D-Limonene Affects the Feeding Behavior and the Acquisition and Transmission of Tomato Yellow Leaf Curl Virus by Bemisia tabaci
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Key Volatile Compounds in A. graveolens, A. rugosa, and C. sativum and Their Impact on the Preference of B. tabaci
2.1.1. B. tabaci MEDs and Plant Rearing
2.1.2. The Preference of B. tabaci MEDs Assessed by Y-Tube Olfactometer Behavioral Experiments
2.1.3. Extraction and Identification of Volatile Compounds in A. graveolens, A. rugosa, and C. sativum
2.2. Effects of D-Limonene on Stylet Activities, the Acquisition, and Transmission of TYLCV by B. tabaci
2.2.1. Feeding Behavior of Nonviruliferous and Viruliferous Whitefly after Treatment of D-Limonene
2.2.2. Influence of D-Limonene on the Acquisition and Transmission of TYLCV by B. tabaci
2.3. Binding Analysis of OBP Associated with D-Limonene Recognition in B. tabaci
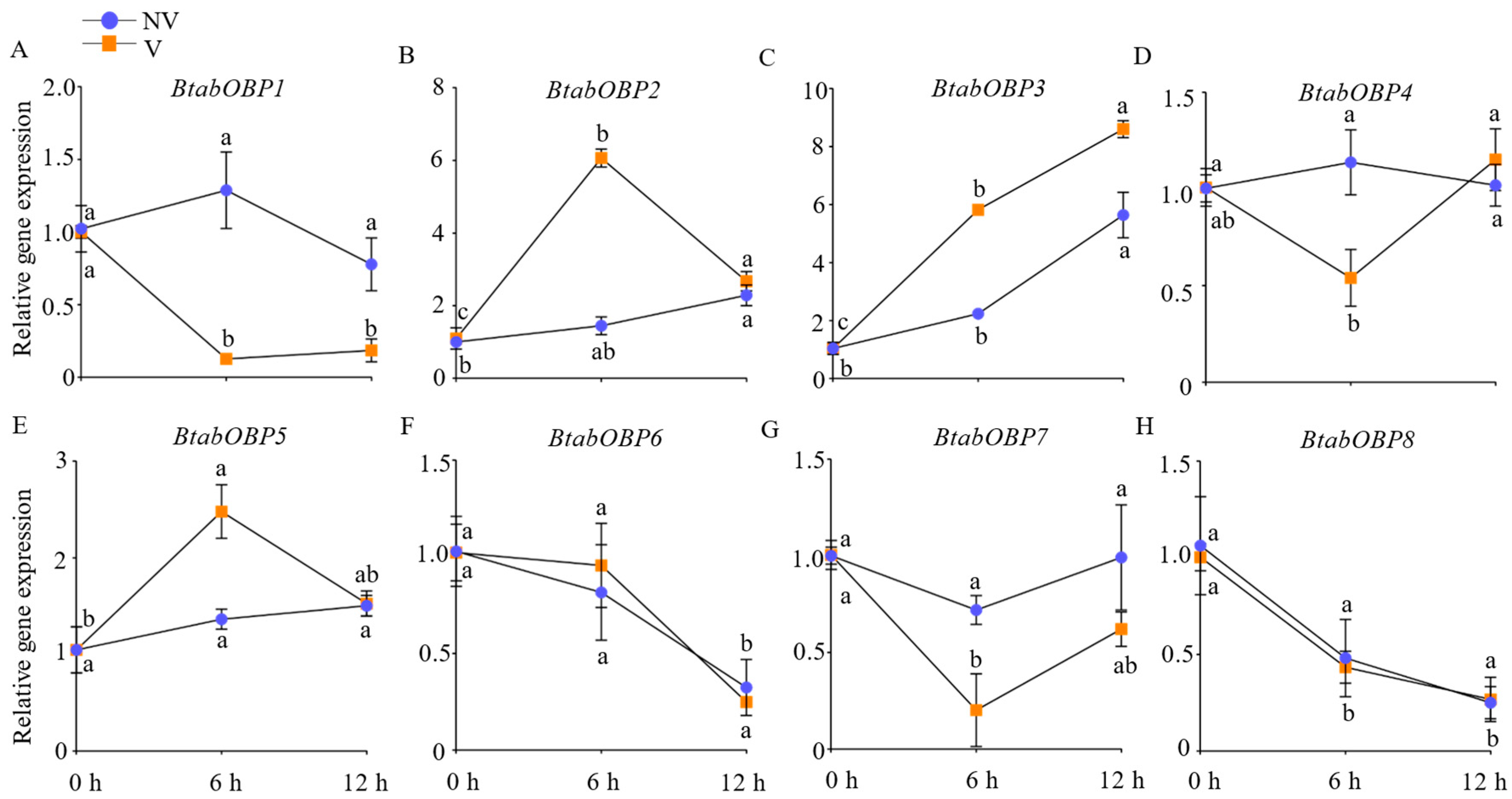
2.3.1. The Relative Gene Expression of BtabOBPs
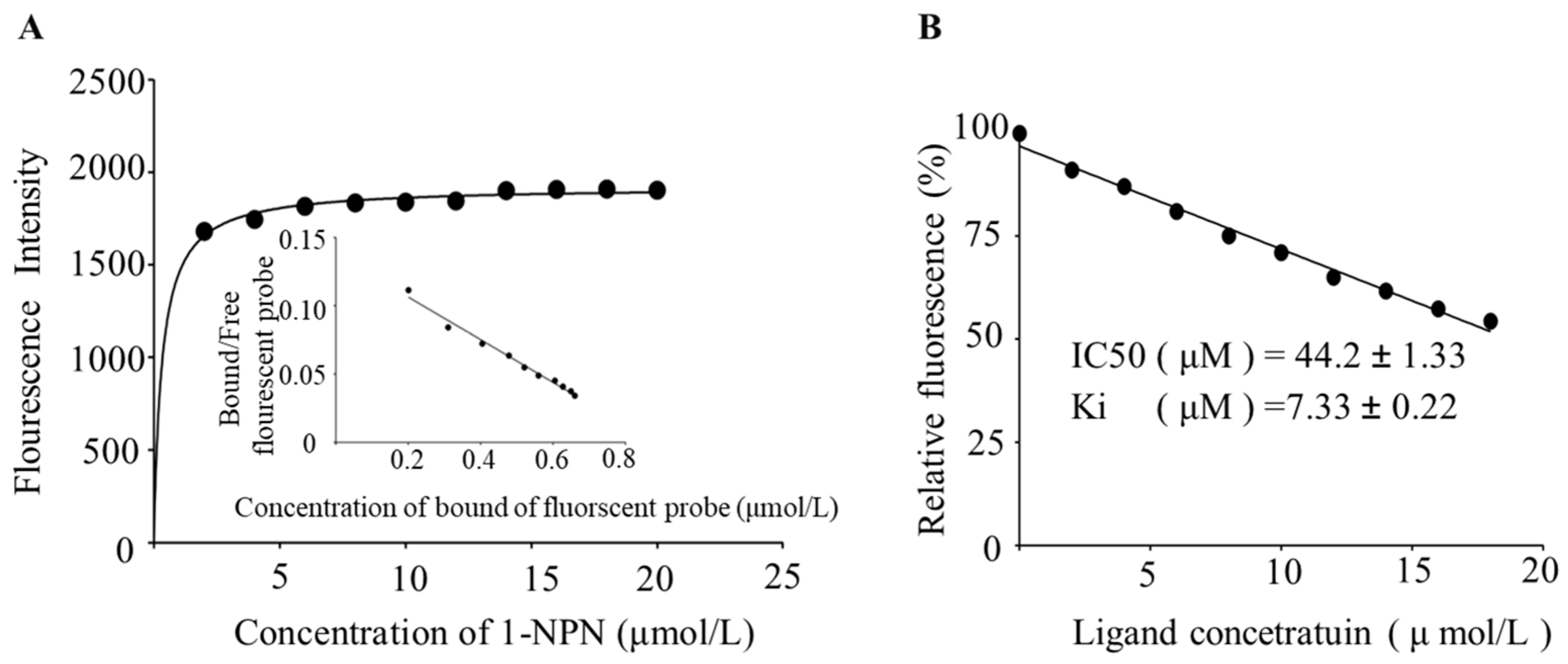
2.3.2. Competitive Fluorescence Binding Assay
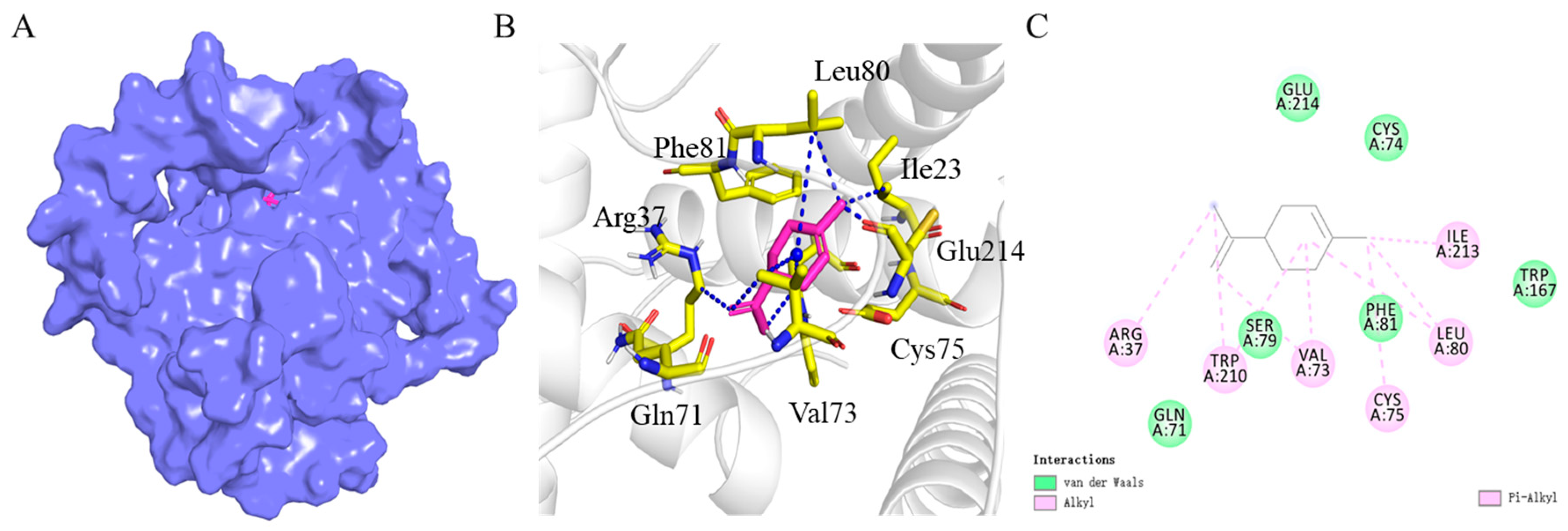
2.4. Homology Modeling of BtabOBP3 and Molecular Docking with D-Limonene
2.5. Data Analysis
3. Results
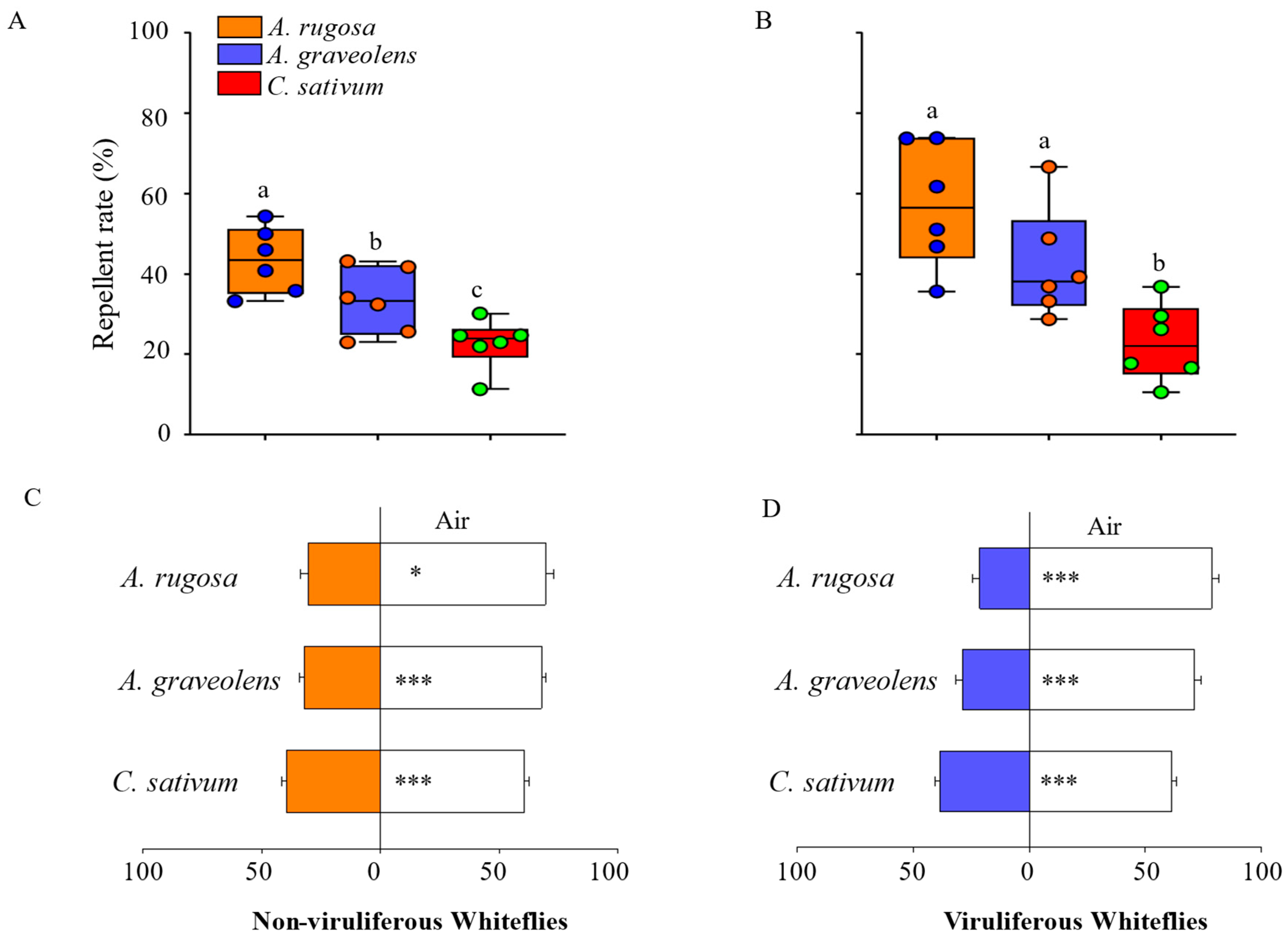
3.1. Key Volatile Compounds in A. graveolens, A. rugosa, and C. sativum and Their Impact on the Preference of B. tabaci
3.1.1. Y-Tube Olfactometer Behavioral Experiments of B. tabaci and Plants
3.1.2. Extraction and Identification of Volatiles from Three Plants
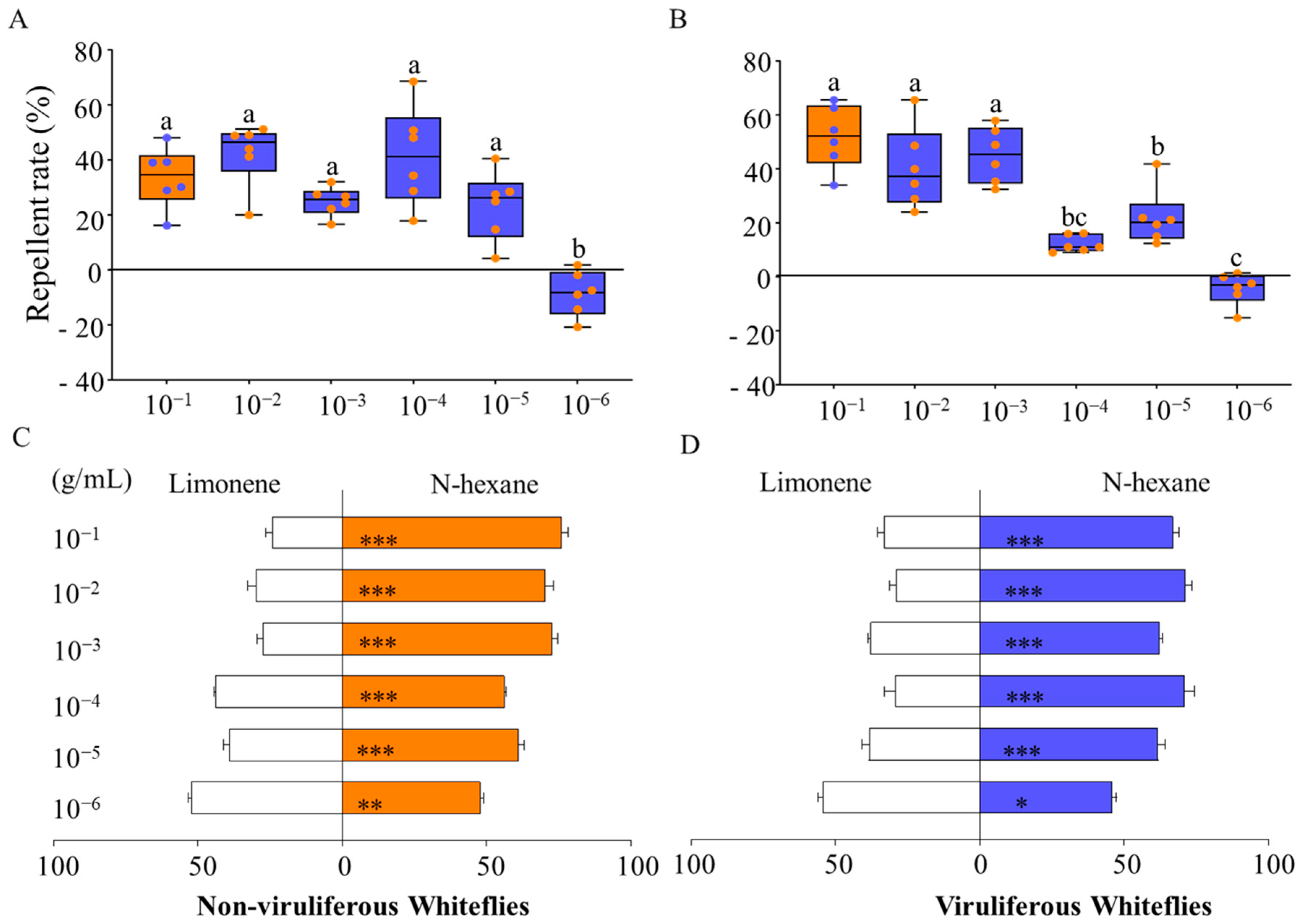
3.1.3. Y-Tube Olfactometer Behavioral Experiments of B. tabaci and D-Limonene
3.2. Effects of D-Limonene on Stylet Activities, Acquisition, and Transmission of TYLCV by B. tabaci
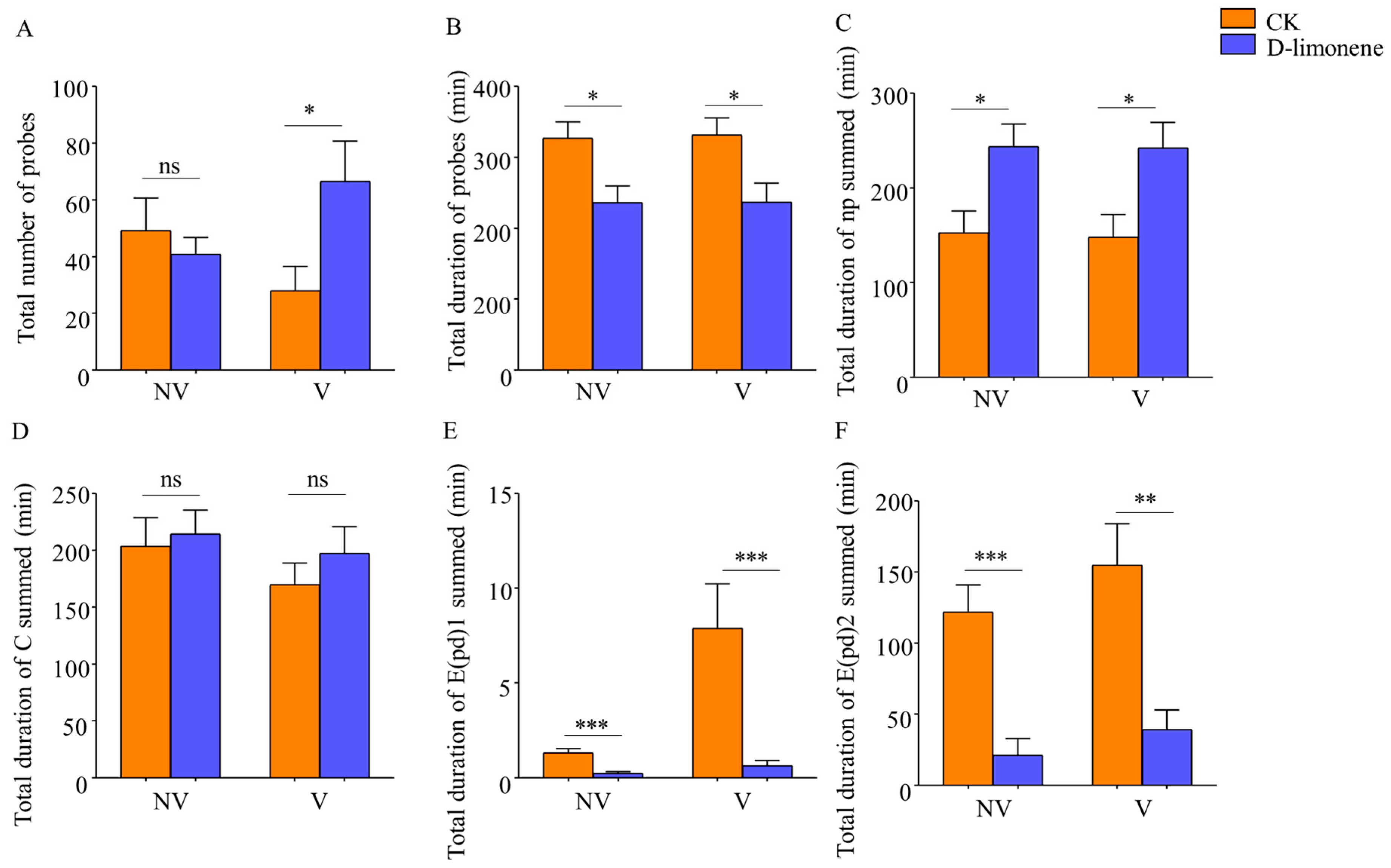
3.2.1. Feeding Behavior of B. tabaci after D-Limonene Treatment
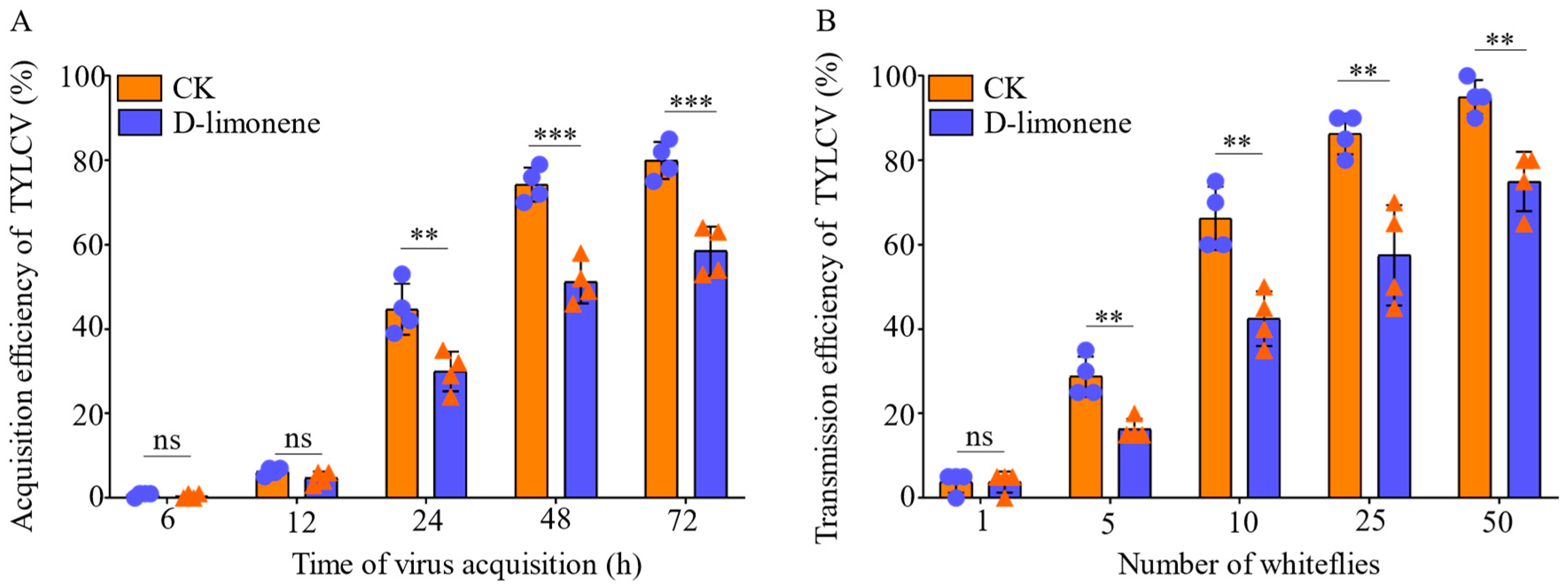
3.2.2. The Acquisition and Transmission of TYLCV after D-Limonene Treatment
3.3. Binding Analysis of BtabOBPs Associated with D-Limonene Recognition in B. tabaci
3.3.1. The Relative Gene Expression of BtabOBPs
3.3.2. Competitive Fluorescence Binding Assay of BtabOBP3
3.4. Homology Modeling of BtabOBP3 and Molecular Docking with D-Limonene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.B.; Zhang, Z.; Zhang, C.; Zhou, X.G.; Zhang, D.Y.; Liu, Y. The molecular mechanism of efficient transmission of plant viruses in variable virus–vector–plant interactions. Hortic. Plant J. 2021, 7, 501–508. [Google Scholar] [CrossRef]
- Visser, J.H. Host-plant finding by insects: Orientation, sensory input and search patterns. J. Insect Physiol. 1988, 34, 259–268. [Google Scholar] [CrossRef]
- Polston, J.E.; De Barro, P.; Boykin, L.M. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag. Sci. 2014, 70, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Zhang, Y.J.; Brown, J.K.; Cong, B.; Xu, B.Y.; Wu, Q.J.; Zhu, G.R. The Introduction of the exotic Q biotype of Bemisia tabaci from the mediterranean region into China on ornamental crops. Fla. Entomol. 2006, 89, 168–174. Available online: http://www.jstor.org/stable/4092462 (accessed on 12 November 2023). [CrossRef]
- Pan, H.P.; Chu, D.; Yan, W.Q.; Su, Q.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Xie, W.; Jiao, X.G.; Li, R.M.; et al. Rapid spread of tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS ONE. 2012, 7, e34817. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Wei, J.; He, Y.Z.; Guo, Q.; Guo, T.; Liu, Y.Q.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc. Natl. Acad. Sci. USA 2017, 26, 6746–6751. [Google Scholar] [CrossRef]
- Moriones, E.; Navas-Castillo, J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2000, 71, 123–134. [Google Scholar] [CrossRef]
- Cohen, J.; Gera, A. Lisianthus leaf curl-a new disease of lisianthus caused by tomato yellow leaf curl virus. Plant Dis. 1995, 4, 416–420. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Sánchez-Campos, S.; Díaz, J.A.; Sáez-Alonso, E.; Moriones, E. Tomato yellow leaf curl virus-Is causes a novel disease of common bean and severe epidemics in tomato in Spain. Plant Dis. 1999, 1, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.; Morilla, G.; Bejarano, E.R.; Rodríguez, M.D.; Janssen, D. First report of Capsicum annuum plants infected by tomato yellow leaf curl virus. Plant Dis. 1999, 12, 1176. [Google Scholar] [CrossRef]
- Anfoka, G.; Haj Ahmad, F.; Abhary, M.; Hussein, A. Detection and molecular characterization of viruses associated with tomato yellow leaf curl disease in cucurbit crops in Jordan. Plant Pathol. 2009, 4, 754–762. [Google Scholar] [CrossRef]
- Antignus, Y.; Cohen, S. Complete nucleotide sequence of an infectious clone of a mild isolate of tomato yellow leaf curl virus (TYLCV). Phytopathology 1994, 7, 707–712. [Google Scholar] [CrossRef]
- Cohen, S.; Harpaz, I. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci gennadius). Entomol. Exp. Appl. 1964, 2, 155–166. [Google Scholar] [CrossRef]
- Ioannou, N. Yellow leaf curl and other virus diseases of tomato in Cyprus. Plant Pathol. 1985, 34, 428–434. [Google Scholar] [CrossRef]
- Romero-Rodríguez, B.; Petek, M.; Jiao, C.; Križnik, M.; Zagorščak, M.; Fei, Z.; Bejarano, E.R.; Gruden, K.; Castillo, A.G. Transcriptional and epigenetic changes during tomato yellow leaf curl virus infection in tomato. BMC Plant Biol. 2023, 23, 651. [Google Scholar] [CrossRef] [PubMed]
- Czosnek, H.; Laterrot, H. A worldwide survey of tomato yellow leaf curl viruses. Arch. Virol. 1997, 142, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Dângelo, R.; Michereff-Filho, M.; Campos, M.; da Silva, P.; Guedes, R. Insecticide resistance and control failure likelihood of the whitefly Bemisia tabaci (MEAM1; B biotype): A Neotropical scenario. Ann. Appl. Biol. 2018, 172, 88–99. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, A.C.; Athanassiou, C. Natural insecticides from native plants of the mediterranean basin and their activity for the control of major insect pests in vegetable crops: Shifting from the past to the future. J. Pest Sci. 2021, 94, 187–202. [Google Scholar] [CrossRef]
- Wang, T.Y.; Luan, J.B. Silencing horizontally transferred genes for the control of the whitefly Bemisia tabaci. J. Pest Sci. 2023, 96, 195–208. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schutz, S.; de Both, M.T.; Haring, M.A.; Schuurink, R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef]
- Luan, J.B.; Yao, D.M.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.J.; Liu, S.S. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Lövei, G.L.; Ferrante, M.; Yang, N.W.; Wan, F.H. The potential of trap and barrier cropping to decrease densities of the whitefly Bemisia tabaci MED on cotton in China. Pest Manag. Sci. 2020, 76, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.F.; Zhang, S.; Cui, J.J.; Wang, D.J.; Wang, C.Y.; Luo, J.Y.; Lv, L.M.; Ma, Y. Functional characterizations of one odorant binding protein and three chemosensory proteins from Apolygus lucorum (Meyer-Dur) (Hemiptera: Miridae) legs. J. Insect Physiol. 2013, 59, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, Y.G.; Jiang, J.; Xu, Y.; Francis, F.; Fan, J.; Chen, J.L. Spatial expression analysis of odorant binding proteins in both sexes of the aphid parasitoid Aphidius gifuensis and their ligand binding properties. Front Physiol. 2022, 13, 877133. [Google Scholar] [CrossRef]
- Xu, X.; Xu, W.; Ishida, Y.; Li, Y.; Leal, W.S.; Ames, J.B. 1H, 15N, and 13C chemical shift assignments of the mosquito odorant binding protein-1 (CquiOBP1) bound to the mosquito oviposition pheromone. Biomol. NMR Assign. 2009, 3, 195–197. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Grossi, G.; Falabella, P.; Liu, Y.; Yan, S.; Lu, J.; Xi, J.; Wang, G. Molecular basis of alarm pheromone detection in aphids. Curr. Biol. 2017, 27, 55–61. [Google Scholar] [CrossRef]
- Wang, B.; Dong, W.; Li, H.; D’onofrio, C.; Bai, P.; Chen, R.; Yang, L.; Wu, J.; Wang, X.; Wang, B.; et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae. Curr. Biol. 2022, 32, 951–962.e7. [Google Scholar] [CrossRef]
- Kil, E.J.; Kim, S.; Lee, Y.J.; Byun, H.S.; Park, J.; Seo, H.; Kim, C.S.; Shim, J.K.; Lee, J.H.; Kim, J.K. Tomato yellow leaf curl virus (TYLCV-IL): A seed-transmissible geminivirus in tomatoes. Sci. Rep. 2016, 6, 19013. [Google Scholar] [CrossRef]
- Lu, D.Y.H.; Yue, H.; Huang, L.P.; Zhang, D.Y.; Zhang, Z.H.; Zhang, Z.; Zhang, Y.J.; Li, F.; Yan, F.; Zhou, X.G.; et al. Suppression of Bta11975, an α-glucosidase, by RNA interference reduces transmission of tomato chlorosis virus by Bemisia tabaci. Pest Manag. Sci. 2021, 77, 5294–5303. [Google Scholar] [CrossRef]
- Oluwafemi, S.; Bruce, T.J.; Pickett, J.A.; Ton, J.; Birkett, M.A. Behavioral responses of the leafhopper, Cicadulina storeyi China, a major vector of maize streak virus, to volatile cues from intact and leafhopper-damaged maize. J. Chem. Ecol. 2011, 37, 40–48. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, S.H.; Ronderos, D.S.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Smith, D.P.; Montell, C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 2010, 20, 1672–1678. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Possible role of plant volatiles in tolerance against huanglongbing in citrus. Plant Signal. Behav. 2016, 11, e1138193. [Google Scholar] [CrossRef]
- Dong, G.Y.; Bai, X.H.; Aimila, A.; Aisa, H.A.; Maiwulanjiang, M. Study on lavender essential oil chemical compositions by GC-MS and improved pGC. Molecules 2020, 25, 3166. [Google Scholar] [CrossRef]
- Fan, X.F.; Liu, Y.; Zhang, Z.; Zhang, Z.H.; Peng, J.; Gao, Y.; Zheng, L.M.; Chen, J.B.; Du, J.; Yan, S. Bta06987, Encoding a Peptide of the AKH/RPCH Family: A Role of Energy Mobilization in Bemisia tabaci. Insects 2022, 13, 834. [Google Scholar] [CrossRef]
- Maluta, N.K.P.; Lopes, J.R.S.; Fiallo-Olivé, E.; Navas-Castillo, J.; Lourenção, A.L. Foliar spraying of tomato plants with systemic insecticides: Effects on feeding behavior, mortality and oviposition of Bemisia tabaci (Hemiptera: Aleyrodidae) and inoculation efficiency of Tomato chlorosis virus. Insects 2020, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Maluta, N.; Fereres, A.; Lopes, J.R.S. Plant-mediated indirect effects of two viruses with different transmission modes on Bemisia tabaci feeding behavior and fitness. J. Pest Sci. 2019, 92, 405–416. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Mei, X.D.; Feng, J.N.; Berg, B.G.; Zhang, Y.J.; Guo, Y.Y. Characterization of three pheromone-binding proteins (PBPs) of Helicoverpa armigera (Hübner) and their binding properties. J. Insect Physiol. 2012, 58, 941–948. [Google Scholar] [CrossRef]
- Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 1999, 10, 411–421. [Google Scholar] [CrossRef]
- Qu, C.; Yang, Z.K.; Wang, S.; Zhao, H.P.; Li, F.Q.; Yang, X.L.; Luo, C.J. Binding affinity characterization of four antennae-enriched odorant-binding proteins from Harmonia axyridis (Coleoptera: Coccinellidae). Front. Physiol. 2022, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Excel Workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Garzo, E.; Moreno, A.; Hernando, S.; Mariño, V.; Torne, M.; Santamaria, E.; Díaz, I.; Fereres, A. Electrical penetration graph technique as a tool to monitor the early stages of aphid resistance to insecticides. Pest Manag. Sci. 2016, 72, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.T.; Qin, Y.C. Repellent effects of different celery varieties in Bemisia tabaci (Hemiptera: Aleyrodidae) Biotype Q. J. Econ. Entomol. 2017, 110, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, S.; Cao, J.; Qiao, J.; Zhao, G.R. Systematic Optimization of limonene production in engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Rossi, Y.E.; Palacios, S.M. Fumigant toxicity of Citrus sinensis essential oil on Musca domestica L. adults in the absence and presence of a P450 inhibitor. Acta Trop. 2013, 127, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; An, Y.; Hou, Z.B.; Wang, X.D.; Zhou, F.; Zhang, J.; Wang, J.L. Acute toxicity of Zanthoxylum bungeanum against two stored product insects and synergistic interactions between two major compounds limonene and linalool. J. Environ. Sci. Health B 2022, 57, 739–744. [Google Scholar] [CrossRef]
- Díaz-Pendón, J.A.; Cañizares, M.C.; Moriones, E.; Bejarano, E.R.; Czosnek, H.; Navas-Castillo, J. Tomato yellow leaf curl viruses: Ménage à trois between the virus complex, the plant and the whitefly vector. Mol. Plant Pathol. 2010, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, M.; Medina, V. Localization of Tomato yellow leaf curl virus in its whitefly vector Bemisia tabaci. In Tomato Yellow Leaf Curl Virus Disease; Springer: Dordrecht, The Netherlands, 2007; pp. 171–183. [Google Scholar] [CrossRef]
- Guo, H.; Qu, Y.; Liu, X.; Zhong, W.; Fang, J. Female-biased symbionts and tomato yellow leaf curl virus infections in Bemisia tabaci. PLoS ONE 2014, 9, e84538. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; de Blas, C.; Barrios, L.; Fereres, A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of tomato yellow leaf curl virus. Ann. Entomol. Soc. Am. 2000, 93, 573–579. [Google Scholar] [CrossRef]
- Zhan, H.X.; Dewer, Y.; Zhang, J.P.; Tian, J.H.; Li, D.; Qu, C.; Yang, Z.; Li, F.Q.; Luo, C. Odorant-binding protein 1 plays a crucial role in the olfactory response of Bemisia tabaci to R-Curcumene. J. Agric. Food Chem. 2021, 69, 12785–12793. [Google Scholar] [CrossRef]
- Li, F.Q.; Li, D.; Dewer, Y.; Qu, C.; Yang, Z.; Tian, J.H.; Luo, C. Discrimination of oviposition deterrent volatile β-Ionone by odorant-binding proteins 1 and 4 in the whitefly Bemisia tabaci. Biomolecules 2019, 9, 563. [Google Scholar] [CrossRef]
- Shi, X.B.; Wang, X.Z.; Zhang, D.Y.; Zhang, Z.H.; Zhang, Z.; Cheng, J.B.; Zheng, L.M.; Zhou, X.G.; Tan, X.Q.; Liu, Y. Silencing of odorant-binding protein gene OBP3 using RNA interference reduced virus transmission of tomato chlorosis virus. Int. J. Mol. Sci. 2019, 20, 4969. [Google Scholar] [CrossRef]
| Number | Compound | CAS | Formula | Relative Content (%) |
|---|---|---|---|---|
| 1 | Allyl phenoxyacetate | 7493-74-5 | C11H12O3 | 35.4 |
| 2 | o-Phenylenediamine | 95-54-5 | C6H8N2 | 26.6 |
| 3 | D-Limonene | 5989-27-5 | C10H16 | 13.7 |
| 4 | Trans-ligustilide | 100036-59-8 | C12H14O2 | 5.7 |
| 5 | 1-Methoxy-3-(2-hydroxyethyl) nonane | 70928-44-8 | C12H26O2 | 5.2 |
| 6 | Trans-β-Ocimene | 3779-61-1 | C10H16 | 4.1 |
| 7 | (+)-β-Selinene | 17066-67-0 | C15H24 | 3.5 |
| 8 | 4-Ethylbenzoic acid, allyl ester | 100029-33-7 | C12H14O2 | 3.1 |
| 9 | 1-Acetyl-2-phenylhydrazine | 114-83-0 | C8H10N2O | 1.9 |
| 10 | 3-Methyl-1-heptene | 4810-09-7 | C8H16 | 0.8 |
| Number | Compound | CAS | Formula | Relative Content (%) |
|---|---|---|---|---|
| 1 | Pulegone | 15932-80-6 | C10H16O | 85.3 |
| 2 | D-Limonene | 5989-27-5 | C10H16 | 5.7 |
| 3 | Isopulegone | 29606-79-9 | C10H16O | 4.5 |
| 4 | 1,3,4-Trimethylcyclohex-3-enecarbaldehyde | 40702-26-9 | C10H16O | 4.5 |
| Number | Compound | CAS | Formula | Relative Content (%) |
|---|---|---|---|---|
| 1 | Allyl phenoxyacetate | 7493-74-5 | C11H12O3 | 40.2 |
| 2 | D-Limonene | 5989-27-5 | C10H16 | 13.6 |
| 3 | Myrcene | 123-35-3 | C10H16 | 13.0 |
| 4 | 1,2-Benzenediamine | 95-54-5 | C6H8N2 | 12.9 |
| 5 | Trans-β-Ocimene | 3779-61-1 | C10H16 | 4.9 |
| 6 | 3-Methylcyclohex-4-ene-1,2-dicarboxylic acid | 40469-16-7 | C9H12O4 | 4.8 |
| 7 | (+)-β-Selinene | 17066-67-0 | C15H24 | 3.0 |
| 8 | 2,3-Dimethylnonadecane | 75163-99-4 | C21H44 | 1.5 |
| 9 | 3-Methylphenol acetate | 122-46-3 | C9H10O2 | 3.0 |
| 10 | 1-Acetyl-2-phenylhydrazine | 114-83-0 | C8H10N2O | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Gao, L.; Zhang, Z.; Li, K.; Zhang, Z.; Zhang, D.; Chen, J.; Peng, J.; Gao, Y.; Du, J.; et al. D-Limonene Affects the Feeding Behavior and the Acquisition and Transmission of Tomato Yellow Leaf Curl Virus by Bemisia tabaci. Viruses 2024, 16, 300. https://doi.org/10.3390/v16020300
Wei Y, Gao L, Zhang Z, Li K, Zhang Z, Zhang D, Chen J, Peng J, Gao Y, Du J, et al. D-Limonene Affects the Feeding Behavior and the Acquisition and Transmission of Tomato Yellow Leaf Curl Virus by Bemisia tabaci. Viruses. 2024; 16(2):300. https://doi.org/10.3390/v16020300
Chicago/Turabian StyleWei, Yan, Liming Gao, Zhanhong Zhang, Kailong Li, Zhuo Zhang, Deyong Zhang, Jianbin Chen, Jing Peng, Yang Gao, Jiao Du, and et al. 2024. "D-Limonene Affects the Feeding Behavior and the Acquisition and Transmission of Tomato Yellow Leaf Curl Virus by Bemisia tabaci" Viruses 16, no. 2: 300. https://doi.org/10.3390/v16020300
APA StyleWei, Y., Gao, L., Zhang, Z., Li, K., Zhang, Z., Zhang, D., Chen, J., Peng, J., Gao, Y., Du, J., Yan, S., Shi, X., & Liu, Y. (2024). D-Limonene Affects the Feeding Behavior and the Acquisition and Transmission of Tomato Yellow Leaf Curl Virus by Bemisia tabaci. Viruses, 16(2), 300. https://doi.org/10.3390/v16020300







