J, Schreiner S. DMSO and Its Role in Differentiation Impact Efficacy of Human Adenovirus (HAdV) Infection in HepaRG Cells. Viruses. 2024; 16(4):633. https://doi.org/10.3390/v16040633
Chicago/Turabian StyleHofmann, Katharina, Samuel Hofmann, Franziska Weigl, Julia Mai, and Sabrina Schreiner. 2024. "DMSO and Its Role in Differentiation Impact Efficacy of Human Adenovirus (HAdV) Infection in HepaRG Cells" Viruses 16, no. 4: 633. https://doi.org/10.3390/v16040633
APA StyleHofmann, K., Hofmann, S., Weigl, F., Mai, J., & Schreiner, S. (2024). DMSO and Its Role in Differentiation Impact Efficacy of Human Adenovirus (HAdV) Infection in HepaRG Cells. Viruses, 16(4), 633. https://doi.org/10.3390/v16040633





