What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment Prescription
2.2. Outcomes
2.3. Statistical Analysis
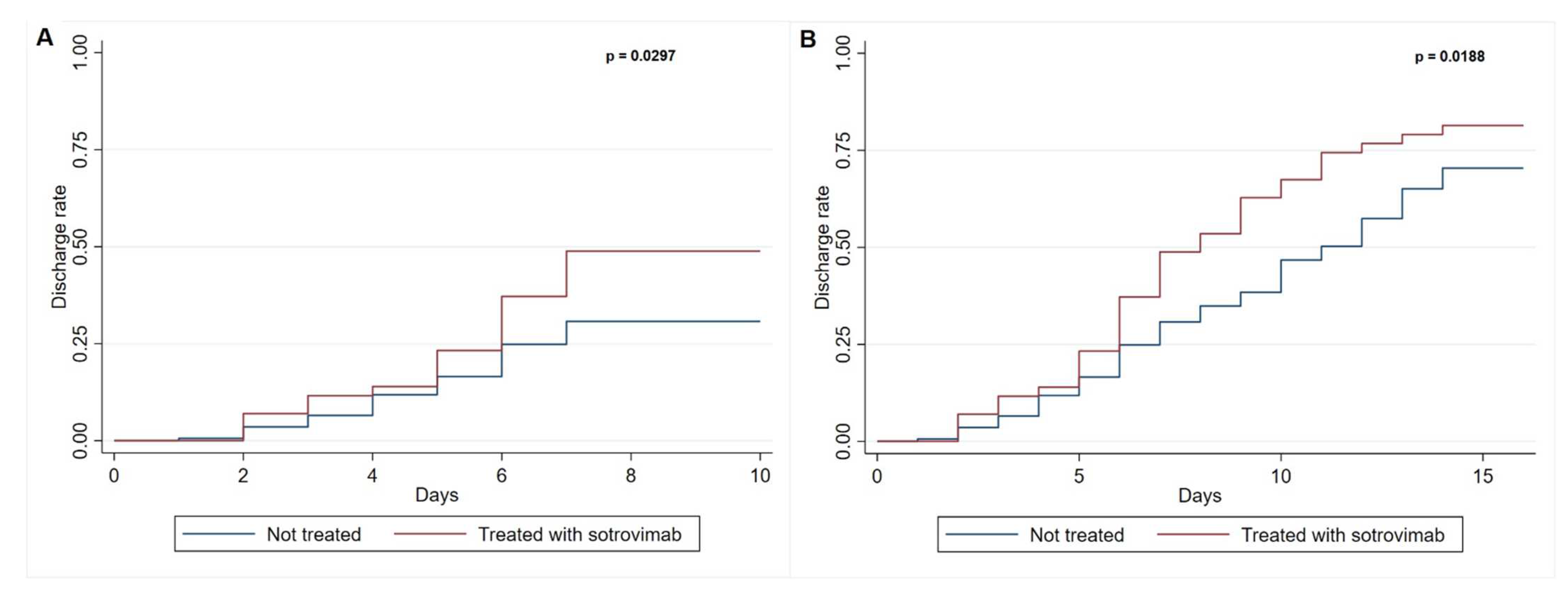
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.F.W.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.W.; Yuan, S.; Yuen, K.Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta-Analysis. Lancet Microbe 2020, 2, e13–e22. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Ghorbani, S.; Khatami, A.; Farzi, R.; Baradaran, B.; Turner, D.L.; Turner, R.J.; Bahr, N.C.; Idrovo, J. Comparison of Confirmed COVID-19 with SARS and MERS Cases—Clinical Characteristics, Laboratory Findings, Radiographic Signs and Outcomes: A Systematic Review and Meta-analysis. Rev. Med. Virol. 2020, 30, e2112. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 Pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Manjunath, K.; Ranjan, R.K.; Kaushik, S.; Kumar, S.; Verma, V. COVID-19 Pandemic: Insights into Structure, Function, and HACE2 Receptor Recognition by SARS-CoV-2. PLoS Pathog. 2020, 16, e1008762. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Fiore, V.; Princic, E.; Geremia, N.; Panu Napodano, C.M.; Muredda, A.A.; Maida, I.; Madeddu, G.; Babudieri, S. Predictors of Infection, Symptoms Development, and Mortality in People with SARS-CoV-2 Living in Retirement Nursing Homes. PLoS ONE 2021, 16, e0248009. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective Evaluation of Anosmia and Ageusia in COVID-19 Patients: A Single-center Experience on 72 Cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef]
- Geremia, N.; De Vito, A.; Gunnella, S.; Fiore, V.; Princic, E.; Panu Napodano, C.; Madeddu, G.; Babudieri, S. A Case of Vasculitis-Like Skin Eruption Associated With COVID-19. Infect. Dis. Clin. Pract. 2020, 28, e30–e31. [Google Scholar] [CrossRef]
- Grant, M.C.; Geoghegan, L.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The Prevalence of Symptoms in 24,410 Adults Infected by the Novel Coronavirus (SARS-CoV-2; COVID-19): A Systematic Review and Meta-Analysis of 148 Studies from 9 Countries. PLoS ONE 2020, 15, e0234765. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; De Vito, A.; Deiana, G.; Pes, C.; Giovanditto, F.; Fiore, V.; Lechien, J.R.; Saussez, S.; Policicchio, D.; Boccaletti, R.; et al. Systemic Inflammatory Markers and Psychophysical Olfactory Scores in Coronavirus Disease 2019 Patients: Is There Any Correlation? J. Laryngol. Otol. 2021, 135, 723–728. [Google Scholar] [CrossRef]
- Vaira, L.A.; De Vito, A.; Deiana, G.; Pes, C.; Giovanditto, F.; Fiore, V.; Lechien, J.R.; Le Bon, S.D.; Saussez, S.; Madeddu, G.; et al. Correlations between IL-6 Serum Level and Olfactory Dysfunction Severity in COVID-19 Patients: A Preliminary Study. Eur. Arch. Otorhinolaryngol 2022, 279, 811–816. [Google Scholar] [CrossRef]
- Drake, T.M.; Riad, A.M.; Fairfield, C.J.; Egan, C.; Knight, S.R.; Pius, R.; Hardwick, H.E.; Norman, L.; Shaw, C.A.; Mclean, K.A.; et al. Characterisation of In-Hospital Complications Associated with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol UK: A Prospective, Multicentre Cohort Study. Lancet 2021, 398, 223–237. [Google Scholar] [CrossRef]
- De Vito, A.; Geremia, N.; Fiore, V.; Princic, E.; Babudieri, S.; Madeddu, G. Clinical Features, Laboratory Findings and Predictors of Death in Hospitalized Patients with COVID-19 in Sardinia, Italy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7861–7868. [Google Scholar]
- Zinellu, A.; De Vito, A.; Scano, V.; Paliogiannis, P.; Fiore, V.; Madeddu, G.; Maida, I.; Zinellu, E.; Mangoni, A.A.; Arru, L.B.; et al. The PaO2/FiO2 Ratio on Admission Is Independently Associated with Prolonged Hospitalization in COVID-19 Patients. J. Infect. Dev. Ctries. 2021, 15, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Duong, D. Alpha, Beta, Delta, Gamma: What’s Important to Know about SARS-CoV-2 Variants of Concern? CMAJ Can. Med. Assoc. J. 2021, 193, E1059. [Google Scholar] [CrossRef] [PubMed]
- Scovino, A.M.; Dahab, E.C.; Vieira, G.F.; Freire-de-Lima, L.; Freire-de-Lima, C.G.; Morrot, A. SARS-CoV-2’s Variants of Concern: A Brief Characterization. Front. Immunol. 2022, 13, 3541. [Google Scholar] [CrossRef]
- Nealon, J.; Cowling, B.J. Omicron Severity: Milder but Not Mild. Lancet 2022, 399, 412–413. [Google Scholar] [CrossRef]
- Sigal, A.; Milo, R.; Jassat, W. Estimating Disease Severity of Omicron and Delta SARS-CoV-2 Infections. Nat. Rev. Immunol. 2022, 22, 267–269. [Google Scholar] [CrossRef]
- Mohsin, M.; Mahmud, S. Omicron SARS-CoV-2 Variant of Concern: A Review on Its Transmissibility, Immune Evasion, Reinfection, and Severity. Medicine 2022, 101, E29165. [Google Scholar] [CrossRef] [PubMed]
- Mader, A.L.; Tydykov, L.; Glück, V.; Bertok, M.; Weidlich, T.; Gottwald, C.; Stefl, A.; Vogel, M.; Plentz, A.; Köstler, J.; et al. Omicron’s Binding to Sotrovimab, Casirivimab, Imdevimab, CR3022, and Sera from Previously Infected or Vaccinated Individuals. iScience 2022, 25, 104076. [Google Scholar] [CrossRef]
- De Vito, A.; Colpani, A.; Bitti, A.; Zauli, B.; Meloni, M.C.; Fois, M.; Denti, L.; Bacciu, S.; Marcia, C.; Maida, I.; et al. Safety and Efficacy of Molnupiravir in SARS-CoV-2 Infected Patients: A Real-Life Experience. J. Med. Virol. 2022, 94, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Colpani, A.; Saderi, L.; Puci, M.; Zauli, B.; Fiore, V.; Fois, M.; Meloni, M.C.; Bitti, A.; Di Castri, C.; et al. Impact of Early SARS-CoV-2 Antiviral Therapy on Disease Progression. Viruses 2023, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Mengato, D.; Sasset, L.; Ferrari, A.; Gardin, S.; Scaglione, V.; Bonadiman, N.; Calandrino, L.; Cavinato, S.; Trivellato, S.; et al. Molnupiravir and Nirmatrelvir/Ritonavir: Tolerability, Safety, and Adherence in a Retrospective Cohort Study. Viruses 2023, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.; Torreele, E.; Vaillant, M. COVID-19 Vaccine Efficacy and Effectiveness—The Elephant (Not) in the Room. Lancet Microbe 2021, 2, e279–e280. [Google Scholar] [CrossRef]
- De Vito, A.; Colpani, A.; Trunfio, M.; Fiore, V.; Moi, G.; Fois, M.; Leoni, N.; Ruiu, S.; Babudieri, S.; Calcagno, A.; et al. Living with HIV and Getting Vaccinated: A Narrative Review. Vaccines 2023, 11, 896. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An Infectious SARS-CoV-2 B.1.1.529 Omicron Virus Escapes Neutralization by Therapeutic Monoclonal Antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar] [CrossRef]
- Touret, F.; Baronti, C.; Bouzidi, H.S.; de Lamballerie, X. In Vitro Evaluation of Therapeutic Antibodies against a SARS-CoV-2 Omicron B.1.1.529 Isolate. Sci. Rep. 2022, 12, 4683. [Google Scholar] [CrossRef]
- Takashita, E.; Yamayoshi, S.; Halfmann, P.; Wilson, N.; Ries, H.; Richardson, A.; Bobholz, M.; Vuyk, W.; Maddox, R.; Baker, D.A.; et al. In Vitro Efficacy of Antiviral Agents against Omicron Subvariant BA.4.6. N. Engl. J. Med. 2022, 387, 2094–2097. [Google Scholar] [CrossRef]
- Araf, Y.; Akter, F.; Tang, Y.d.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron Variant of SARS-CoV-2: Genomics, Transmissibility, and Responses to Current COVID-19 Vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current Evidence on Efficacy of COVID-19 Booster Dose Vaccination against the Omicron Variant: A Systematic Review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Post, L.A.; Lorenzo-Redondo, R. Omicron: Fewer Adverse Outcomes Come with New Dangers. Lancet 2022, 399, 1280. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Brunkard, J.M.; Boehmer, T.K.; Peterson, E.; Adjei, S.; Binder, A.M.; Cobb, S.; Graff, P.; Hidalgo, P.; Panaggio, M.J.; et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods-United States, December 2020–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 146–152. [Google Scholar] [CrossRef]
- Ministero Della Salute—Istituto Superiore di Sanità. Aggiornamento Casi COVID-19—Dati AggregatiQuotidianiRegioni/PPAA; Istituto Superiore di Sanità: Rome, Italy, 2022. [Google Scholar]
- Istituto Superiore di Sanità. Comunicato Stampa N°08/2022-Covid-19, Flash Survey Iss: Il 17 Gennaio Il 95,8% Dei Campioni Positivi a Omicron. Available online: https://www.iss.it/web/guest/cov19-cosa-fa-iss-varianti/-/asset_publisher/yJS4xO2fauqM/content/comunicato%C2%A0stampa-n%C2%B008-2022-covid-19-flash-survey-iss-il-17-gennaio-il-95-8-dei-campioni-positivi-a-omicron?_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId=6608164&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_redirect=https%3A%2F%2Fwww.iss.it%2Fweb%2Fguest%2Fcov19-cosa-fa-iss-varianti%3Fp_p_id%3Dcom_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_assetEntryId%3D6608164%26_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_cur%3D0%26p_r_p_resetCur%3Dfalse (accessed on 11 August 2023).
- Poliseno, M.; Drago, E.P.; Poli, M.A.; Altamura, M.; Bruno, S.R.; Calamo, A.; Giannelli, A.; Infante, G.; Mazzola, M.; Moschetta, D.; et al. Clinical Characteristics, Outcomes, and Risk Factors of Patients Hospitalized for COVID-19 across the Latest Pandemic Waves: Has Something Changed? BioMed 2023, 3, 272–281. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The Omicron Variant Is Highly Resistant against Antibody-Mediated Neutralization: Implications for Control of the COVID-19 Pandemic. Cell 2022, 185, 447. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Piccicacco, N.; Zeitler, K.; Ing, A.; Montero, J.; Faughn, J.; Silbert, S.; Kim, K. Real-World Effectiveness of Early Remdesivir and Sotrovimab in the Highest-Risk COVID-19 Outpatients during the Omicron Surge. J. Antimicrob. Chemother. 2022, 77, 2693–2700. [Google Scholar] [CrossRef]
- Cheng, M.M.; Reyes, C.; Satram, S.; Birch, H.; Gibbons, D.C.; Drysdale, M.; Bell, C.F.; Suyundikov, A.; Ding, X.; Maher, M.C.; et al. Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA. Infect. Dis. Ther. 2023, 12, 607–621. [Google Scholar] [CrossRef]
- Chavarot, N.; Melenotte, C.; Amrouche, L.; Rouzaud, C.; Sberro-Soussan, R.; Pavie, J.; Martinez, F.; Pouvaret, A.; Leruez-Ville, M.; Cantin, D.; et al. Early Treatment with Sotrovimab Monoclonal Antibody in Kidney Transplant Recipients with Omicron Infection. Kidney Int. 2022, 101, 1290. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, C.; Palacios, C.F.; Azar, M.M.; Cohen, E.; Malinis, M. Real-World Experience with Available, Outpatient COVID-19 Therapies in Solid Organ Transplant Recipients during the Omicron Surge. Am. J. Transplant. 2022, 22, 2458–2463. [Google Scholar] [CrossRef]
- Pinchera, B.; Buonomo, A.R.; Scotto, R.; Carrano, R.; Salemi, F.; Galluccio, F.; Guarino, M.; Viceconte, G.; Schiano Moriello, N.; Giaccone, A.; et al. Sotrovimab in Solid Organ Transplant Patients With Early, Mild/Moderate SARS-CoV-2 Infection: A Single-Center Experience. Transplantation 2022, 106, e345. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Qi, C.; Adebayo, J.O.; Underwood, J.; Coulson, J.; Bailey, R.; Lyons, R.; Edwards, A.; Cooper, A.; John, G.; et al. Real-World Effectiveness of Molnupiravir, Nirmatrelvir-Ritonavir, and Sotrovimab on Preventing Hospital Admission among Higher-Risk Patients with COVID-19 in Wales: A Retrospective Cohort Study. J. Infect. 2023, 86, 352. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Brehm, T.T.; Fischer, M.; Heyer, A.; Wichmann, D.; Jordan, S.; Nörz, D.; Lütgehetmann, M.; Addo, M.M.; Lohse, A.W.; et al. Sotrovimab in Hospitalized Patients with SARS-CoV-2 Omicron Variant Infection: A Propensity Score-Matched Retrospective Cohort Study. Microbiol. Spectr. 2023, 11, e0410322. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; Beaty, L.E.; Bennett, T.D.; Carlson, N.E.; Davis, C.B.; Kwan, B.M.; Mayer, D.A.; Ong, T.C.; Russell, S.; Steele, J.; et al. Real-World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients. J. Infect. Dis. 2022, 226, 2129. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Colpani, A.; Madeddu, G. Shedding New Light on COVID-19 Therapeutics during the Omicron Era: A Deeper Dive into Real-World Data. Lancet Reg. Health Eur. 2023, 31, 100694. [Google Scholar] [CrossRef] [PubMed]
| Overall (689) | Sotrovimab (341) | Not Treated (348) | p Value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 72.4 (60.9–80.0) | 71.1 (60.0–79.1) | 74.9 (61.9–84.3) | 0.0012 |
| Age ≥ 65 years | 478 (69.4) | 231 (67.7) | 247 (71.0) | 0.357 |
| Male gender, n (%) | 393 (55.6) | 181 (53.1) | 202 (58.0) | 0.190 |
| Comorbidities, n (%) | ||||
| Obesity | 149 (21.6) | 68 (19.9) | 92 (23.3) | 0.288 |
| CKD | 138 (20.0) | 73 (21.4) | 65 (18.7) | 0.371 |
| Immune depression | 151 (21.9) | 113 (33.1) | 38 (10.9) | <0.001 |
| Transplant recipients | 30 (4.3) | 24 (7.0) | 6 (1.7) | 0.001 |
| Diabetes | 155 (22.5) | 75 (22.0) | 80 (23.0) | 0.755 |
| Chronic liver disease | 47 (6.8) | 28 (8.2) | 19 (5.5) | 0.152 |
| COPD | 114 (16.5) | 68 (19.9) | 46 (13.2) | 0.018 |
| Previous CVA | 185 (26.8) | 134 (39.3) | 51 (14.7) | <0.001 |
| Oncological disease | 143 (20.8) | 93 (27.3) | 50 (14.4) | <0.001 |
| Heamatological cancer | 66 (9.6) | 44 (12.9) | 22 (6.3) | 0.003 |
| Heamatological cancer with chemotherapy treatment | 45 (6.5) | 30 (8.8) | 15 (4.3) | 0.017 |
| Cardiovascular disease | 203 (29.5) | 76 (22.3) | 127 (36.5) | <0.001 |
| Vaccine, n (%) | 564 (81.8) | 291 (85.3) | 273 (78.4) | 0.019 |
| Time between last vaccine dose and SARS-CoV-2 positivity, median (IQR) | 155 (96–213) | 173 (105–223.5) | 149 (85–199) | 0.001 |
| Time between last vaccine dose and of SARS-CoV-2 positivity ≤ 155 days, n (%) | 282/564 (50.0) | 132/291 (45.4) | 150/273 (54.9) | 0.023 |
| Symptoms, n (%) | 553 (80.3) | 341 (100) | 212 (60.9) | <0.001 |
| Fever, n (%) | 324 (47.0) | 198 (58.1) | 126 (36.2) | <0.001 |
| Cough, n (%) | 276 (40.1) | 192 (56.3) | 84 (24.1) | <0.001 |
| Ageusia, n (%) | 13 (1.9) | 11 (3.2) | 2 (0.6) | 0.011 |
| Sore throat, n (%) | 145 (21.0) | 121 (35.5) | 24 (6.9) | <0.001 |
| Asthenia, n (%) | 233 (33.8) | 150 (44.0) | 83 (23.8) | <0.001 |
| Myalgia, n (%) | 102 (14.8) | 74 (21.7) | 28 (8.0) | <0.001 |
| GI symptoms, n (%) | 74 (10.7) | 35 (10.3) | 39 (11.2) | 0.689 |
| Dyspnea, n (%) | 123 (17.8) | 37 (10.8) | 86 (24.7) | <0.001 |
| Days between symptoms onset and SARS-CoV-2 diagnosis | 1 (0–2) | 1 (0–2) | 1.5 (0–3) | <0.001 |
| Monlupiravir | 61 (8.85) | 61 (17.9) | 0 | - |
| Nirmatrelvir/ritonavir | 9 (1.3) | 9 (2.6) | 0 | - |
| Remdesivir | 26 (3.8) | 26 (7.6) | 0 | - |
| Disease progression, n (%) | 161 (23.4) | 17 (5.0) | 144 (41.4) | <0.001 |
| Death, n (%) | 81 (11.8) | 18 (5.3) | 63 (18.1) | <0.001 |
| Death in people with disease progression, n (%) | 65 (9.4) | 9 (2.6) | 56 (16.1) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | OR (95%CI) | p-Value | OR (CI95%) | p-Value |
| Age (10 years) | 1.26 (1.11–1.43) | <0.001 | 1.23 (1.04–1.45) | 0.015 |
| Obesity | 1.53 (1.02–2.29) | 0.038 | 1.19 (0.69–2.06) | 0.528 |
| Immunodeficit | 0.55 (0.34–0.88) | 0.012 | 1.59 (0.82–3.01) | 0.168 |
| Previous CVA | 0.47 (0.30–0.74) | 0.001 | 1.12 (0.62–2.03) | 0.701 |
| Oncological disease | 0.47 (0.28–0.78) | 0.003 | 0.95 (0.49–1.86) | 0.891 |
| Cardiovascular disease | 2.20 (1.52–3.19) | <0.001 | 1.69 (1.01–2.80) | 0.044 |
| Vaccine, n (%) | 0.22 (0.14–0.33) | <0.001 | 0.21 (0.12–0.37) | <0.001 |
| Symptoms, n (%) | 3.15 (1.76–5.66) | <0.001 | ||
| Fever, n (%) | 1.76 (1.23–2.51) | 0.002 | 3.88 (2.35–6.38) | <0.001 |
| Dyspnea, n (%) | 9.15 (5.94–14.07) | <0.001 | 7.24 (4.17–12.58) | <0.001 |
| Sotrovimab | 0.07 (0.4–0.13) | <0.001 | 0.05 (0.02–0.11) | <0.001 |
| Sotrovimab + Antiviral | 0.22 (0.10–0.49) | <0.001 | 1.47 (0.51–4.21) | 0.476 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | OR (95%CI) | p-Value | OR (95%CI) | p-Value |
| Age (10 years) | 1.48 (1.20–1.83) | <0.001 | 1.36 (1.09–1.69) | 0.006 |
| Haematological cancer with chemotherapy treatment | 1.86 (0.79–4.35) | 0.152 | 4.07 (1.45–11.4) | 0.008 |
| Cardiovascular disease | 2.75 (1.64–4.62) | <0.001 | 1.76 (0.96–3.22) | 0.065 |
| Vaccine, n (%) | 0.33 (0.19–0.57) | <0.001 | 0.37 (0.20–0.68) | 0.002 |
| Dyspnea, n (%) | 5.27 (3.09–9.00) | <0.001 | 3.63 (2.02–6.50) | <0.001 |
| Sotrovimab | 0.14 (0.07–0.29) | <0.001 | 0.16 (0.06–0.42) | <0.001 |
| Sotrovimab + Antiviral | 0.38 (0.13–1.07) | 0.066 | 1.79 (0.45–7.16) | 0.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vito, A.; Colpani, A.; Poliseno, M.; Diella, L.; Ieva, F.R.P.; Belati, A.; Papale, R.; Babudieri, S.; De Santis, L.; Saracino, A.; et al. What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study. Viruses 2023, 15, 1757. https://doi.org/10.3390/v15081757
De Vito A, Colpani A, Poliseno M, Diella L, Ieva FRP, Belati A, Papale R, Babudieri S, De Santis L, Saracino A, et al. What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study. Viruses. 2023; 15(8):1757. https://doi.org/10.3390/v15081757
Chicago/Turabian StyleDe Vito, Andrea, Agnese Colpani, Mariacristina Poliseno, Lucia Diella, Francesco Rosario Paolo Ieva, Alessandra Belati, Roberto Papale, Sergio Babudieri, Laura De Santis, Annalisa Saracino, and et al. 2023. "What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study" Viruses 15, no. 8: 1757. https://doi.org/10.3390/v15081757
APA StyleDe Vito, A., Colpani, A., Poliseno, M., Diella, L., Ieva, F. R. P., Belati, A., Papale, R., Babudieri, S., De Santis, L., Saracino, A., Lo Caputo, S., & Madeddu, G. (2023). What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study. Viruses, 15(8), 1757. https://doi.org/10.3390/v15081757








