Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications
Abstract
1. A Historical Introduction into the Discovery of the Adenovirus
2. Adenovirus Biology and Clinical Presentation
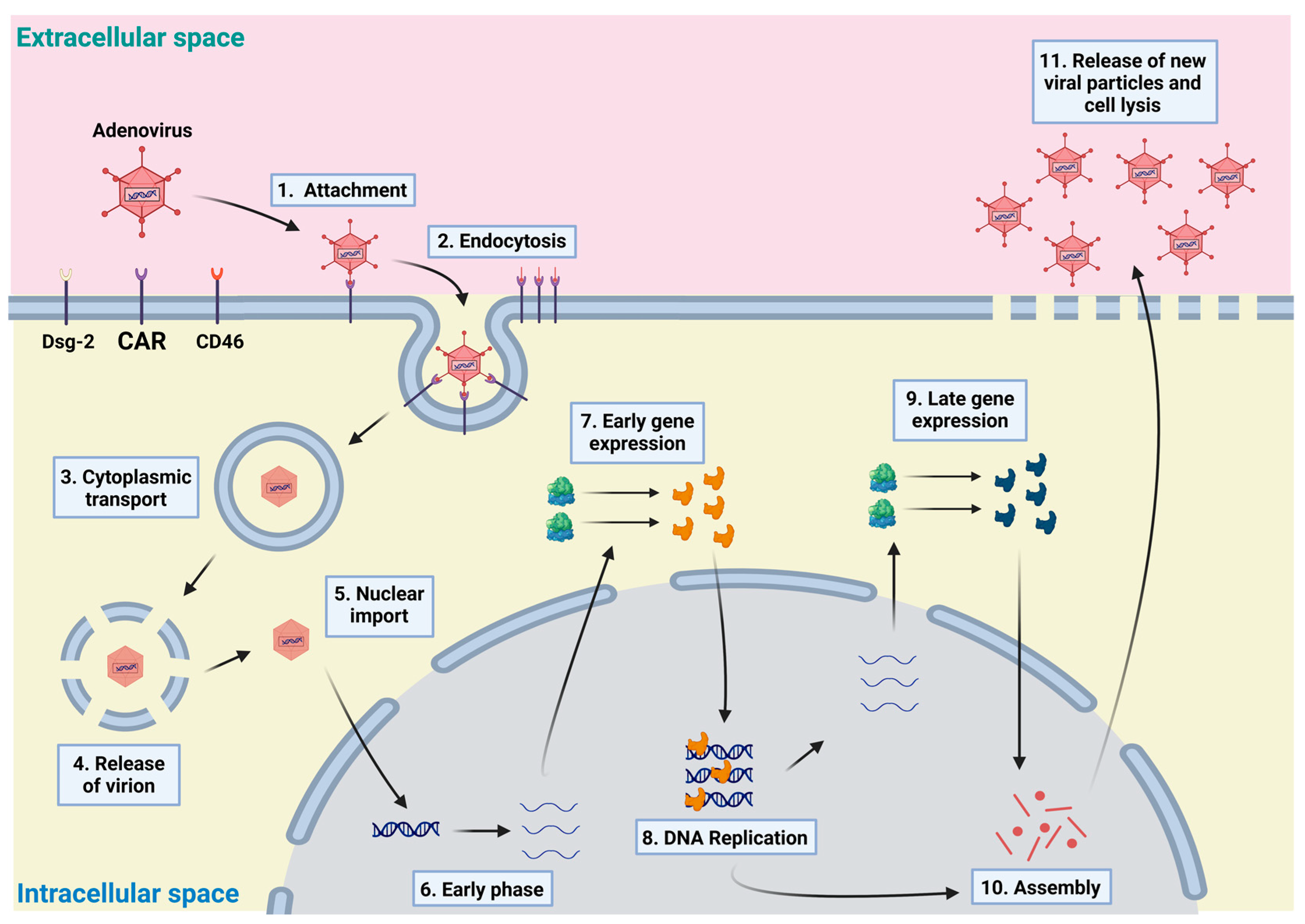
3. Adenovirus as a Vector and Its Production on a Large Scale
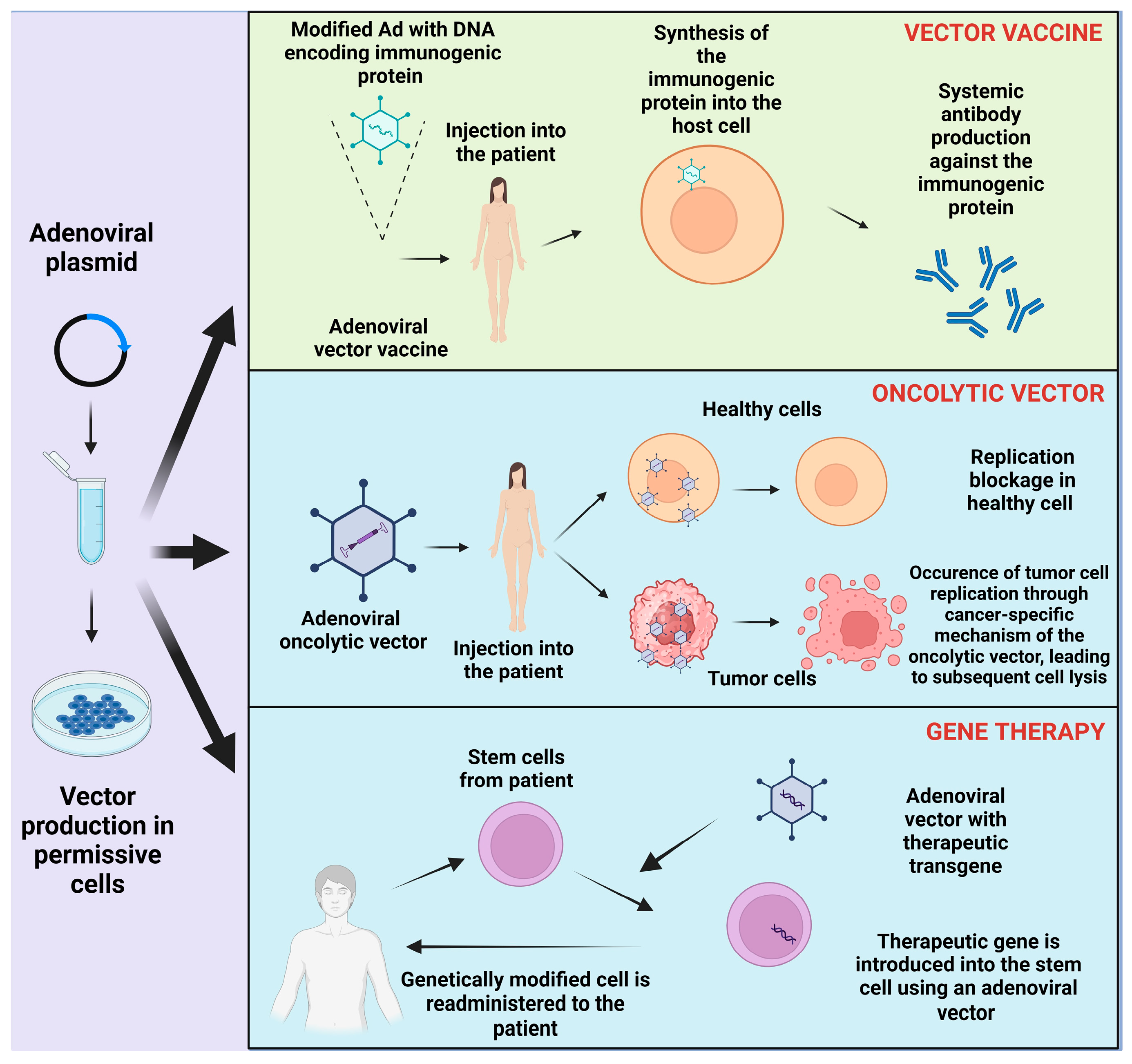
4. Clinical Application
4.1. Regenerative Medicine
4.2. Adenoviral Vector Vaccine
4.3. Adenoviral Vectors and Gene Editing
4.4. Oncolytic Adenovirus and Cancer Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowe, W.P.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.H.; Ward, T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953, 84, 570–573. [Google Scholar] [CrossRef]
- Harrach, B.; Tarján, Z.L.; Benkő, M. Adenoviruses across the animal kingdom: A walk in the zoo. FEBS Lett. 2019, 593, 3660–3673. [Google Scholar] [CrossRef]
- Pereira, H.G. Adenoviruses of man and animals. Dev. Biol. Stand. 1975, 28, 28–41. [Google Scholar]
- Gray, G.C.; Callahan, J.D.; Hawksworth, A.W.; Fisher, C.A.; Gaydos, J.C. Respiratory diseases among U.S. military personnel: Countering emerging threats. Emerg. Infect. Dis. 1999, 5, 379–385. [Google Scholar] [CrossRef]
- Hilleman, M.R.; Werner, J.H. Recovery of new agent from patients with acute respiratory illness. Proc. Soc. Exp. Biol. Med. 1954, 85, 183–188. [Google Scholar] [CrossRef]
- Huebner, R.J.; Rowe, W.P.; Ward, T.G.; Parrott, R.H.; Bell, J.A. Adenoidal-pharyngeal-conjunctival agents: A newly recognized group of common viruses of the respiratory system. N. Engl. J. Med. 1954, 251, 1077–1086. [Google Scholar] [CrossRef]
- Enders, J.F.; Bell, J.A.; Dingle, J.H.; Francis, T.; Hilleman, M.R.; Huebner, R.J.; Payne, A.M. Adenoviruses: Group name proposed for new respiratory-tract viruses. Science 1956, 124, 119–120. [Google Scholar] [CrossRef]
- Shenk, T. Adenoviridae: The Viruses and Their Replication, Fields Virology, 3rd ed.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; Volume 2. [Google Scholar]
- Roelvink, P.W.; Mi Lee, G.; Einfeld, D.A.; Kovesdi, I.; Wickham, T.J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 1999, 286, 1568–1571. [Google Scholar] [CrossRef]
- Smith, J.G.; Wiethoff, C.M.; Stewart, P.L.; Nemerow, G.R. Adenovirus. Curr. Top. Microbiol. Immunol. 2010, 343, 195–224. [Google Scholar] [CrossRef]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003, 9, 1408–1412. [Google Scholar] [CrossRef]
- Hanaoka, N.; Hazama, M.; Fukushima, K.; Fujimoto, T. Sensitivity of Human Mastadenovirus, the Causal Agent of Pharyngoconjunctival Fever, Epidemic Keratoconjunctivitis, and Hemorrhagic Cystitis in Immunocompromised Individuals, to Brincidofovir. Microbiol. Spectr. 2022, 10, e0156921. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.-Y.; Liu, Y.; Persson, J.; Beyer, I.; Möller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.-B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Lu, R.; Zhao, Y.; Xie, Z.; Shen, J.; Tan, W. Phylogenetic evidence for intratypic recombinant events in a novel human adenovirus C that causes severe acute respiratory infection in children. Sci. Rep. 2016, 6, 23014. [Google Scholar] [CrossRef]
- Robinson, C.M.; Singh, G.; Lee, J.Y.; Dehghan, S.; Rajaiya, J.; Liu, E.B.; Yousuf, M.A.; Betensky, R.A.; Jones, M.S.; Dyer, D.W.; et al. Molecular evolution of human adenoviruses. Sci. Rep. 2013, 3, 1812. [Google Scholar] [CrossRef]
- van Oostrum, J.; Burnett, R.M. Molecular composition of the adenovirus type 2 virion. J. Virol. 1985, 56, 439–448. [Google Scholar] [CrossRef]
- Nemerow, G.R.; Stewart, P.L.; Reddy, V.S. Structure of human adenovirus. Curr. Opin. Virol. 2012, 2, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rux, J.J.; Kuser, P.R.; Burnett, R.M. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J. Virol. 2003, 77, 9553–9566. [Google Scholar] [CrossRef]
- Burnett, R.M.; Grütter, M.G.; White, J.L. The structure of the adenovirus capsid. I. An envelope model of hexon at 6 A resolution. J. Mol. Biol. 1985, 185, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Condezo, G.N.; Martín-González, N.; Pérez-Illana, M.; Hernando-Pérez, M.; Gallardo, J.; San Martín, C. Adenoviruses (Adenoviridae) and their structural relatives. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–344. [Google Scholar]
- Crawford-Miksza, L.; Schnurr, D.P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 1996, 70, 1836–1844. [Google Scholar] [CrossRef]
- Sevvana, M.; Klose, T.; Rossmann, M.G. Principles of Virus Structure. In Encyclopedia of Virology; Elsevier Academic Press: Amsterdam, The Netherlands, 2021; pp. 257–277. ISBN 9780128145166. [Google Scholar]
- Caspar, D.L.; Klug, A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol. 1962, 27, 1–24. [Google Scholar] [CrossRef]
- Liu, H.; Jin, L.; Koh, S.B.S.; Atanasov, I.; Schein, S.; Wu, L.; Zhou, Z.H. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science 2010, 329, 1038–1043. [Google Scholar] [CrossRef]
- Zubieta, C.; Schoehn, G.; Chroboczek, J.; Cusack, S. The structure of the human adenovirus 2 penton. Mol. Cell 2005, 17, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.L.; Fuller, S.D.; Burnett, R.M. Difference imaging of adenovirus: Bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993, 12, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Menéndez-Conejero, R.; San Martín, C.; van Raaij, M.J. Crystal structure of the fibre head domain of the Atadenovirus Snake Adenovirus 1. PLoS ONE 2014, 9, e114373. [Google Scholar] [CrossRef] [PubMed]
- Chroboczek, J.; Ruigrok, R.W.; Cusack, S. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 1995, 199 Pt 1, 163–200. [Google Scholar] [CrossRef]
- Gallardo, J.; Pérez-Illana, M.; Martín-González, N.; San Martín, C. Adenovirus Structure: What Is New? Int. J. Mol. Sci. 2021, 22, 5240. [Google Scholar] [CrossRef]
- Ma, H.-C.; Hearing, P. Adenovirus structural protein IIIa is involved in the serotype specificity of viral DNA packaging. J. Virol. 2011, 85, 7849–7855. [Google Scholar] [CrossRef]
- Davison, A.J.; Benkő, M.; Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef]
- Martín-González, N.; Gómez-González, A.; Hernando-Pérez, M.; Bauer, M.; Greber, U.F.; San Martín, C.; de Pablo, P.J. Adenovirus core protein V reinforces the capsid and enhances genome release from disrupted particles. Sci. Adv. 2023, 9, eade9910. [Google Scholar] [CrossRef]
- Pérez-Berná, A.J.; Marion, S.; Chichón, F.J.; Fernández, J.J.; Winkler, D.C.; Carrascosa, J.L.; Steven, A.C.; Šiber, A.; San Martín, C. Distribution of DNA-condensing protein complexes in the adenovirus core. Nucleic Acids Res. 2015, 43, 4274–4283. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [CrossRef]
- Tomko, R.P.; Xu, R.; Philipson, L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 1997, 94, 3352–3356. [Google Scholar] [CrossRef] [PubMed]
- Bewley, M.C.; Springer, K.; Zhang, Y.B.; Freimuth, P.; Flanagan, J.M. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 1999, 286, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Marttila, M.; Persson, D.; Gustafsson, D.; Liszewski, M.K.; Atkinson, J.P.; Wadell, G.; Arnberg, N. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 2005, 79, 14429–14436. [Google Scholar] [CrossRef]
- Nilsson, E.C.; Storm, R.J.; Bauer, J.; Johansson, S.M.C.; Lookene, A.; Ångström, J.; Hedenström, M.; Eriksson, T.L.; Frängsmyr, L.; Rinaldi, S.; et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011, 17, 105–109. [Google Scholar] [CrossRef]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Frängsmyr, L.; Frank, M.; Blaum, B.S.; Chai, W.; Podgorski, I.I.; Harrach, B.; Benkő, M.; et al. Polysialic acid is a cellular receptor for human adenovirus 52. Proc. Natl. Acad. Sci. USA 2018, 115, E4264–E4273. [Google Scholar] [CrossRef]
- Lenman, A.; Müller, S.; Nygren, M.I.; Frängsmyr, L.; Stehle, T.; Arnberg, N. Coagulation factor IX mediates serotype-specific binding of species A adenoviruses to host cells. J. Virol. 2011, 85, 13420–13431. [Google Scholar] [CrossRef]
- Arnberg, N. Adenovirus receptors: Implications for targeting of viral vectors. Trends Pharmacol. Sci. 2012, 33, 442–448. [Google Scholar] [CrossRef]
- Findlay, J.S.; Cook, G.P.; Blair, G.E. Blood Coagulation Factor X Exerts Differential Effects on Adenovirus Entry into Human Lymphocytes. Viruses 2018, 10, 20. [Google Scholar] [CrossRef]
- Jonsson, M.I.; Lenman, A.E.; Frängsmyr, L.; Nyberg, C.; Abdullahi, M.; Arnberg, N. Coagulation factors IX and X enhance binding and infection of adenovirus types 5 and 31 in human epithelial cells. J. Virol. 2009, 83, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Doszpoly, A.; Harrach, B.; LaPatra, S.; Benkő, M. Unconventional gene arrangement and content revealed by full genome analysis of the white sturgeon adenovirus, the single member of the genus Ichtadenovirus. Infect. Genet. Evol. 2019, 75, 103976. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Wright, K.M.; Harrach, B. DNA sequence of frog adenovirus. J. Gen. Virol. 2000, 81, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Ostapchuk, P.; Hearing, P. Control of adenovirus packaging. J. Cell. Biochem. 2005, 96, 25–35. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.J.; Hearing, P. The C-terminal 70 amino acids of the adenovirus E4-ORF6/7 protein are essential and sufficient for E2F complex formation. Nucleic Acids Res. 1991, 19, 6579–6586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lutz, P.; Rosa-Calatrava, M.; Kedinger, C. The product of the adenovirus intermediate gene IX is a transcriptional activator. J. Virol. 1997, 71, 5102–5109. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.M.; Hearing, P. The adenovirus early region 4 open reading frame 6/7 protein regulates the DNA binding activity of the cellular transcription factor, E2F, through a direct complex. Genes Dev. 1989, 3, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.; Engel, D.A.; Shenk, T. An adenovirus early region 4 gene product is required for induction of the infection-specific form of cellular E2F activity. Genes Dev. 1989, 3, 1062–1074. [Google Scholar] [CrossRef]
- Chang, L.S.; Shenk, T. The adenovirus DNA-binding protein stimulates the rate of transcription directed by adenovirus and adeno-associated virus promoters. J. Virol. 1990, 64, 2103–2109. [Google Scholar] [CrossRef]
- Fessler, S.P.; Young, C.S. Control of adenovirus early gene expression during the late phase of infection. J. Virol. 1998, 72, 4049–4056. [Google Scholar] [CrossRef] [PubMed]
- Berk, A.J. Functions of adenovirus E1A. Cancer Surv. 1986, 5, 367–387. [Google Scholar] [PubMed]
- Kulanayake, S.; Tikoo, S.K. Adenovirus Core Proteins: Structure and Function. Viruses 2021, 13, 388. [Google Scholar] [CrossRef]
- Hall, K.; Blair Zajdel, M.E.; Blair, G.E. Unity and diversity in the human adenoviruses: Exploiting alternative entry pathways for gene therapy. Biochem. J. 2010, 431, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Giberson, A.N.; Davidson, A.R.; Parks, R.J. Chromatin structure of adenovirus DNA throughout infection. Nucleic Acids Res. 2012, 40, 2369–2376. [Google Scholar] [CrossRef]
- Ishida, S.; Fujinaga, Y.; Fujinaga, K.; Sakamoto, N.; Hashimoto, S. Unusual splice sites in the E1A-E1B cotranscripts synthesized in adenovirus type 40-infected A549 cells. Arch. Virol. 1994, 139, 389–402. [Google Scholar] [CrossRef]
- Lillie, J.W.; Loewenstein, P.M.; Green, M.R.; Green, M. Functional domains of adenovirus type 5 E1a proteins. Cell 1987, 50, 1091–1100. [Google Scholar] [CrossRef]
- Avvakumov, N.; Wheeler, R.; D’Halluin, J.C.; Mymryk, J.S. Comparative sequence analysis of the largest E1A proteins of human and simian adenoviruses. J. Virol. 2002, 76, 7968–7975. [Google Scholar] [CrossRef]
- Steegenga, W.T.; van Laar, T.; Riteco, N.; Mandarino, A.; Shvarts, A.; van der Eb, A.J.; Jochemsen, A.G. Adenovirus E1A proteins inhibit activation of transcription by p53. Mol. Cell. Biol. 1996, 16, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, P.; Houweling, A.; van der Eb, A. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology 1983, 128, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Boulakia, C.A.; Chen, G.; Ng, F.W.; Teodoro, J.G.; Branton, P.E.; Nicholson, D.W.; Poirier, G.G.; Shore, G.C. Bcl-2 and adenovirus E1B 19 kDA protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymerase. Oncogene 1996, 12, 529–535. [Google Scholar]
- Hidalgo, P.; Ip, W.H.; Dobner, T.; Gonzalez, R.A. The biology of the adenovirus E1B 55K protein. FEBS Lett. 2019, 593, 3504–3517. [Google Scholar] [CrossRef] [PubMed]
- Caravokyri, C.; Leppard, K.N. Human adenovirus type 5 variants with sequence alterations flanking the E2A gene: Effects on E2 expression and DNA replication. Virus Genes 1996, 12, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Deryckere, F.; Ebenau-Jehle, C.; Wold, W.S.; Burgert, H.G. Tumor necrosis factor alpha increases expression of adenovirus E3 proteins. Immunobiology 1995, 193, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Sparer, T.E.; Tripp, R.A.; Dillehay, D.L.; Hermiston, T.W.; Wold, W.S.; Gooding, L.R. The role of human adenovirus early region 3 proteins (gp19K, 10.4K, 14.5K, and 14.7K) in a murine pneumonia model. J. Virol. 1996, 70, 2431–2439. [Google Scholar] [CrossRef]
- Täuber, B.; Dobner, T. Molecular regulation and biological function of adenovirus early genes: The E4 ORFs. Gene 2001, 278, 1–23. [Google Scholar] [CrossRef]
- Lichtenstein, D.L.; Toth, K.; Doronin, K.; Tollefson, A.E.; Wold, W.S.M. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 2004, 23, 75–111. [Google Scholar] [CrossRef]
- Sandler, A.B.; Ketner, G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J. Virol. 1989, 63, 624–630. [Google Scholar] [CrossRef]
- Seto, D.; Chodosh, J.; Brister, J.R.; Jones, M.S. Using the whole-genome sequence to characterize and name human adenoviruses. J. Virol. 2011, 85, 5701–5702. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Characterization of an upstream regulatory element of adenovirus L1 poly (A) site. Virology 2005, 337, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Seggern, D.J.V.; Chiu, C.Y.; Fleck, S.K.; Stewart, P.L.; Nemerow, G.R. A helper-independent adenovirus vector with E1, E3, and fiber deleted: Structure and infectivity of fiberless particles. J. Virol. 1999, 73, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.J.; Nemerow, G.R. Adenovirus tales: From the cell surface to the nuclear pore complex. PLoS Pathog. 2015, 11, e1004821. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Flatt, J.W. Adenovirus Entry: From Infection to Immunity. Annu. Rev. Virol. 2019, 6, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R. Cell receptors involved in adenovirus entry. Virology 2000, 274, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Nestić, D.; Božinović, K.; Pehar, I.; Wallace, R.; Parker, A.L.; Majhen, D. The Revolving Door of Adenovirus Cell Entry: Not All Pathways Are Equal. Pharmaceutics 2021, 13, 1585. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005, 79, 1992–2000. [Google Scholar] [CrossRef]
- Cassany, A.; Ragues, J.; Guan, T.; Bégu, D.; Wodrich, H.; Kann, M.; Nemerow, G.R.; Gerace, L. Nuclear import of adenovirus DNA involves direct interaction of hexon with an N-terminal domain of the nucleoporin Nup214. J. Virol. 2015, 89, 1719–1730. [Google Scholar] [CrossRef]
- Smith, J.G.; Silvestry, M.; Lindert, S.; Lu, W.; Nemerow, G.R.; Stewart, P.L. Insight into the mechanisms of adenovirus capsid disassembly from studies of defensin neutralization. PLoS Pathog. 2010, 6, e1000959. [Google Scholar] [CrossRef]
- Pied, N.; Wodrich, H. Imaging the adenovirus infection cycle. FEBS Lett. 2019, 593, 3419–3448. [Google Scholar] [CrossRef]
- Wang, I.-H.; Burckhardt, C.J.; Yakimovich, A.; Morf, M.K.; Greber, U.F. The nuclear export factor CRM1 controls juxta-nuclear microtubule-dependent virus transport. J. Cell Sci. 2017, 130, 2185–2195. [Google Scholar] [CrossRef]
- Trotman, L.C.; Mosberger, N.; Fornerod, M.; Stidwill, R.P.; Greber, U.F. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat. Cell Biol. 2001, 3, 1092–1100. [Google Scholar] [CrossRef]
- Greber, U.F.; Suomalainen, M. Adenovirus entry: Stability, uncoating, and nuclear import. Mol. Microbiol. 2022, 118, 309–320. [Google Scholar] [CrossRef]
- Charman, M.; Herrmann, C.; Weitzman, M.D. Viral and cellular interactions during adenovirus DNA replication. FEBS Lett. 2019, 593, 3531–3550. [Google Scholar] [CrossRef]
- Condezo, G.N.; San Martín, C. Localization of adenovirus morphogenesis players, together with visualization of assembly intermediates and failed products, favor a model where assembly and packaging occur concurrently at the periphery of the replication center. PLoS Pathog. 2017, 13, e1006320. [Google Scholar] [CrossRef]
- Hammarskjöld, M.L.; Winberg, G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell 1980, 20, 787–795. [Google Scholar] [CrossRef]
- Murali, V.K.; Ornelles, D.A.; Gooding, L.R.; Wilms, H.T.; Huang, W.; Tollefson, A.E.; Wold, W.S.M.; Garnett-Benson, C. Adenovirus death protein (ADP) is required for lytic infection of human lymphocytes. J. Virol. 2014, 88, 903–912. [Google Scholar] [CrossRef]
- Doronin, K.; Toth, K.; Kuppuswamy, M.; Krajcsi, P.; Tollefson, A.E.; Wold, W.S.M. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology 2003, 305, 378–387. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, T.; Wang, C.-B.; Liang, W.-L.; Lian, G.-W.; Zanin, M.; Wong, S.-S.; Tian, X.-G.; Zhong, J.-Y.; Zhang, Y.-Y.; et al. Human adenovirus (HAdV) infection in children with acute respiratory tract infections in Guangzhou, China, 2010-2021: A molecular epidemiology study. World J. Pediatr. 2022, 18, 545–552. [Google Scholar] [CrossRef]
- Radke, J.R.; Cook, J.L. Human adenovirus infections: Update and consideration of mechanisms of viral persistence. Curr. Opin. Infect. Dis. 2018, 31, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.S.; Taylor, B.; Reimels, W.; Barrett, F.F.; Devincenzo, J.P. Adenovirus 7a: A community-acquired outbreak in a children’s hospital. Pediatr. Infect. Dis. J. 2000, 19, 996–1000. [Google Scholar] [CrossRef]
- Lynch, J.P.; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Kajon, A.E.; Moseley, J.M.; Metzgar, D.; Huong, H.-S.; Wadleigh, A.; Ryan, M.A.K.; Russell, K.L. Molecular epidemiology of adenovirus type 4 infections in US military recruits in the postvaccination era (1997–2003). J. Infect. Dis. 2007, 196, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.A.K.; Gray, G.C.; Smith, B.; McKeehan, J.A.; Hawksworth, A.W.; Malasig, M.D. Large Epidemic of Respiratory Illness Due to Adenovirus Types 7 and 3 in Healthy Young Adults. Clin. Infect. Dis. 2002, 34, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G. Adenovirus infections in transplant recipients. Clin. Infect. Dis. 2006, 43, 331–339. [Google Scholar] [CrossRef]
- Echavarría, M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008, 21, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Schwartz, R.H.; Insel, R.A.; Menegus, M.A. Severe diffuse adenovirus 7a pneumonia in a child with combined immunodeficiency: Possible therapeutic effect of human immune serum globulin containing specific neutralizing antibody. Pediatr. Infect. Dis. 1984, 3, 246–251. [Google Scholar] [CrossRef]
- Ferdman, R.M.; Ross, L.; Inderlied, C.; Church, J.A. Adenovirus viremia in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 1997, 16, 413–415. [Google Scholar] [CrossRef]
- Ismail, A.M.; Zhou, X.; Dyer, D.W.; Seto, D.; Rajaiya, J.; Chodosh, J. Genomic foundations of evolution and ocular pathogenesis in human adenovirus species D. FEBS Lett. 2019, 593, 3583–3608. [Google Scholar] [CrossRef]
- Georgi, F.; Greber, U.F. The Adenovirus Death Protein—A small membrane protein controls cell lysis and disease. FEBS Lett. 2020, 594, 1861–1878. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L. Genome Analysis of A Novel Recombinant Human Adenovirus Type 1 in China. Sci. Rep. 2019, 9, 4298. [Google Scholar] [CrossRef]
- Biserni, G.B.; Dondi, A.; Masetti, R.; Bandini, J.; Dormi, A.; Conti, F.; Pession, A.; Lanari, M. Immune Response against Adenovirus in Acute Upper Respiratory Tract Infections in Immunocompetent Children. Vaccines 2020, 8, 602. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Atasheva, S.; Shayakhmetov, D.M. Cytokine Responses to Adenovirus and Adenovirus Vectors. Viruses 2022, 14, 888. [Google Scholar] [CrossRef]
- Kühl, U.; Pauschinger, M.; Seeberg, B.; Lassner, D.; Noutsias, M.; Poller, W.; Schultheiss, H.-P. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005, 112, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Proenca-Modena, J.L.; de Souza Cardoso, R.; Criado, M.F.; Milanez, G.P.; de Souza, W.M.; Parise, P.L.; Bertol, J.W.; de Jesus, B.L.S.; Prates, M.C.M.; Silva, M.L.; et al. Human adenovirus replication and persistence in hypertrophic adenoids and palatine tonsils in children. J. Med. Virol. 2019, 91, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Kosulin, K.; Geiger, E.; Vécsei, A.; Huber, W.-D.; Rauch, M.; Brenner, E.; Wrba, F.; Hammer, K.; Innerhofer, A.; Pötschger, U.; et al. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin. Microbiol. Infect. 2016, 22, 381.e1–381.e8. [Google Scholar] [CrossRef]
- Kosulin, K.; Haberler, C.; Hainfellner, J.A.; Amann, G.; Lang, S.; Lion, T. Investigation of adenovirus occurrence in pediatric tumor entities. J. Virol. 2007, 81, 7629–7635. [Google Scholar] [CrossRef]
- Kosulin, K.; Rauch, M.; Ambros, P.F.; Pötschger, U.; Chott, A.; Jäger, U.; Drach, J.; Nader, A.; Lion, T. Screening for adenoviruses in haematological neoplasia: High prevalence in mantle cell lymphoma. Eur. J. Cancer 2014, 50, 622–627. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Yant, S.R.; Storm, T.A.; Fuess, S.; Meuse, L.; Kay, M.A. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 2001, 75, 6969–6976. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef]
- Duigou, G.J.; Young, C.S.H. Replication-competent adenovirus formation in 293 cells: The recombination-based rate is influenced by structure and location of the transgene cassette and not increased by overproduction of HsRad51, Rad51-interacting, or E2F family proteins. J. Virol. 2005, 79, 5437–5444. [Google Scholar] [CrossRef][Green Version]
- McConnell, M.J.; Imperiale, M.J. Biology of adenovirus and its use as a vector for gene therapy. Hum. Gene Ther. 2004, 15, 1022–1033. [Google Scholar] [CrossRef]
- Ewer, K.J.; Lambe, T.; Rollier, C.S.; Spencer, A.J.; Hill, A.V.; Dorrell, L. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr. Opin. Immunol. 2016, 41, 47–54. [Google Scholar] [CrossRef]
- Gorziglia, M.I.; Lapcevich, C.; Roy, S.; Kang, Q.; Kadan, M.; Wu, V.; Pechan, P.; Kaleko, M. Generation of an adenovirus vector lacking E1, e2a, E3, and all of E4 except open reading frame 3. J. Virol. 1999, 73, 6048–6055. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Kumari, R.; Mittal, S.K. Current Use of Adenovirus Vectors and Their Production Methods. Methods Mol. Biol. 2019, 1937, 155–175. [Google Scholar] [CrossRef]
- Vemula, S.V.; Mittal, S.K. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin. Biol. Ther. 2010, 10, 1469–1487. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Brough, D.E.; Lizonova, A.; Hsu, C.; Kulesa, V.A.; Kovesdi, I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J. Virol. 1996, 70, 6497–6501. [Google Scholar] [CrossRef]
- Zhang, X.; Godbey, W.T. Viral vectors for gene delivery in tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 515–534. [Google Scholar] [CrossRef]
- Lusky, M.; Christ, M.; Rittner, K.; Dieterle, A.; Dreyer, D.; Mourot, B.; Schultz, H.; Stoeckel, F.; Pavirani, A.; Mehtali, M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J. Virol. 1998, 72, 2022–2032. [Google Scholar] [CrossRef]
- Mitani, K.; Graham, F.L.; Caskey, C.T.; Kochanek, S. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc. Natl. Acad. Sci. USA 1995, 92, 3854–3858. [Google Scholar] [CrossRef]
- Parks, R.J.; Chen, L.; Anton, M.; Sankar, U.; Rudnicki, M.A.; Graham, F.L. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 1996, 93, 13565–13570. [Google Scholar] [CrossRef]
- Palmer, D.J.; Ng, P. Methods for the production of helper-dependent adenoviral vectors. Methods Mol. Biol. 2008, 433, 33–53. [Google Scholar] [CrossRef]
- Cervantes-García, D.; Ortiz-López, R.; Mayek-Pérez, N.; Rojas-Martínez, A. Oncolytic virotherapy. Ann. Hepatol. 2008, 7, 34–45. [Google Scholar] [CrossRef]
- Gouvarchin Ghaleh, H.E.; Bolandian, M.; Dorostkar, R.; Jafari, A.; Pour, M.F. Concise review on optimized methods in production and transduction of lentiviral vectors in order to facilitate immunotherapy and gene therapy. Biomed. Pharmacother. 2020, 128, 110276. [Google Scholar] [CrossRef]
- Available online: https://www.researchamerica.org/blog/ad-ding-value-the-science-behind-adenovirus-vector-vaccines/ (accessed on 1 September 2023).
- He, T.C.; Zhou, S.; da Costa, L.T.; Yu, J.; Kinzler, K.W.; Vogelstein, B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 1998, 95, 2509–2514. [Google Scholar] [CrossRef]
- Ng, P.; Parks, R.J.; Cummings, D.T.; Evelegh, C.M.; Sankar, U.; Graham, F.L. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum. Gene Ther. 1999, 10, 2667–2672. [Google Scholar] [CrossRef]
- Syyam, A.; Nawaz, A.; Ijaz, A.; Sajjad, U.; Fazil, A.; Irfan, S.; Muzaffar, A.; Shahid, M.; Idrees, M.; Malik, K.; et al. Adenovirus vector system: Construction, history and therapeutic applications. Biotechniques 2022, 73, 297–305. [Google Scholar] [CrossRef]
- Von Seggern, D.J.; Nemerow, G.R. Adenoviral Vectors for Protein Expression. Gene Expr. Syst. 2007, 5, 111–156. [Google Scholar] [CrossRef]
- Elahi, S.M.; Jiang, J.; Nazemi-Moghaddam, N.; Gilbert, R. A Method to Generate and Rescue Recombinant Adenovirus Devoid of Replication-Competent Particles in Animal-Origin-Free Culture Medium. Viruses 2023, 15, 2152. [Google Scholar] [CrossRef] [PubMed]
- Jalšić, L.; Lytvyn, V.; Elahi, S.M.; Hrapovic, S.; Nassoury, N.; Chahal, P.S.; Gaillet, B.; Gilbert, R. Inducible HEK293 AAV packaging cell lines expressing Rep proteins. Mol. Ther. Methods Clin. Dev. 2023, 30, 259–275. [Google Scholar] [CrossRef]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977, 36, 59–74. [Google Scholar] [CrossRef]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef]
- Fallaux, F.J.; Kranenburg, O.; Cramer, S.J.; Houweling, A.; van Ormondt, H.; Hoeben, R.C.; van der Eb, A.J. Characterization of 911: A new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther. 1996, 7, 215–222. [Google Scholar] [CrossRef]
- Imler, J.L.; Chartier, C.; Dreyer, D.; Dieterle, A.; Sainte-Marie, M.; Faure, T.; Pavirani, A.; Mehtali, M. Novel complementation cell lines derived from human lung carcinoma A549 cells support the growth of E1-deleted adenovirus vectors. Gene Ther. 1996, 3, 75–84. [Google Scholar]
- Murakami, P.; Pungor, E.; Files, J.; Do, L.; van Rijnsoever, R.; Vogels, R.; Bout, A.; McCaman, M. A single short stretch of homology between adenoviral vector and packaging cell line can give rise to cytopathic effect-inducing, helper-dependent E1-positive particles. Hum. Gene Ther. 2002, 13, 909–920. [Google Scholar] [CrossRef]
- Krougliak, V.; Graham, F.L. Development of cell lines capable of complementing E1, E4, and protein IX defective adenovirus type 5 mutants. Hum. Gene Ther. 1995, 6, 1575–1586. [Google Scholar] [CrossRef]
- Zhou, H.; Beaudet, A.L. A new vector system with inducible E2a cell line for production of higher titer and safer adenoviral vectors. Virology 2000, 275, 348–357. [Google Scholar] [CrossRef]
- Klessig, D.F.; Grodzicker, T.; Cleghon, V. Construction of human cell lines which contain and express the adenovirus DNA binding protein gene by cotransformation with the HSV-1 tk gene. Virus Res. 1984, 1, 169–188. [Google Scholar] [CrossRef]
- Zhou, H.; O’Neal, W.; Morral, N.; Beaudet, A.L. Development of a complementing cell line and a system for construction of adenovirus vectors with E1 and E2a deleted. J. Virol. 1996, 70, 7030–7038. [Google Scholar] [CrossRef]
- Segura, M.M.; Alba, R.; Bosch, A.; Chillón, M. Advances in helper-dependent adenoviral vector research. Curr. Gene Ther. 2008, 8, 222–235. [Google Scholar] [CrossRef]
- Hardy, S.; Kitamura, M.; Harris-Stansil, T.; Dai, Y.; Phipps, M.L. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997, 71, 1842–1849. [Google Scholar] [CrossRef]
- Kovesdi, I.; Hedley, S.J. Adenoviral producer cells. Viruses 2010, 2, 1681–1703. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Li, C.; Jiang, S.; Ma, L. A novel and simple method for construction of recombinant adenoviruses. Nucleic Acids Res. 2006, 34, e89. [Google Scholar] [CrossRef]
- Su, Q.; Sena-Esteves, M.; Gao, G. Purification of the Recombinant Adenovirus by Cesium Chloride Gradient Centrifugation. Cold Spring Harb. Protoc. 2019, 5, 374–378. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Chekhonin, V.P. A new insight into aggregation of oncolytic adenovirus Ad5-delta-24-RGD during CsCl gradient ultracentrifugation. Sci. Rep. 2021, 11, 16088. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Gordon, N.F.; Stock, R.; Petrides, D. Adenoviral Vector COVID-19 Vaccines: Process and Cost Analysis. Processes 2021, 9, 1430. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Da Mello, J.F.R.E.; Blasi, M. The use of viral vectors in vaccine development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Kajon, A.E.; Lamson, D.M.; St George, K. Emergence and re-emergence of respiratory adenoviruses in the United States. Curr. Opin. Virol. 2019, 34, 63–69. [Google Scholar] [CrossRef]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Siegfried, W.; Yoshimura, K.; Yoneyama, K.; Fukayama, M.; Stier, L.E.; Pääkkö, P.K.; Gilardi, P.; Stratford-Perricaudet, L.D.; Perricaudet, M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science 1991, 252, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, H.L.; Thorsteinsdottir, H.; Perry, G.; Renihan, J.; Singer, P.A.; Daar, A.S. Regenerative medicine: New opportunities for developing countries. IJBT 2006, 8, 60. [Google Scholar] [CrossRef]
- Jacques, E.; Suuronen, E.J. The Progression of Regenerative Medicine and its Impact on Therapy Translation. Clin. Transl. Sci. 2020, 13, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Domb, A.J.; Sharifzadeh, G.; Nahum, V. Gene Therapy for Regenerative Medicine. Pharmaceutics 2023, 15, 856. [Google Scholar] [CrossRef]
- Bukharova, T.B.; Nedorubova, I.A.; Mokrousova, V.O.; Meglei, A.Y.; Basina, V.P.; Nedorubov, A.A.; Vasilyev, A.V.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; et al. Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery. Cells 2023, 12, 1762. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.A.; Patel, S.R.; Schwarz, E.A.; Zanzonico, P.; Hahn, R.T.; Ilercil, A.; Devereux, R.B.; Goldsmith, S.J.; Christian, T.F.; Sanborn, T.A.; et al. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J. Thorac. Cardiovasc. Surg. 1998, 115, 168–176; discussion 176–177. [Google Scholar] [CrossRef]
- Schwarz, E.R.; Speakman, M.T.; Patterson, M.; Hale, S.S.; Isner, J.M.; Kedes, L.H.; Kloner, R.A. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat--angiogenesis and angioma formation. J. Am. Coll. Cardiol. 2000, 35, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Pajula, J.; Lähteenvuo, J.; Lähteenvuo, M.; Honkonen, K.; Halonen, P.; Hätinen, O.-P.; Kuivanen, A.; Heikkilä, M.; Nurro, J.; Hartikainen, J.; et al. Adenoviral VEGF-DΔN ΔC gene therapy for myocardial ischemia. Front. Bioeng. Biotechnol. 2022, 10, 999226. [Google Scholar] [CrossRef] [PubMed]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef] [PubMed]
- Appledorn, D.M.; Patial, S.; McBride, A.; Godbehere, S.; van Rooijen, N.; Parameswaran, N.; Amalfitano, A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 2008, 181, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Chéneau, C.; Eichholz, K.; Tran, T.H.; Tran, T.T.P.; Paris, O.; Henriquet, C.; Bajramovic, J.J.; Pugniere, M.; Kremer, E.J. Lactoferrin Retargets Human Adenoviruses to TLR4 to Induce an Abortive NLRP3-Associated Pyroptotic Response in Human Phagocytes. Front. Immunol. 2021, 12, 685218. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.G.; Blattman, J.N.; Kasturi, S.P.; Kelley, R.P.; Kaufman, D.R.; Lynch, D.M.; La Porte, A.; Simmons, N.L.; Clark, S.L.; Pulendran, B.; et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J. Virol. 2011, 85, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Yao, J.; Shayakhmetov, D.M. Innate immunity to adenovirus: Lessons from mice. FEBS Lett. 2019, 593, 3461–3483. [Google Scholar] [CrossRef]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef]
- Elkashif, A.; Alhashimi, M.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Adenoviral vector-based platforms for developing effective vaccines to combat respiratory viral infections. Clin. Transl. Immunol. 2021, 10, e1345. [Google Scholar] [CrossRef]
- Marquez-Martinez, S.; Vijayan, A.; Khan, S.; Zahn, R. Cell entry and innate sensing shape adaptive immune responses to adenovirus-based vaccines. Curr. Opin. Immunol. 2023, 80, 102282. [Google Scholar] [CrossRef]
- Tatsis, N.; Ertl, H.C.J. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Baden, L.R.; Liu, J.; Li, H.; Johnson, J.A.; Walsh, S.R.; Kleinjan, J.A.; Engelson, B.A.; Peter, L.; Abbink, P.; Milner, D.A.; et al. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J. Infect. Dis. 2015, 211, 518–528. [Google Scholar] [CrossRef]
- Provine, N.M.; Amini, A.; Garner, L.C.; Spencer, A.J.; Dold, C.; Hutchings, C.; Silva Reyes, L.; FitzPatrick, M.E.B.; Chinnakannan, S.; Oguti, B.; et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science 2021, 371, 521–526. [Google Scholar] [CrossRef]
- Abbink, P.; Lemckert, A.A.C.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef]
- Holterman, L.; Vogels, R.; van der Vlugt, R.; Sieuwerts, M.; Grimbergen, J.; Kaspers, J.; Geelen, E.; van der Helm, E.; Lemckert, A.; Gillissen, G.; et al. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: Low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 2004, 78, 13207–13215. [Google Scholar] [CrossRef]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.E.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef]
- Barouch, D.H.; Liu, J.; Lynch, D.M.; O’Brien, K.L.; La Porte, A.; Simmons, N.L.; Riggs, A.M.; Clark, S.; Abbink, P.; Montefiori, D.C.; et al. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J. Virol. 2009, 83, 9584–9590. [Google Scholar] [CrossRef]
- Farina, S.F.; Gao, G.P.; Xiang, Z.Q.; Rux, J.J.; Burnett, R.M.; Alvira, M.R.; Marsh, J.; Ertl, H.C.; Wilson, J.M. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 2001, 75, 11603–11613. [Google Scholar] [CrossRef]
- O’Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999, 10, 1349–1358. [Google Scholar] [CrossRef]
- Yotnda, P.; Chen, D.-H.; Chiu, W.; Piedra, P.A.; Davis, A.; Templeton, N.S.; Brenner, M.K. Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Mol. Ther. 2002, 5, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Zhang, H.; Wei, J.; Wu, J. Extracellular Vesicles-Mimetic Encapsulation Improves Oncolytic Viro-Immunotherapy in Tumors With Low Coxsackie and Adenovirus Receptor. Front. Bioeng. Biotechnol. 2020, 8, 574007. [Google Scholar] [CrossRef]
- Kremer, E.J. Pros and Cons of Adenovirus-Based SARS-CoV-2 Vaccines. Mol. Ther. 2020, 28, 2303–2304. [Google Scholar] [CrossRef]
- Hasanpourghadi, M.; Novikov, M.; Ertl, H.C.J. COVID-19 Vaccines Based on Adenovirus Vectors. Trends Biochem. Sci. 2021, 46, 429–430. [Google Scholar] [CrossRef]
- Tumban, E. Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval. Viruses 2020, 13, 54. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, S.; Liu, J.; Wu, L.; Qiu, J.; Wang, N.; Ren, J.; Li, Z.; Guo, X.; Tao, F.; et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022, 20, 400. [Google Scholar] [CrossRef]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef]
- Stanley, D.A.; Honko, A.N.; Asiedu, C.; Trefry, J.C.; Lau-Kilby, A.W.; Johnson, J.C.; Hensley, L.; Ammendola, V.; Abbate, A.; Grazioli, F.; et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014, 20, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Salisch, N.C.; Stephenson, K.E.; Williams, K.; Cox, F.; van der Fits, L.; Heerwegh, D.; Truyers, C.; Habets, M.N.; Kanjilal, D.G.; Larocca, R.A.; et al. A Double-Blind, Randomized, Placebo-Controlled Phase 1 Study of Ad26.ZIKV.001, an Ad26-Vectored Anti-Zika Virus Vaccine. Ann. Intern. Med. 2021, 174, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.; Buchbinder, S.; Duerr, A. Overview of STEP and Phambili trial results: Two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr. Opin. HIV AIDS 2010, 5, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Stieh, D.J.; Sarnecki, M.; Walsh, S.R.; Tomaras, G.D.; Kublin, J.G.; McElrath, M.J.; Alter, G.; Ferrari, G.; Montefiori, D.; et al. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): A randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet HIV 2020, 7, e688–e698. [Google Scholar] [CrossRef]
- van Kampen, K.R.; Shi, Z.; Gao, P.; Zhang, J.; Foster, K.W.; Chen, D.-T.; Marks, D.; Elmets, C.A.; Tang, D.C. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 2005, 23, 1029–1036. [Google Scholar] [CrossRef]
- Khalil, A.M. The genome editing revolution: Review. J. Genet. Eng. Biotechnol. 2020, 18, 68. [Google Scholar] [CrossRef]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Maggio, I.; Liu, J.; Janssen, J.M.; Chen, X.; Gonçalves, M.A.F.V. Adenoviral vectors encoding CRISPR/Cas9 multiplexes rescue dystrophin synthesis in unselected populations of DMD muscle cells. Sci. Rep. 2016, 6, 37051. [Google Scholar] [CrossRef]
- Stephens, C.J.; Kashentseva, E.; Everett, W.; Kaliberova, L.; Curiel, D.T. Targeted in vivo knock-in of human alpha-1-antitrypsin cDNA using adenoviral delivery of CRISPR/Cas9. Gene Ther. 2018, 25, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Sakai, E.; Iizuka, S.; Taracena-Gándara, M.; Sakurai, F.; Mizuguchi, H. Generation of the Adenovirus Vector-Mediated CRISPR/Cpf1 System and the Application for Primary Human Hepatocytes Prepared from Humanized Mice with Chimeric Liver. Biol. Pharm. Bull. 2018, 41, 1089–1095. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.; Ehrhardt, A. Expanding the Spectrum of Adenoviral Vectors for Cancer Therapy. Cancers 2020, 12, 1139. [Google Scholar] [CrossRef]
- Mulvihill, S.; Warren, R.; Venook, A.; Adler, A.; Randlev, B.; Heise, C.; Kirn, D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: A phase I trial. Gene Ther. 2001, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Gryciuk, A.; Rogalska, M.; Baran, J.; Kuryk, L.; Staniszewska, M. Oncolytic Adenoviruses Armed with Co-Stimulatory Molecules for Cancer Treatment. Cancers 2023, 15, 1947. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.R.; Kirn, D.H.; Williams, A.; Heise, C.; Horn, S.; Muna, M.; Ng, L.; Nye, J.A.; Sampson-Johannes, A.; Fattaey, A.; et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996, 274, 373–376. [Google Scholar] [CrossRef]
- Heise, C.; Hermiston, T.; Johnson, L.; Brooks, G.; Sampson-Johannes, A.; Williams, A.; Hawkins, L.; Kirn, D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000, 6, 1134–1139. [Google Scholar] [CrossRef]
- Kitajima, S.; Li, F.; Takahashi, C. Tumor Milieu Controlled by RB Tumor Suppressor. Int. J. Mol. Sci. 2020, 21, 2450. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Mach, N.; Gao, J.; Schaffarczyk, L.; Janz, S.; Ehrke-Schulz, E.; Dittmar, T.; Ehrhardt, A.; Zhang, W. Spectrum-Wide Exploration of Human Adenoviruses for Breast Cancer Therapy. Cancers 2020, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Senac, J.S.; Weaver, E.A.; May, S.M.; Jelinek, D.F.; Greipp, P.; Witzig, T.; Barry, M.A. Species D adenoviruses as oncolytics against B-cell cancers. Clin. Cancer Res. 2011, 17, 6712–6722. [Google Scholar] [CrossRef]
- Persson, B.D.; John, L.; Rafie, K.; Strebl, M.; Frängsmyr, L.; Ballmann, M.Z.; Mindler, K.; Havenga, M.; Lemckert, A.; Stehle, T.; et al. Human species D adenovirus hexon capsid protein mediates cell entry through a direct interaction with CD46. Proc. Natl. Acad. Sci. USA 2021, 118, e2020732118. [Google Scholar] [CrossRef]
- Daussy, C.F.; Pied, N.; Wodrich, H. Understanding Post Entry Sorting of Adenovirus Capsids; A Chance to Change Vaccine Vector Properties. Viruses 2021, 13, 1221. [Google Scholar] [CrossRef]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Bett, A.J.; Haddara, W.; Prevec, L.; Graham, F.L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc. Natl. Acad. Sci. USA 1994, 91, 8802–8806. [Google Scholar] [CrossRef] [PubMed]
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021, 22, 10522. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.V.; Galoforo, S.S.; Corry, P.M.; Lee, Y.J. Adenoviral-mediated transfer of a heat-inducible double suicide gene into prostate carcinoma cells. Cancer Res. 1998, 58, 1358–1362. [Google Scholar] [PubMed]
- Robinson, M.; Ge, Y.; Ko, D.; Yendluri, S.; Laflamme, G.; Hawkins, L.; Jooss, K. Comparison of the E3 and L3 regions for arming oncolytic adenoviruses to achieve a high level of tumor-specific transgene expression. Cancer Gene Ther. 2008, 15, 9–17. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Olszanski, A.J.; Nyakas, M.; Hornyak, T.J.; Wolchok, J.D.; Levitsky, V.; Kuryk, L.; Hansen, T.B.; Jäderberg, M. Pilot Study of ONCOS-102 and Pembrolizumab: Remodeling of the Tumor Microenvironment and Clinical Outcomes in Anti-PD-1-Resistant Advanced Melanoma. Clin. Cancer Res. 2023, 29, 100–109. [Google Scholar] [CrossRef]
- Havunen, R.; Kalliokoski, R.; Siurala, M.; Sorsa, S.; Santos, J.M.; Cervera-Carrascon, V.; Anttila, M.; Hemminki, A. Cytokine-Coding Oncolytic Adenovirus TILT-123 Is Safe, Selective, and Effective as a Single Agent and in Combination with Immune Checkpoint Inhibitor Anti-PD-1. Cells 2021, 10, 246. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Tian, Y.; Zhu, G.; Liu, S.; Liu, F. Efficacy of a novel double-controlled oncolytic adenovirus driven by the Ki67 core promoter and armed with IL-15 against glioblastoma cells. Cell Biosci. 2020, 10, 124. [Google Scholar] [CrossRef]
- Salzwedel, A.O.; Han, J.; LaRocca, C.J.; Shanley, R.; Yamamoto, M.; Davydova, J. Combination of interferon-expressing oncolytic adenovirus with chemotherapy and radiation is highly synergistic in hamster model of pancreatic cancer. Oncotarget 2018, 9, 18041–18052. [Google Scholar] [CrossRef]
- Cervera-Carrascon, V.; Siurala, M.; Santos, J.M.; Havunen, R.; Tähtinen, S.; Karell, P.; Sorsa, S.; Kanerva, A.; Hemminki, A. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. Oncoimmunology 2018, 7, e1412902. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol. Ther. 2023, 31, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Quixabeira, D.C.A.; Pakola, S.; Jirovec, E.; Havunen, R.; Basnet, S.; Santos, J.M.; Kudling, T.V.; Clubb, J.H.A.; Haybout, L.; Arias, V.; et al. Boosting cytotoxicity of adoptive allogeneic NK cell therapy with an oncolytic adenovirus encoding a human vIL-2 cytokine for the treatment of human ovarian cancer. Cancer Gene Ther. 2023, 30, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, J.; Ji, W.; Wang, G.; Fang, L.; Zhang, Q.; Ang, L.; Zhao, M.; Sen, Y.; Chen, L.; et al. Triple-serotype chimeric oncolytic adenovirus exerts multiple synergistic mechanisms against solid tumors. J. Immunother. Cancer 2022, 10, e004691. [Google Scholar] [CrossRef]
| Disease | Phase | Clinical Trial Number |
|---|---|---|
| HIV | I | NCT00479999 |
| Ebola | I | NCT02289027 |
| HIV | I | NCT01989533 |
| Malaria | I | NCT00371189 |
| COVID-19 disease | II | NCT05027672 |
| COVID-19 disease | I | NCT04568811 |
| Prostata cancer | II | NCT00583752 |
| Tuberculosis | I | NCT00800670 |
| Hepatitis C | I | NCT01094873 |
| Melanoma | II | NCT00010309 |
| OAd | Transgene | Disease | Phase | Clinical Trial Number |
|---|---|---|---|---|
| Ad5-yCD/mut TKSR39rep- hIL-12 | yCD, TK and hIL-12 | Prostate adenocarcinoma | I | NCT02555397 |
| Ad11p/Ad3 | No | Solid epithelial tumors | I | NCT02636036 |
| VCN-01/Ad5 based) | No | Solid tumors | I | NCT02045602 |
| AdAPT-001 | TGF-ß | Solid tumors | I | NCT04673942 |
| Ad5/3-E2F-D24-hTNFa-IRES-hIL2 (TILT-123) | TNF-α, IL-2 | Solid tumors | I | NCT04695327 |
| OAdTILT-123 | No | Melanoma | I | NCT04217473 |
| Ad5-yCD/mut TKSR39rep-ADP | Astrocytoma | I | NCT05686798 | |
| VCN-01 | No | Retinoblastoma | I | NCT03284268 |
| Ad5-yCD/mutTKSR39rep-ADP | No | Pancreas Adenocarcinoma | I | NCT04739046 |
| NG-641 | FAP, CXCL9/ CXCL10/IFNa2 | Solid epithelial tumors | I | NCT04053283 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarsella, L.; Ehrke-Schulz, E.; Paulussen, M.; Thal, S.C.; Ehrhardt, A.; Aydin, M. Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications. Viruses 2024, 16, 377. https://doi.org/10.3390/v16030377
Scarsella L, Ehrke-Schulz E, Paulussen M, Thal SC, Ehrhardt A, Aydin M. Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications. Viruses. 2024; 16(3):377. https://doi.org/10.3390/v16030377
Chicago/Turabian StyleScarsella, Luca, Eric Ehrke-Schulz, Michael Paulussen, Serge C. Thal, Anja Ehrhardt, and Malik Aydin. 2024. "Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications" Viruses 16, no. 3: 377. https://doi.org/10.3390/v16030377
APA StyleScarsella, L., Ehrke-Schulz, E., Paulussen, M., Thal, S. C., Ehrhardt, A., & Aydin, M. (2024). Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications. Viruses, 16(3), 377. https://doi.org/10.3390/v16030377





