Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency
Abstract
1. Transcriptional Noise Is a Ubiquitous Phenomenon
2. Key Features of HIV-1 Latency
3. The HIV-1 Promoter and the Tat-Positive Feedback Loop
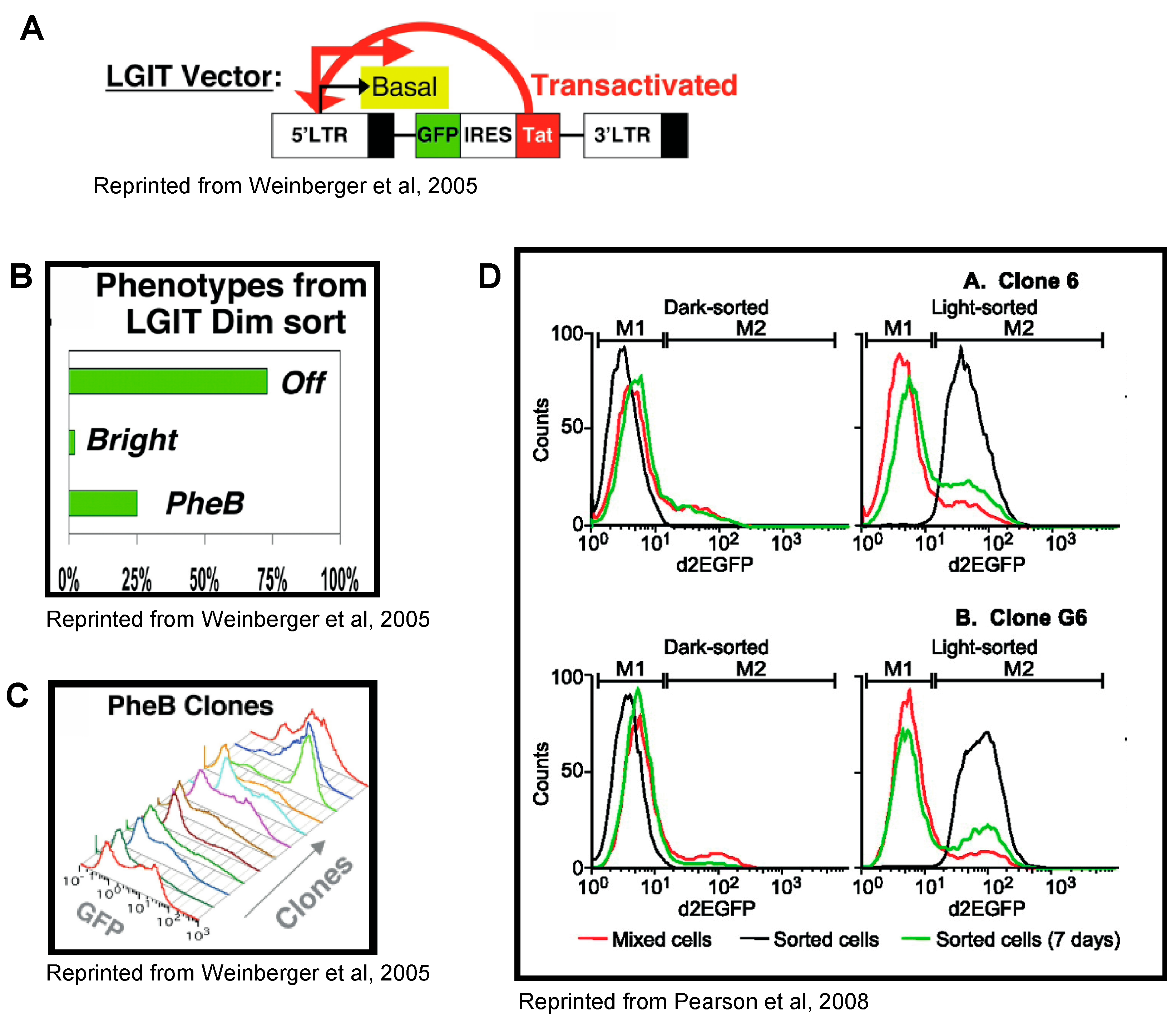
4. Tat Feedback Induces Stochastic Switches between High and Low Viral Expression States
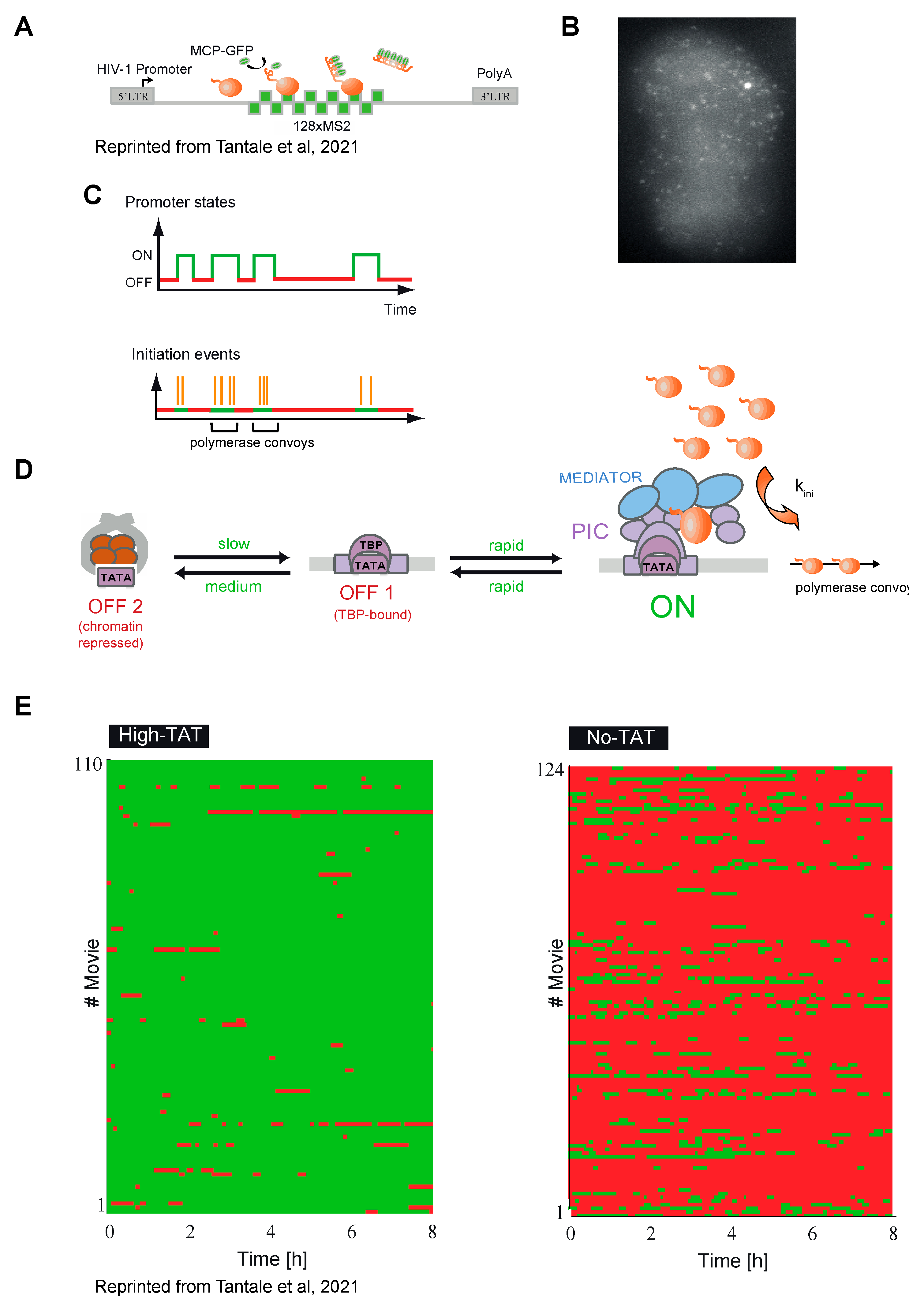
5. HIV-1 Transcription Is Stochastic in Basal and Tat-Activated Conditions
6. Stochastic Transcriptional Response to Activation
7. Factors Influencing HIV-1 Transcriptional Noise
7.1. Promoter Architecture Controls Transcription Stochasticity
7.2. Influence of Chromatin and Integration Sites on the Stochasticity of Transcription
7.3. Polymerase Pausing and Viral Transcriptional Stochasticity
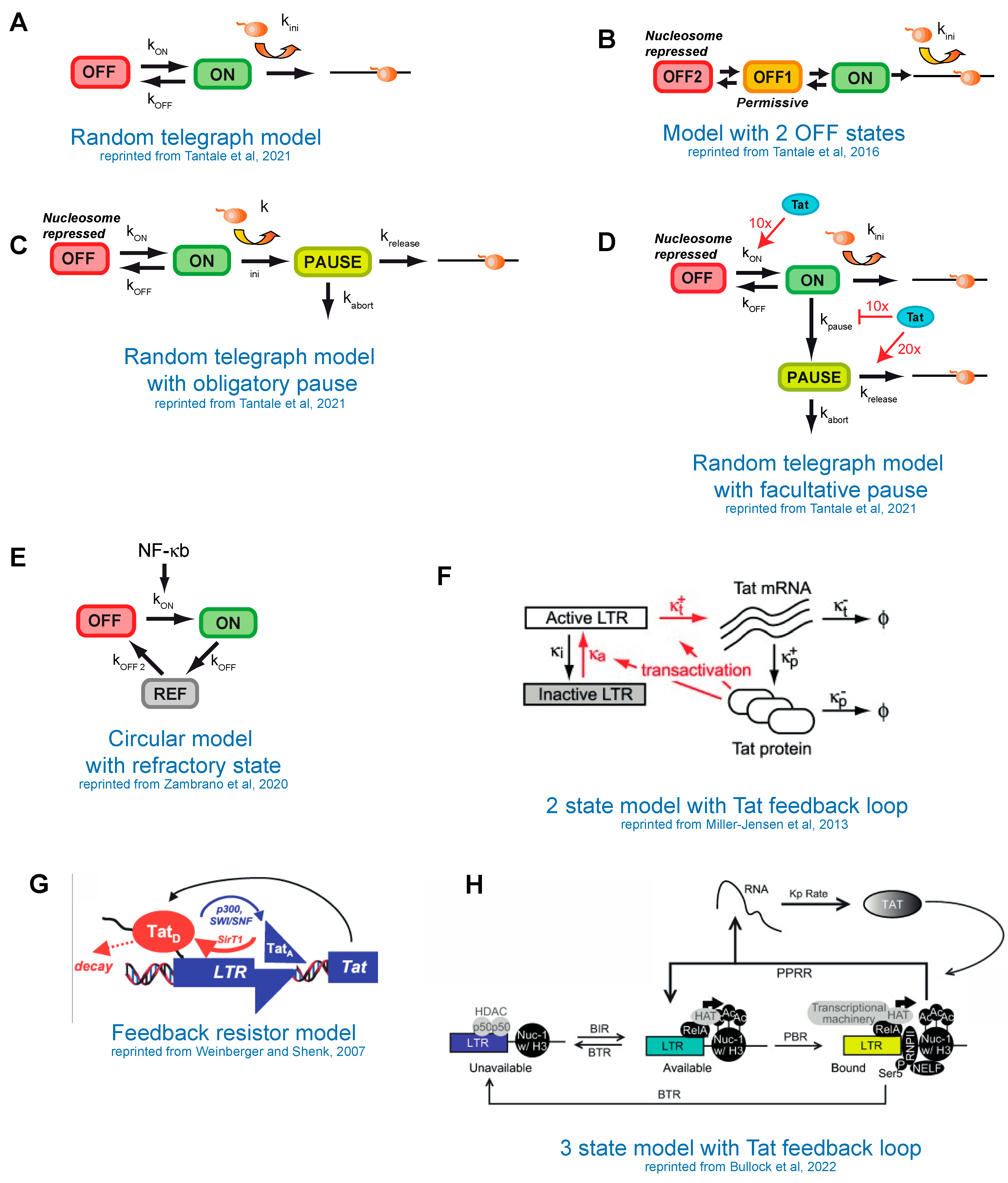
8. Modeling of HIV-1 Transcription
9. Stochastic Models of HIV-1 Transcription without the Tat Feedback Loop
10. Modeling the Tat Feedback Loop and Bistability versus Bimodality
11. Manipulating HIV-1 Transcriptional Noise as a Strategy for a Cure
12. Perspectives
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
References
- Balázsi, G.; van Oudenaarden, A.; Collins, J.J. Cellular Decision Making and Biological Noise: From Microbes to Mammals. Cell 2011, 144, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Raser, J.M.; O’Shea, E.K. Control of Stochasticity in Eukaryotic Gene Expression. Science 2004, 304, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Raser, J.M.; O’Shea, E.K. Noise in Gene Expression: Origins, Consequences, and Control. Science 2005, 309, 2010–2013. [Google Scholar] [CrossRef]
- Thattai, M.; van Oudenaarden, A. Intrinsic Noise in Gene Regulatory Networks. Proc. Natl. Acad. Sci. USA 2001, 98, 8614–8619. [Google Scholar] [CrossRef] [PubMed]
- Bar-Even, A.; Paulsson, J.; Maheshri, N.; Carmi, M.; O’Shea, E.; Pilpel, Y.; Barkai, N. Noise in Protein Expression Scales with Natural Protein Abundance. Nat. Genet. 2006, 38, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Munsky, B.; Neuert, G.; van Oudenaarden, A. Using Gene Expression Noise to Understand Gene Regulation. Science 2012, 336, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P.S. Stochastic Gene Expression in a Single Cell. Science 2002, 297, 1183–1186. [Google Scholar] [CrossRef]
- Bertaux, F.; Marguerat, S.; Shahrezaei, V. Division Rate, Cell Size and Proteome Allocation: Impact on Gene Expression Noise and Implications for the Dynamics of Genetic Circuits. R. Soc. Open Sci. 2018, 5, 172234. [Google Scholar] [CrossRef]
- Peccoud, J.; Ycart, B. Markovian Modeling of Gene-Product Synthesis. Theor. Popul. Biol. 1995, 48, 222–234. [Google Scholar] [CrossRef]
- Ozbudak, E.M.; Thattai, M.; Kurtser, I.; Grossman, A.D.; van Oudenaarden, A. Regulation of Noise in the Expression of a Single Gene. Nat. Genet. 2002, 31, 69–73. [Google Scholar] [CrossRef]
- Tunnacliffe, E.; Chubb, J.R. What Is a Transcriptional Burst? Trends Genet. 2020, 36, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, O.; Muller, A.; Crudu, A. Théorèmes Limites Pour Les Processus de Markov à Sauts. Tech. Sci. Inform. 2007, 26, 443–469. [Google Scholar] [CrossRef]
- Friedman, N.; Cai, L.; Xie, X.S. Linking Stochastic Dynamics to Population Distribution: An Analytical Framework of Gene Expression. Phys. Rev. Lett. 2006, 97, 168302. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Peskin, C.S.; Tranchina, D.; Vargas, D.Y.; Tyagi, S. Stochastic mRNA Synthesis in Mammalian Cells. PLoS Biol. 2006, 4, e309. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.V.; Finkenstädt, B.; Woodcock, D.J.; Friedrichsen, S.; Semprini, S.; Ashall, L.; Spiller, D.G.; Mullins, J.J.; Rand, D.A.; Davis, J.R.E.; et al. Dynamic Analysis of Stochastic Transcription Cycles. PLoS Biol. 2011, 9, e1000607. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.D.; Razooky, B.S.; Singh, A.; Trimeloni, T.V.; McCollum, J.M.; Cox, C.D.; Simpson, M.L.; Weinberger, L.S. Transcriptional Burst Frequency and Burst Size Are Equally Modulated across the Human Genome. Proc. Natl. Acad. Sci. USA 2012, 109, 17454–17459. [Google Scholar] [CrossRef]
- Golding, I.; Paulsson, J.; Zawilski, S.M.; Cox, E.C. Real-Time Kinetics of Gene Activity in Individual Bacteria. Cell 2005, 123, 1025–1036. [Google Scholar] [CrossRef]
- Suter, D.M.; Molina, N.; Gatfield, D.; Schneider, K.; Schibler, U.; Naef, F. Mammalian Genes Are Transcribed with Widely Different Bursting Kinetics. Science 2011, 332, 472–474. [Google Scholar] [CrossRef]
- Tunnacliffe, E.; Corrigan, A.M.; Chubb, J.R. Promoter-Mediated Diversification of Transcriptional Bursting Dynamics Following Gene Duplication. Proc. Natl. Acad. Sci. USA 2018, 115, 8364–8369. [Google Scholar] [CrossRef]
- Tantale, K.; Mueller, F.; Kozulic-Pirher, A.; Lesne, A.; Victor, J.-M.; Robert, M.-C.; Capozi, S.; Chouaib, R.; Bäcker, V.; Mateos-Langerak, J.; et al. A Single-Molecule View of Transcription Reveals Convoys of RNA Polymerases and Multi-Scale Bursting. Nat. Commun. 2016, 7, 12248. [Google Scholar] [CrossRef]
- Tantale, K.; Garcia-Oliver, E.; Robert, M.-C.; L’Hostis, A.; Yang, Y.; Tsanov, N.; Topno, R.; Gostan, T.; Kozulic-Pirher, A.; Basu-Shrivastava, M.; et al. Stochastic Pausing at Latent HIV-1 Promoters Generates Transcriptional Bursting. Nat. Commun. 2021, 12, 4503. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, A.M.; Tunnacliffe, E.; Cannon, D.; Chubb, J.R. A Continuum Model of Transcriptional Bursting. Elife 2016, 5, e13051. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, S.; Loffreda, A.; Carelli, E.; Stefanelli, G.; Colombo, F.; Bertrand, E.; Tacchetti, C.; Agresti, A.; Bianchi, M.E.; Molina, N.; et al. First Responders Shape a Prompt and Sharp NF-κB-Mediated Transcriptional Response to TNF-α. iScience 2020, 23, 101529. [Google Scholar] [CrossRef] [PubMed]
- Maamar, H.; Raj, A.; Dubnau, D. Noise in Gene Expression Determines Cell Fate in Bacillus Subtilis. Science 2007, 317, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Blake, W.J.; Balázsi, G.; Kohanski, M.A.; Isaacs, F.J.; Murphy, K.F.; Kuang, Y.; Cantor, C.R.; Walt, D.R.; Collins, J.J. Phenotypic Consequences of Promoter-Mediated Transcriptional Noise. Mol. Cell 2006, 24, 853–865. [Google Scholar] [CrossRef]
- Wernet, M.F.; Mazzoni, E.O.; Çelik, A.; Duncan, D.M.; Duncan, I.; Desplan, C. Stochastic Spineless Expression Creates the Retinal Mosaic for Colour Vision. Nature 2006, 440, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Rifkin, S.A.; Andersen, E.; van Oudenaarden, A. Variability in Gene Expression Underlies Incomplete Penetrance. Nature 2010, 463, 913–918. [Google Scholar] [CrossRef]
- Perez-Carrasco, R.; Guerrero, P.; Briscoe, J.; Page, K.M. Intrinsic Noise Profoundly Alters the Dynamics and Steady State of Morphogen-Controlled Bistable Genetic Switches. PLoS Comput. Biol. 2016, 12, e1005154. [Google Scholar] [CrossRef]
- Ballouz, S.; Pena, M.T.; Knight, F.M.; Adams, L.B.; Gillis, J.A. The Transcriptional Legacy of Developmental Stochasticity. bioRxiv 2019. [Google Scholar] [CrossRef]
- Arias, A.M.; Hayward, P. Filtering Transcriptional Noise during Development: Concepts and Mechanisms. Nat. Rev. Genet. 2006, 7, 34–44. [Google Scholar] [CrossRef]
- Hornstein, E.; Shomron, N. Canalization of Development by microRNAs. Nat. Genet. 2006, 38 (Suppl. 6), S20–S24. [Google Scholar] [CrossRef] [PubMed]
- Herranz, H.; Cohen, S.M. MicroRNAs and Gene Regulatory Networks: Managing the Impact of Noise in Biological Systems. Genes Dev. 2010, 24, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, H.; Lampert, F.; Stojanovski, K.; Regot, S.; Vaga, S.; Buser, R.; Lee, S.S.; Koeppl, H.; Posas, F.; Pelet, S.; et al. Parallel Feedback Loops Control the Basal Activity of the HOG MAPK Signaling Cascade. Integr. Biol. 2015, 7, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Azpeitia, E.; Wagner, A. Short Residence Times of DNA-Bound Transcription Factors Can Reduce Gene Expression Noise and Increase the Transmission of Information in a Gene Regulation System. Front. Mol. Biosci. 2020, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Urban, E.A.; Johnston, R.J. Buffering and Amplifying Transcriptional Noise During Cell Fate Specification. Front. Genet. 2018, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.V.; Chen, X.; Martin, B.; Chaturvedi, S.; Hwang, D.W.; Li, W.; Yu, C.; Ding, S.; Thomson, M.; Singer, R.H.; et al. A DNA Repair Pathway Can Regulate Transcriptional Noise to Promote Cell Fate Transitions. Science 2021, 373, eabc6506. [Google Scholar] [CrossRef]
- Rouzine, I.M.; Weinberger, A.D.; Weinberger, L.S. An Evolutionary Role for HIV Latency in Enhancing Viral Transmission. Cell 2015, 160, 1002–1012. [Google Scholar] [CrossRef]
- Arkin, A.; Ross, J.; McAdams, H.H. Stochastic Kinetic Analysis of Developmental Pathway Bifurcation in Phage Lambda-Infected Escherichia Coli Cells. Genetics 1998, 149, 1633–1648. [Google Scholar] [CrossRef]
- Singh, N.; Tscharke, D.C. Herpes Simplex Virus Latency Is Noisier the Closer We Look. J. Virol. 2020, 94, e01701-19. [Google Scholar] [CrossRef]
- Pai, A.; Weinberger, L.S. Fate-Regulating Circuits in Viruses: From Discovery to New Therapy Targets. Annu. Rev. Virol. 2017, 4, 469–490. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Klein, J.; Vardi, N.; Bolovan-Fritts, C.; Wolf, M.; Du, K.; Mlera, L.; Calvert, M.; Moorman, N.J.; Goodrum, F.; et al. A Molecular Mechanism for Probabilistic Bet Hedging and Its Role in Viral Latency. Proc. Natl. Acad. Sci. USA 2020, 117, 17240–17248. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Gantner, P.; Fromentin, R.; Chomont, N. The Multifaceted Nature of HIV Latency. J. Clin. Investig. 2020, 130, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.J.; Trautmann, L.; Pinyakorn, S.; Leyre, L.; Pagliuzza, A.; Kroon, E.; Rolland, M.; Takata, H.; Buranapraditkun, S.; Intasan, J.; et al. Rapid HIV RNA Rebound after Antiretroviral Treatment Interruption in Persons Durably Suppressed in Fiebig I Acute HIV Infection. Nat. Med. 2018, 24, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Pannus, P.; Rutsaert, S.; De Wit, S.; Allard, S.D.; Vanham, G.; Cole, B.; Nescoi, C.; Aerts, J.; De Spiegelaere, W.; Tsoumanis, A.; et al. Rapid Viral Rebound after Analytical Treatment Interruption in Patients with Very Small HIV Reservoir and Minimal On-Going Viral Transcription. J. Int. AIDS Soc. 2020, 23, e25453. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Aga, E.; Bosch, R.J.; Pilkinton, M.; Kroon, E.; MacLaren, L.; Keefer, M.; Fox, L.; Barr, L.; Acosta, E.; et al. Time to Viral Rebound After Interruption of Modern Antiretroviral Therapies. Clin. Infect. Dis. 2022, 74, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.B.; Chomont, N.; Deeks, S.G. The Biology of the HIV-1 Latent Reservoir and Implications for Cure Strategies. Cell Host Microbe 2020, 27, 519–530. [Google Scholar] [CrossRef]
- Eisele, E.; Siliciano, R.F. Redefining the Viral Reservoirs That Prevent HIV-1 Eradication. Immunity 2012, 37, 377–388. [Google Scholar] [CrossRef]
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The Challenge of Viral Reservoirs in HIV-1 Infection. Annu. Rev. Med. 2002, 53, 557–593. [Google Scholar] [CrossRef]
- Finzi, D.; Blankson, J.; Siliciano, J.D.; Margolick, J.B.; Chadwick, K.; Pierson, T.; Smith, K.; Lisziewicz, J.; Lori, F.; Flexner, C.; et al. Latent Infection of CD4+ T Cells Provides a Mechanism for Lifelong Persistence of HIV-1, Even in Patients on Effective Combination Therapy. Nat. Med. 1999, 5, 512–517. [Google Scholar] [CrossRef]
- Finzi, D.; Hermankova, M.; Pierson, T.; Carruth, L.M.; Buck, C.; Chaisson, R.E.; Quinn, T.C.; Chadwick, K.; Margolick, J.; Brookmeyer, R.; et al. Identification of a Reservoir for HIV-1 in Patients on Highly Active Antiretroviral Therapy. Science 1997, 278, 1295–1300. [Google Scholar] [CrossRef]
- Chun, T.W.; Engel, D.; Berrey, M.M.; Shea, T.; Corey, L.; Fauci, A.S. Early Establishment of a Pool of Latently Infected, Resting CD4+ T Cells during Primary HIV-1 Infection. Proc. Natl. Acad. Sci. USA 1998, 95, 8869–8873. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Deng, K.; Gao, H.; Xing, S.; Capoferri, A.A.; Durand, C.M.; Rabi, S.A.; Laird, G.M.; Kim, M.; Hosmane, N.N.; et al. Transcriptional Reprogramming during Effector-to-Memory Transition Renders CD4+ T Cells Permissive for Latent HIV-1 Infection. Immunity 2017, 47, 766–775.e3. [Google Scholar] [CrossRef] [PubMed]
- Vanhamel, J.; Bruggemans, A.; Debyser, Z. Establishment of Latent HIV-1 Reservoirs: What Do We Really Know? J. Virus Erad. 2019, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hosmane, N.N.; Kwon, K.J.; Bruner, K.M.; Capoferri, A.A.; Beg, S.; Rosenbloom, D.I.S.; Keele, B.F.; Ho, Y.-C.; Siliciano, J.D.; Siliciano, R.F. Proliferation of Latently Infected CD4+ T Cells Carrying Replication-Competent HIV-1: Potential Role in Latent Reservoir Dynamics. J. Exp. Med. 2017, 214, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gurule, E.E.; Brennan, T.P.; Gerold, J.M.; Kwon, K.J.; Hosmane, N.N.; Kumar, M.R.; Beg, S.A.; Capoferri, A.A.; Ray, S.C.; et al. Expanded Cellular Clones Carrying Replication-Competent HIV-1 Persist, Wax, and Wane. Proc. Natl. Acad. Sci. USA 2018, 115, E2575–E2584. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Simonetti, F.R.; Ho, Y.-C. The Forces Driving Clonal Expansion of the HIV-1 Latent Reservoir. Virol. J. 2020, 17, 4. [Google Scholar] [CrossRef]
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.-Y.; Archer, J.; Pond, S.L.K.; Chung, Y.-S.; Penugonda, S.; Chipman, J.G.; Fletcher, C.V.; et al. Persistent HIV-1 Replication Maintains the Tissue Reservoir during Therapy. Nature 2016, 530, 51–56. [Google Scholar] [CrossRef]
- Brodin, J.; Zanini, F.; Thebo, L.; Lanz, C.; Bratt, G.; Neher, R.A.; Albert, J. Establishment and Stability of the Latent HIV-1 DNA Reservoir. Elife 2016, 5, e18889. [Google Scholar] [CrossRef]
- Kearney, M.F.; Wiegand, A.; Shao, W.; McManus, W.R.; Bale, M.J.; Luke, B.; Maldarelli, F.; Mellors, J.W.; Coffin, J.M. Ongoing HIV Replication During ART Reconsidered. Open Forum Infect. Dis. 2017, 4, ofx173. [Google Scholar] [CrossRef]
- Bachmann, N.; von Siebenthal, C.; Vongrad, V.; Turk, T.; Neumann, K.; Beerenwinkel, N.; Bogojeska, J.; Fellay, J.; Roth, V.; Kok, Y.L.; et al. Determinants of HIV-1 Reservoir Size and Long-Term Dynamics during Suppressive ART. Nat. Commun. 2019, 10, 3193. [Google Scholar] [CrossRef]
- Hokello, J.; Sharma, A.L.; Tyagi, M. Efficient Non-Epigenetic Activation of HIV Latency through the T-Cell Receptor Signalosome. Viruses 2020, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Weinberger, L.S. Stochastic Gene Expression as a Molecular Switch for Viral Latency. Curr. Opin. Microbiol. 2009, 12, 460–466. [Google Scholar] [CrossRef][Green Version]
- Weinberger, L.S.; Burnett, J.C.; Toettcher, J.E.; Arkin, A.P.; Schaffer, D.V. Stochastic Gene Expression in a Lentiviral Positive-Feedback Loop: HIV-1 Tat Fluctuations Drive Phenotypic Diversity. Cell 2005, 122, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, L.S.; Dar, R.D.; Simpson, M.L. Transient-Mediated Fate Determination in a Transcriptional Circuit of HIV. Nat. Genet. 2008, 40, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Razooky, B.S.; Cao, Y.; Hansen, M.M.K.; Perelson, A.S.; Simpson, M.L.; Weinberger, L.S. Nonlatching Positive Feedback Enables Robust Bimodality by Decoupling Expression Noise from the Mean. PLoS Biol. 2017, 15, e2000841. [Google Scholar] [CrossRef] [PubMed]
- Razooky, B.S.; Pai, A.; Aull, K.; Rouzine, I.M.; Weinberger, L.S. A Hardwired HIV Latency Program. Cell 2015, 160, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.S.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-Competent Noninduced Proviruses in the Latent Reservoir Increase Barrier to HIV-1 Cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.L.; Rosenbloom, D.I.S.; Fu, F.; Nowak, M.A.; Siliciano, R.F. Predicting the Outcomes of Treatment to Eradicate the Latent Reservoir for HIV-1. Proc. Natl. Acad. Sci. USA 2014, 111, 13475–13480. [Google Scholar] [CrossRef]
- Dar, R.D.; Hosmane, N.N.; Arkin, M.R.; Siliciano, R.F.; Weinberger, L.S. Screening for Noise in Gene Expression Identifies Drug Synergies. Science 2014, 344, 1392–1396. [Google Scholar] [CrossRef]
- Pearson, R.; Kim, Y.K.; Hokello, J.; Lassen, K.; Friedman, J.; Tyagi, M.; Karn, J. Epigenetic Silencing of Human Immunodeficiency Virus (HIV) Transcription by Formation of Restrictive Chromatin Structures at the Viral Long Terminal Repeat Drives the Progressive Entry of HIV into Latency. J. Virol. 2008, 82, 12291–12303. [Google Scholar] [CrossRef]
- Ne, E.; Palstra, R.-J.; Mahmoudi, T. Transcription: Insights from the HIV-1 Promoter. Int. Rev. Cell Mol. Biol. 2018, 335, 191–243. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Ramirez, N.-G.P.; D’Orso, I. HIV-1 Proviral Transcription and Latency in the New Era. Viruses 2020, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Paras, P.; Van Lint, C. Chromatin Disruption in the Promoter of Human Immunodeficiency Virus Type 1 during Transcriptional Activation. EMBO J. 1993, 12, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, C.; Emiliani, S.; Ott, M.; Verdin, E. Transcriptional Activation and Chromatin Remodeling of the HIV-1 Promoter in Response to Histone Acetylation. EMBO J. 1996, 15, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.Y.; Calman, A.F.; Luciw, P.A.; Peterlin, B.M. Anti-Termination of Transcription within the Long Terminal Repeat of HIV-1 by Tat Gene Product. Nature 1987, 330, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.B.; Baltimore, D.; Frankel, A.D. The Role of Tat in the Human Immunodeficiency Virus Life Cycle Indicates a Primary Effect on Transcriptional Elongation. Proc. Natl. Acad. Sci. USA 1991, 88, 4045–4049. [Google Scholar] [CrossRef]
- Wei, P.; Garber, M.E.; Fang, S.M.; Fischer, W.H.; Jones, K.A. A Novel CDK9-Associated C-Type Cyclin Interacts Directly with HIV-1 Tat and Mediates Its High-Affinity, Loop-Specific Binding to TAR RNA. Cell 1998, 92, 451–462. [Google Scholar] [CrossRef]
- Schulze-Gahmen, U.; Hurley, J.H. Structural Mechanism for HIV-1 TAR Loop Recognition by Tat and the Super Elongation Complex. Proc. Natl. Acad. Sci. USA 2018, 115, 12973–12978. [Google Scholar] [CrossRef]
- He, N.; Liu, M.; Hsu, J.; Xue, Y.; Chou, S.; Burlingame, A.; Krogan, N.J.; Alber, T.; Zhou, Q. HIV-1 Tat and Host AFF4 Recruit Two Transcription Elongation Factors into a Bifunctional Complex for Coordinated Activation of HIV-1 Transcription. Mol. Cell 2010, 38, 428–438. [Google Scholar] [CrossRef]
- Sobhian, B.; Laguette, N.; Yatim, A.; Nakamura, M.; Levy, Y.; Kiernan, R.; Benkirane, M. HIV-1 Tat Assembles a Multifunctional Transcription Elongation Complex and Stably Associates with the 7SK snRNP. Mol. Cell 2010, 38, 439–451. [Google Scholar] [CrossRef]
- Fujinaga, K.; Irwin, D.; Huang, Y.; Taube, R.; Kurosu, T.; Peterlin, B.M. Dynamics of Human Immunodeficiency Virus Transcription: P-TEFb Phosphorylates RD and Dissociates Negative Effectors from the Transactivation Response Element. Mol. Cell. Biol. 2004, 24, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.H.; Rana, T.M. DSIF and NELF Interact with RNA Polymerase II Elongation Complex and HIV-1 Tat Stimulates P-TEFb-Mediated Phosphorylation of RNA Polymerase II and DSIF during Transcription Elongation. J. Biol. Chem. 2001, 276, 12951–12958. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Deng, L.; Lacoste, V.; Park, H.U.; Pumfery, A.; Kashanchi, F.; Brady, J.N.; Kumar, A. Coordination of Transcription Factor Phosphorylation and Histone Methylation by the P-TEFb Kinase during Human Immunodeficiency Virus Type 1 Transcription. J. Virol. 2004, 78, 13522–13533. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.M.; Farnung, L.; Urlaub, H.; Cramer, P. Structure of Paused Transcription Complex Pol II-DSIF-NELF. Nature 2018, 560, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, T.; Parra, M.; Vries, R.G.J.; Kauder, S.E.; Verrijzer, C.P.; Ott, M.; Verdin, E. The SWI/SNF Chromatin-Remodeling Complex Is a Cofactor for Tat Transactivation of the HIV Promoter. J. Biol. Chem. 2006, 281, 19960–19968. [Google Scholar] [CrossRef]
- Tréand, C.; du Chéné, I.; Brès, V.; Kiernan, R.; Benarous, R.; Benkirane, M.; Emiliani, S. Requirement for SWI/SNF Chromatin-Remodeling Complex in Tat-Mediated Activation of the HIV-1 Promoter. EMBO J. 2006, 25, 1690–1699. [Google Scholar] [CrossRef]
- Rafati, H.; Parra, M.; Hakre, S.; Moshkin, Y.; Verdin, E.; Mahmoudi, T. Repressive LTR Nucleosome Positioning by the BAF Complex Is Required for HIV Latency. PLoS Biol. 2011, 9, e1001206. [Google Scholar] [CrossRef]
- Benkirane, M.; Chun, R.F.; Xiao, H.; Ogryzko, V.V.; Howard, B.H.; Nakatani, Y.; Jeang, K.T. Activation of Integrated Provirus Requires Histone Acetyltransferase. P300 and P/CAF Are Coactivators for HIV-1 Tat. J. Biol. Chem. 1998, 273, 24898–24905. [Google Scholar] [CrossRef]
- Marzio, G.; Tyagi, M.; Gutierrez, M.I.; Giacca, M. HIV-1 Tat Transactivator Recruits P300 and CREB-Binding Protein Histone Acetyltransferases to the Viral Promoter. Proc. Natl. Acad. Sci. USA 1998, 95, 13519–13524. [Google Scholar] [CrossRef]
- Agbottah, E.; Deng, L.; Dannenberg, L.O.; Pumfery, A.; Kashanchi, F. Effect of SWI/SNF Chromatin Remodeling Complex on HIV-1 Tat Activated Transcription. Retrovirology 2006, 3, 48. [Google Scholar] [CrossRef]
- Gilchrist, D.A.; Dos Santos, G.; Fargo, D.C.; Xie, B.; Gao, Y.; Li, L.; Adelman, K. Pausing of RNA Polymerase II Disrupts DNA-Specified Nucleosome Organization to Enable Precise Gene Regulation. Cell 2010, 143, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Noe Gonzalez, M.; Blears, D.; Svejstrup, J.Q. Causes and Consequences of RNA Polymerase II Stalling during Transcript Elongation. Nat. Rev. Mol. Cell Biol. 2021, 22, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Core, L.; Adelman, K. Promoter-Proximal Pausing of RNA Polymerase II: A Nexus of Gene Regulation. Genes Dev. 2019, 33, 960–982. [Google Scholar] [CrossRef] [PubMed]
- Žumer, K.; Maier, K.C.; Farnung, L.; Jaeger, M.G.; Rus, P.; Winter, G.; Cramer, P. Two Distinct Mechanisms of RNA Polymerase II Elongation Stimulation in Vivo. Mol. Cell 2021, 81, 3096–3109.e8. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Miller-Jensen, K.; Shah, P.S.; Arkin, A.P.; Schaffer, D.V. Control of Stochastic Gene Expression by Host Factors at the HIV Promoter. PLoS Pathog. 2009, 5, e1000260. [Google Scholar] [CrossRef] [PubMed]
- Miller-Jensen, K.; Skupsky, R.; Shah, P.S.; Arkin, A.P.; Schaffer, D.V. Genetic Selection for Context-Dependent Stochastic Phenotypes: Sp1 and TATA Mutations Increase Phenotypic Noise in HIV-1 Gene Expression. PLoS Comput. Biol. 2013, 9, e1003135. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Defechereux, P.; Verdin, E. The Site of HIV-1 Integration in the Human Genome Determines Basal Transcriptional Activity and Response to Tat Transactivation. EMBO J. 2001, 20, 1726–1738. [Google Scholar] [CrossRef]
- Singh, A.; Razooky, B.; Cox, C.D.; Simpson, M.L.; Weinberger, L.S. Transcriptional Bursting from the HIV-1 Promoter Is a Significant Source of Stochastic Noise in HIV-1 Gene Expression. Biophys. J. 2010, 98, L32–L34. [Google Scholar] [CrossRef]
- Skupsky, R.; Burnett, J.C.; Foley, J.E.; Schaffer, D.V.; Arkin, A.P. HIV Promoter Integration Site Primarily Modulates Transcriptional Burst Size Rather than Frequency. PLoS Comput. Biol. 2010, 6, e1000952. [Google Scholar] [CrossRef]
- Dey, S.S.; Foley, J.E.; Limsirichai, P.; Schaffer, D.V.; Arkin, A.P. Orthogonal Control of Expression Mean and Variance by Epigenetic Features at Different Genomic Loci. Mol. Syst. Biol. 2015, 11, 806. [Google Scholar] [CrossRef]
- Carey, J.; Cameron, V.; de Haseth, P.L.; Uhlenbeck, O.C. Sequence-Specific Interaction of R17 Coat Protein with Its Ribonucleic Acid Binding Site. Biochemistry 1983, 22, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, E.; Chartrand, P.; Schaefer, M.; Shenoy, S.M.; Singer, R.H.; Long, R.M. Localization of ASH1 mRNA Particles in Living Yeast. Mol. Cell 1998, 2, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Fusco, D.; Accornero, N.; Lavoie, B.; Shenoy, S.M.; Blanchard, J.-M.; Singer, R.H.; Bertrand, E. Single mRNA Molecules Demonstrate Probabilistic Movement in Living Mammalian Cells. Curr. Biol. 2003, 13, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Boireau, S.; Maiuri, P.; Basyuk, E.; de la Mata, M.; Knezevich, A.; Pradet-Balade, B.; Bäcker, V.; Kornblihtt, A.; Marcello, A.; Bertrand, E. The Transcriptional Cycle of HIV-1 in Real-Time and Live Cells. J. Cell Biol. 2007, 179, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Lesne, A.; Victor, J.-M.; Bertrand, E.; Basyuk, E.; Barbi, M. The Role of Supercoiling in the Motor Activity of RNA Polymerases. Methods Mol. Biol. 2018, 1805, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.C.; Bass, V.L.; Bullock, M.E.; Chavali, A.K.; Lee, R.E.C.; Mothes, W.; Gaudet, S.; Miller-Jensen, K. NF-κB-Chromatin Interactions Drive Diverse Phenotypes by Modulating Transcriptional Noise. Cell Rep. 2018, 22, 585–599. [Google Scholar] [CrossRef]
- Pichon, X.; Lagha, M.; Mueller, F.; Bertrand, E. A Growing Toolbox to Image Gene Expression in Single Cells: Sensitive Approaches for Demanding Challenges. Mol. Cell 2018, 71, 468–480. [Google Scholar] [CrossRef]
- Brouwer, I.; Lenstra, T.L. Visualizing Transcription: Key to Understanding Gene Expression Dynamics. Curr. Opin. Chem. Biol. 2019, 51, 122–129. [Google Scholar] [CrossRef]
- Liu, Z.; Tjian, R. Visualizing Transcription Factor Dynamics in Living Cells. J. Cell Biol. 2018, 217, 1181–1191. [Google Scholar] [CrossRef]
- Lu, F.; Lionnet, T. Transcription Factor Dynamics. Cold Spring Harb. Perspect. Biol. 2021, 13, a040949. [Google Scholar] [CrossRef]
- Mazzocca, M.; Colombo, E.; Callegari, A.; Mazza, D. Transcription Factor Binding Kinetics and Transcriptional Bursting: What Do We Really Know? Curr. Opin. Struct. Biol. 2021, 71, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Struhl, K. Different SP1 Binding Dynamics at Individual Genomic Loci in Human Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2113579118. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Sieben, C.; Benke, A.; Suter, D.M.; Fierz, B.; Mazza, D.; Manley, S. Single-Molecule Dynamics and Genome-Wide Transcriptomics Reveal That NF-kB (P65)-DNA Binding Times Can Be Decoupled from Transcriptional Activation. PLoS Genet. 2019, 15, e1007891. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kabi, M.; Ranga, U. A Stronger Transcription Regulatory Circuit of HIV-1C Drives the Rapid Establishment of Latency with Implications for the Direct Involvement of Tat. J. Virol. 2020, 94, e00503-20. [Google Scholar] [CrossRef]
- Pal, S.; Jaiswal, V.; Nala, N.; Ranga, U. Enhanced Transcriptional Strength of HIV-1 Subtype C Minimizes Gene Expression Noise and Confers Stability to the Viral Latent State. J. Virol. 2023, 97, e0137622. [Google Scholar] [CrossRef] [PubMed]
- Chanou, A.; Hamperl, S. Single-Molecule Techniques to Study Chromatin. Front. Cell Dev. Biol. 2021, 9, 699771. [Google Scholar] [CrossRef] [PubMed]
- Miller-Jensen, K.; Dey, S.S.; Pham, N.; Foley, J.E.; Arkin, A.P.; Schaffer, D.V. Chromatin Accessibility at the HIV LTR Promoter Sets a Threshold for NF-κB Mediated Viral Gene Expression. Integr. Biol. 2012, 4, 661–671. [Google Scholar] [CrossRef]
- Bullock, M.E.; Moreno-Martinez, N.; Miller-Jensen, K. A Transcriptional Cycling Model Recapitulates Chromatin-Dependent Features of Noisy Inducible Transcription. PLoS Comput. Biol. 2022, 18, e1010152. [Google Scholar] [CrossRef]
- Shao, W.; Zeitlinger, J. Paused RNA Polymerase II Inhibits New Transcriptional Initiation. Nat. Genet. 2017, 49, 1045–1051. [Google Scholar] [CrossRef]
- Gressel, S.; Schwalb, B.; Decker, T.M.; Qin, W.; Leonhardt, H.; Eick, D.; Cramer, P. CDK9-Dependent RNA Polymerase II Pausing Controls Transcription Initiation. Elife 2017, 6, e29736. [Google Scholar] [CrossRef]
- Douaihy, M.; Topno, R.; Lagha, M.; Bertrand, E.; Radulescu, O. BurstDECONV: A Signal Deconvolution Method to Uncover Mechanisms of Transcriptional Bursting in Live Cells. Nucleic Acids Res. 2023, 51, e88. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.; Boddington, C.; Downton, P.; Rowe, W.; Bagnall, J.; Lam, C.; Maya-Mendoza, A.; Schmidt, L.; Harper, C.V.; Spiller, D.G.; et al. Signal Transduction Controls Heterogeneous NF-κB Dynamics and Target Gene Expression through Cytokine-Specific Refractory States. Nat. Commun. 2016, 7, 12057. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, L.S.; Shenk, T. An HIV Feedback Resistor: Auto-Regulatory Circuit Deactivator and Noise Buffer. PLoS Biol. 2007, 5, e9. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lei, X.; Ribeiro, R.M.; Perelson, A.S.; Liang, J. Probabilistic Control of HIV Latency and Transactivation by the Tat Gene Circuit. Proc. Natl. Acad. Sci. USA 2018, 115, 12453–12458. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Stochastic Analysis of Genetic Feedback Circuit Controlling HIV Cell-Fate Decision. In Proceedings of the 2012 IEEE 51st IEEE Conference on Decision and Control (CDC), Maui, HI, USA, 10–13 December 2012; pp. 4918–4923. [Google Scholar]
- Kiernan, R.E.; Vanhulle, C.; Schiltz, L.; Adam, E.; Xiao, H.; Maudoux, F.; Calomme, C.; Burny, A.; Nakatani, Y.; Jeang, K.T.; et al. HIV-1 Tat Transcriptional Activity Is Regulated by Acetylation. EMBO J. 1999, 18, 6106–6118. [Google Scholar] [CrossRef]
- Col, E.; Caron, C.; Seigneurin-Berny, D.; Gracia, J.; Favier, A.; Khochbin, S. The Histone Acetyltransferase, hGCN5, Interacts with and Acetylates the HIV Transactivator, Tat. J. Biol. Chem. 2001, 276, 28179–28184. [Google Scholar] [CrossRef] [PubMed]
- Kaehlcke, K.; Dorr, A.; Hetzer-Egger, C.; Kiermer, V.; Henklein, P.; Schnoelzer, M.; Loret, E.; Cole, P.A.; Verdin, E.; Ott, M. Acetylation of Tat Defines a CyclinT1-Independent Step in HIV Transactivation. Mol. Cell 2003, 12, 167–176. [Google Scholar] [CrossRef]
- Ott, M.; Schnölzer, M.; Garnica, J.; Fischle, W.; Emiliani, S.; Rackwitz, H.R.; Verdin, E. Acetylation of the HIV-1 Tat Protein by P300 Is Important for Its Transcriptional Activity. Curr. Biol. 1999, 9, 1489–1492. [Google Scholar] [CrossRef]
- D’Orso, I.; Frankel, A.D. Tat Acetylation Modulates Assembly of a Viral-Host RNA-Protein Transcription Complex. Proc. Natl. Acad. Sci. USA 2009, 106, 3101–3106. [Google Scholar] [CrossRef]
- Pagans, S.; Pedal, A.; North, B.J.; Kaehlcke, K.; Marshall, B.L.; Dorr, A.; Hetzer-Egger, C.; Henklein, P.; Frye, R.; McBurney, M.W.; et al. SIRT1 Regulates HIV Transcription via Tat Deacetylation. PLoS Biol. 2005, 3, e41. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, H. Understanding the Function of Mammalian Sirtuins and Protein Lysine Acylation. Annu. Rev. Biochem. 2021, 90, 245–285. [Google Scholar] [CrossRef] [PubMed]
- Chavali, A.K.; Wong, V.C.; Miller-Jensen, K. Distinct Promoter Activation Mechanisms Modulate Noise-Driven HIV Gene Expression. Sci. Rep. 2015, 5, 17661. [Google Scholar] [CrossRef] [PubMed]
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Lu, Y.; Bohn-Wippert, K.; Pazerunas, P.J.; Moy, J.M.; Singh, H.; Dar, R.D. Screening for Gene Expression Fluctuations Reveals Latency-Promoting Agents of HIV. Proc. Natl. Acad. Sci. USA 2021, 118, e2012191118. [Google Scholar] [CrossRef]
- Dai, W.; Wu, F.; McMyn, N.; Song, B.; Walker-Sperling, V.E.; Varriale, J.; Zhang, H.; Barouch, D.H.; Siliciano, J.D.; Li, W.; et al. Genome-Wide CRISPR Screens Identify Combinations of Candidate Latency Reversing Agents for Targeting the Latent HIV-1 Reservoir. Sci. Transl. Med. 2022, 14, eabh3351. [Google Scholar] [CrossRef]
- Morton, E.L.; Forst, C.V.; Zheng, Y.; DePaula-Silva, A.B.; Ramirez, N.-G.P.; Planelles, V.; D’Orso, I. Transcriptional Circuit Fragility Influences HIV Proviral Fate. Cell Rep. 2019, 27, 154–171.e9. [Google Scholar] [CrossRef]
- Krebs, A.R. Studying Transcription Factor Function in the Genome at Molecular Resolution. Trends Genet. 2021, 37, 798–806. [Google Scholar] [CrossRef]
- Clark, I.C.; Mudvari, P.; Thaploo, S.; Smith, S.; Abu-Laban, M.; Hamouda, M.; Theberge, M.; Shah, S.; Ko, S.H.; Pérez, L.; et al. HIV Silencing and Cell Survival Signatures in Infected T Cell Reservoirs. Nature 2023, 614, 318–325. [Google Scholar] [CrossRef]
- Agosto, L.M.; Henderson, A.J. CD4+ T Cell Subsets and Pathways to HIV Latency. AIDS Res. Hum. Retroviruses 2018, 34, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.M.K.; Wen, W.Y.; Ingerman, E.; Razooky, B.S.; Thompson, C.E.; Dar, R.D.; Chin, C.W.; Simpson, M.L.; Weinberger, L.S. A Post-Transcriptional Feedback Mechanism for Noise Suppression and Fate Stabilization. Cell 2018, 173, 1609–1621.e15. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damour, A.; Slaninova, V.; Radulescu, O.; Bertrand, E.; Basyuk, E. Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency. Viruses 2023, 15, 1969. https://doi.org/10.3390/v15091969
Damour A, Slaninova V, Radulescu O, Bertrand E, Basyuk E. Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency. Viruses. 2023; 15(9):1969. https://doi.org/10.3390/v15091969
Chicago/Turabian StyleDamour, Alexia, Vera Slaninova, Ovidiu Radulescu, Edouard Bertrand, and Eugenia Basyuk. 2023. "Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency" Viruses 15, no. 9: 1969. https://doi.org/10.3390/v15091969
APA StyleDamour, A., Slaninova, V., Radulescu, O., Bertrand, E., & Basyuk, E. (2023). Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency. Viruses, 15(9), 1969. https://doi.org/10.3390/v15091969




