Tixagevimab/Cilgavimab: Still a Valid Prophylaxis against COVID-19 New Variants?
Abstract
1. Background
2. Material and Methods
2.1. Design, Setting, and Participants
2.2. SARS-CoV-2 Strains and Vero E6 Cell Cultures
2.3. SARS-CoV-2 Neutralization Test
2.4. EC50 Determination
2.5. Statistical Analysis
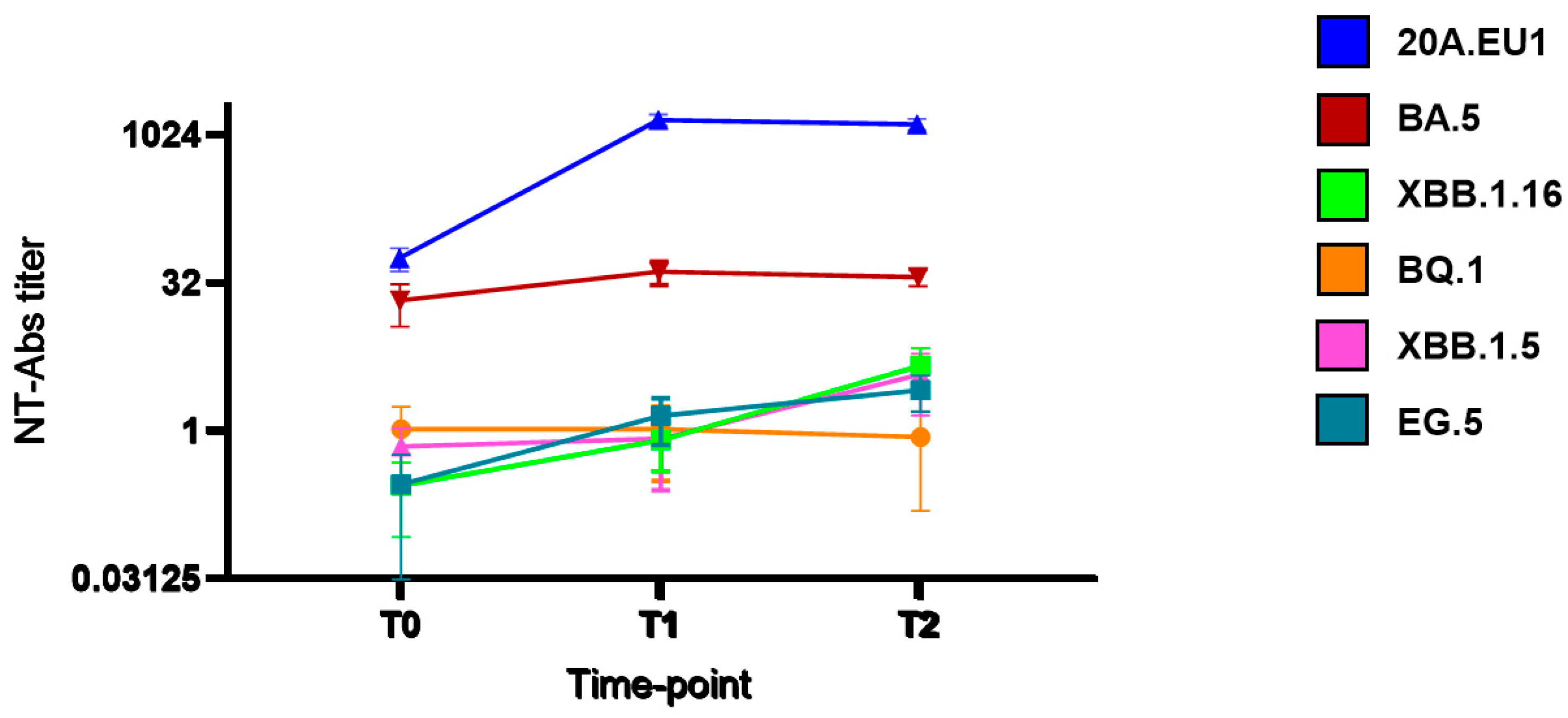
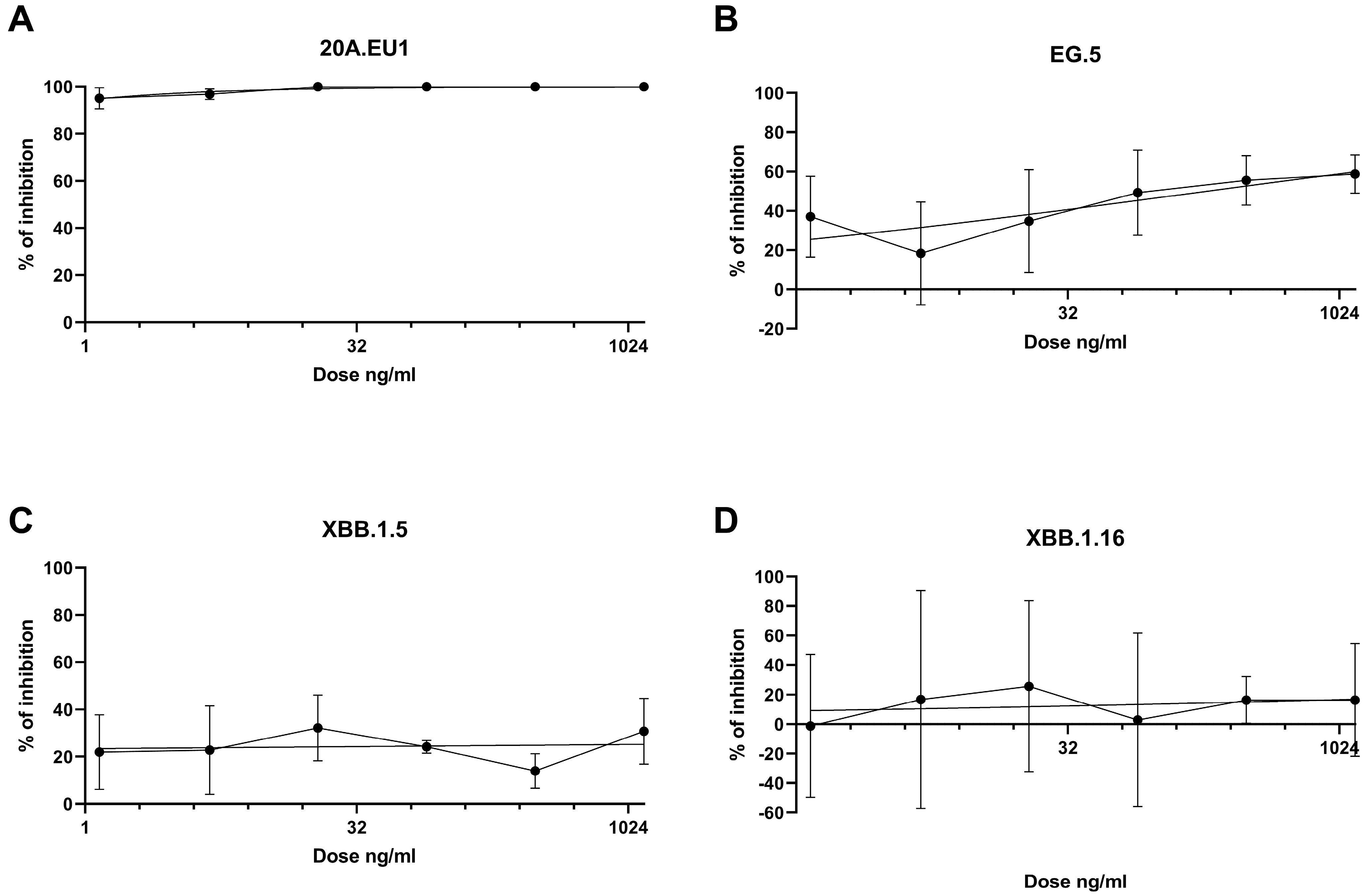
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Variants of Interest and Concern in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-sars-cov-2-omicron-sub-lineages-ba4-and-ba5 (accessed on 1 December 2023).
- Schiaroli, E.; Gidari, A.; Brachelente, G.; Bicchieraro, G.; Spaccapelo, R.; Bastianelli, S.; Pierucci, S.; Busti, C.; Pallotto, C.; Malincarne, L.; et al. Impaired neutralizing antibody efficacy of tixagevimab-cilgavimab 150 + 150 mg as pre-exposure prophylaxis against Omicron BA.5. A real-world experience in booster vaccinated immunocompromised patients. J. Clin. Virol. 2023, 168, 105584. [Google Scholar] [CrossRef]
- Gidari, A.; Nofri, M.; Saccarelli, L.; Bastianelli, S.; Sabbatini, S.; Bozza, S.; Camilloni, B.; Fusco-Moffa, I.; Monari, C.; De Robertis, E.; et al. Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1–12. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Bartolini, D.; Stabile, A.M.; Monari, C.; Galli, F.; Rende, M.; et al. SARS-CoV-2 Survival on Surfaces and the Effect of UV-C Light. Viruses 2021, 13, 408. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Schiaroli, E.; Bastianelli, S.; Pierucci, S.; Busti, C.; Comez, L.; Libera, V.; Macchiarulo, A.; Paciaroni, A.; et al. The Combination of Molnupiravir with Nirmatrelvir or GC376 Has a Synergic Role in the Inhibition of SARS-CoV-2 Replication In Vitro. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Monari, C.; Luciani Pasqua, B.; Dragoni, F.; Schiaroli, E.; Zazzi, M.; et al. Cross-neutralization of SARS-CoV-2 B.1.1.7 and P.1 variants in vaccinated, convalescent and P.1 infected. J. Infect. 2021, 83, 467–472. [Google Scholar] [CrossRef]
- Touret, F.; Baronti, C.; Bouzidi, H.S.; de Lamballerie, X. In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate. Sci. Rep. 2022, 12, 1–5. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Schiaroli, E.; Bastianelli, S.; Pierucci, S.; Busti, C.; Saraca, L.M.; Capogrossi, L.; Pasticci, M.B.; Francisci, D. Synergistic Activity of Remdesivir–Nirmatrelvir Combination on a SARS-CoV-2 In Vitro Model and a Case Report. Viruses 2023, 15, 1577. [Google Scholar] [CrossRef] [PubMed]
- Arshad, U.; Pertinez, H.; Box, H.; Tatham, L.; Rajoli, R.K.R.; Curley, P.; Neary, M.; Sharp, J.; Liptrott, N.J.; Valentijn, A.; et al. Prioritization of Anti-SARS-CoV-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics. Clin. Pharmacol. Ther. 2020, 108, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Ishikawa, K.; Itoh, Y.; Noda, Y.; Eto, T.; Pilla Reddy, V.; Chen, C.C.K.; Gibbs, M.; Johnsson, E. Safety, tolerability, and pharmacokinetics of half-life extended SARS-CoV-2–neutralizing monoclonal antibodies AZD7442 (tixagevimab/cilgavimab) in healthy Japanese adults. J. Infect. Chemother. 2023, 29, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef]
- Montgomery, H.; Hobbs, F.D.R.; Padilla, F.; Arbetter, D.; Templeton, A.; Seegobin, S.; Kim, K.; Campos, J.A.S.; Arends, R.H.; Brodek, B.H.; et al. Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 985–996. [Google Scholar] [CrossRef]
- Loo, Y.M.; McTamney, P.M.; Arends, R.H.; Abram, M.E.; Aksyuk, A.A.; Diallo, S.; Flores, D.J.; Kelly, E.J.; Ren, K.; Roque, R.; et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci. Transl. Med. 2022, 14, eabl8124. [Google Scholar] [CrossRef]
- Thomas, M.; Masson, M.; Bitoun, S.; Hamroun, S.; Seror, R.; Dupuy, H.; Lazaro, E.; Richez, C.; Allanore, Y.; Avouac, J. Prophylaxis with tixagevimab/cilgavimab is associated with lower COVID-19 incidence and severity in patients with autoimmune diseases. Rheumatology 2023. [Google Scholar] [CrossRef]
- Khan, B.A.; Pagsinohin, M.; Lu, L.M.; Tan, P.; Teo, R. Tixagevimab and Cilgavimab Administration for Hemodialysis Patients at Community-Based Dialysis Centers in Singapore as Pre-Exposure Prophylaxis for SARS-CoV-2 Infection. Cureus 2023, 15, e41297. [Google Scholar] [CrossRef]
- Kawashima, I.; Hyuga, H.; Nakadate, A.; Matsuura, M.; Sakamoto, Y.; Suzuki, J.; Kumagai, T.; Suzuki, M.; Koshiishi, M.; Yamamoto, T.; et al. Pre-exposure prophylaxis with tixagevimab/cilgavimab for coronavirus disease 2019 (COVID-19) during the Omicron BA.5 wave at a single institution in Japan. Int. J. Hematol. 2023, 118, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Demel, I.; Skopal, D.; Šafránková, E.; Rozsívalová, P.; Jindra, P.; Šrámek, J.; Turková, A.; Vydra, J.; Labská, K.; Vedrová, J.; et al. Effectiveness of tixagevimab/cilgavimab in patients with hematological malignancies as a pre-exposure prophylaxis to prevent severe COVID-19: A Czech retrospective multicenter study. Ann. Hematol. 2023, 103, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Romano, F.; Coppola, L.; Materazzo, M.; Pedini, D.; Santicchia, M.S.; Cacciola, R.; Toti, L.; Sarmati, L.; Tisone, G. Effects of Anti-COVID-19 Vaccination and Pre-Exposure Prophylaxis with Tixagevimab-Cilgavimab in Kidney and Liver Transplant Recipients. Medicina 2023, 59, 2101. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Granger, K.; Roubal, K.; Smith, D.; Gaffney, K.J.; McGann, M.; Cendagorta, A.; Thurlapati, A.; Herbst, A.; Hendrickson, L.; et al. Efficacy of tixagevimab-cilgavimab in preventing SARS-CoV-2 for patients with B-cell malignancies. Blood 2023, 141, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Leducq, V.; Zafilaza, K.; Fauchois, A.; Ghidaoui, E.; Sayon, S.; Dorival, C.; Meledje, M.-L.; Lusivika-Nzinga, C.; Yordanov, Y.; Martin-Blondel, G.; et al. Spike Protein Genetic Evolution in Patients at High Risk of Severe Coronavirus Disease 2019 Treated by Monoclonal Antibodies. J. Infect. Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, X.; Zhang, Z.; Liu, X.; Wang, P.; Cao, J.; Liang, Q.; Qu, J.; Zhou, M. Serum neutralization of SARS-CoV-2 Omicron BA.2, BA.2.75, BA.2.76, BA.5, BF.7, BQ.1.1 and XBB.1.5 in individuals receiving Evusheld. J. Med. Virol. 2023, 95, e28932. [Google Scholar] [CrossRef] [PubMed]
- Yamasoba, D.; Uriu, K.; Plianchaisuk, A.; Kosugi, Y.; Pan, L.; Zahradnik, J.; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 omicron XBB.1.16 variant. Lancet Infect. Dis. 2023, 23, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Martin-Blondel, G.; de Lamballerie, X.; Dupont, A.; Izopet, J.; Mentré, F.; Kamar, N.; Autran, B.; Paintaud, G.; Caillard, S.; et al. Low to undetectable Omicron BQ.1.1 neutralization by patient’s sera a month after initiation of AZD7442 600 mg. J. Infect. 2023, 86, e126–e129. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Bruel, T.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Maes, P.; Grzelak, L.; Prot, M.; Mougari, S.; Planchais, C.; et al. Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies. Nat. Commun. 2023, 14, 824. [Google Scholar] [CrossRef]
- Pochtovyi, A.A.; Kustova, D.D.; Siniavin, A.E.; Dolzhikova, I.V.; Shidlovskaya, E.V.; Shpakova, O.G.; Vasilchenko, L.A.; Glavatskaya, A.A.; Kuznetsova, N.A.; Iliukhina, A.A.; et al. In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1. Vaccines 2023, 11, 1533. [Google Scholar] [CrossRef]
- Zhang, L.; Kempf, A.; Nehlmeier, I.; Cossmann, A.; Dopfer-Jablonka, A.; Stankov, M.V.; Schulz, S.R.; Jäck, H.-M.; Behrens, G.M.N.; Pöhlmann, S.; et al. Neutralisation sensitivity of SARS-CoV-2 lineages EG.5.1 and XBB.2.3. Lancet Infect. Dis. 2023, 23, e391–e392. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Prevalenza e Distribuzione delle Varianti di SARS-CoV-2 di Interesse per la Sanità Pubblica in Italia. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_3-gennaio-2024.pdf (accessed on 15 January 2024).
| Characteristics | N 72 |
|---|---|
| Age, mean (SD) [range], years | 64.6 (12.0) [34.8-89.3] |
| Sex, male N (%) | 37 (51.4) |
| Comorbidities: | |
| Onco-hematological malignancy, N (%) | 51 (70.8) |
| Primary immunodeficiency, N (%) | 5 (6.9) |
| Kidney transplantation, N (%) | 4 (5.5) |
| Chronic kidney disease, N (%) | 3 (4.1) |
| Secondary immunodeficiency, N (%) | 1 (1.1) |
| Autoimmune disease in immunosuppressant therapy | 8 (11.1) |
| Vaccination, N (%) | 69 (95.8) |
| 3 doses | 52 (75.3) |
| 4 doses | 18 (25.0) |
| 5 doses | 1 (1.4) |
| Time from last vaccine dose to monoclonal injection, days, mean (SD) | 229.9 (94.7) |
| Previous SARS-CoV-2 infection, N (%) | 24 (33.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Svizzeretto, E.; Tommasi, A.; Pallotto, C.; Schiaroli, E.; Francisci, D. Tixagevimab/Cilgavimab: Still a Valid Prophylaxis against COVID-19 New Variants? Viruses 2024, 16, 354. https://doi.org/10.3390/v16030354
Gidari A, Sabbatini S, Bastianelli S, Pierucci S, Busti C, Svizzeretto E, Tommasi A, Pallotto C, Schiaroli E, Francisci D. Tixagevimab/Cilgavimab: Still a Valid Prophylaxis against COVID-19 New Variants? Viruses. 2024; 16(3):354. https://doi.org/10.3390/v16030354
Chicago/Turabian StyleGidari, Anna, Samuele Sabbatini, Sabrina Bastianelli, Sara Pierucci, Chiara Busti, Elisabetta Svizzeretto, Andrea Tommasi, Carlo Pallotto, Elisabetta Schiaroli, and Daniela Francisci. 2024. "Tixagevimab/Cilgavimab: Still a Valid Prophylaxis against COVID-19 New Variants?" Viruses 16, no. 3: 354. https://doi.org/10.3390/v16030354
APA StyleGidari, A., Sabbatini, S., Bastianelli, S., Pierucci, S., Busti, C., Svizzeretto, E., Tommasi, A., Pallotto, C., Schiaroli, E., & Francisci, D. (2024). Tixagevimab/Cilgavimab: Still a Valid Prophylaxis against COVID-19 New Variants? Viruses, 16(3), 354. https://doi.org/10.3390/v16030354







