Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Assessments
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
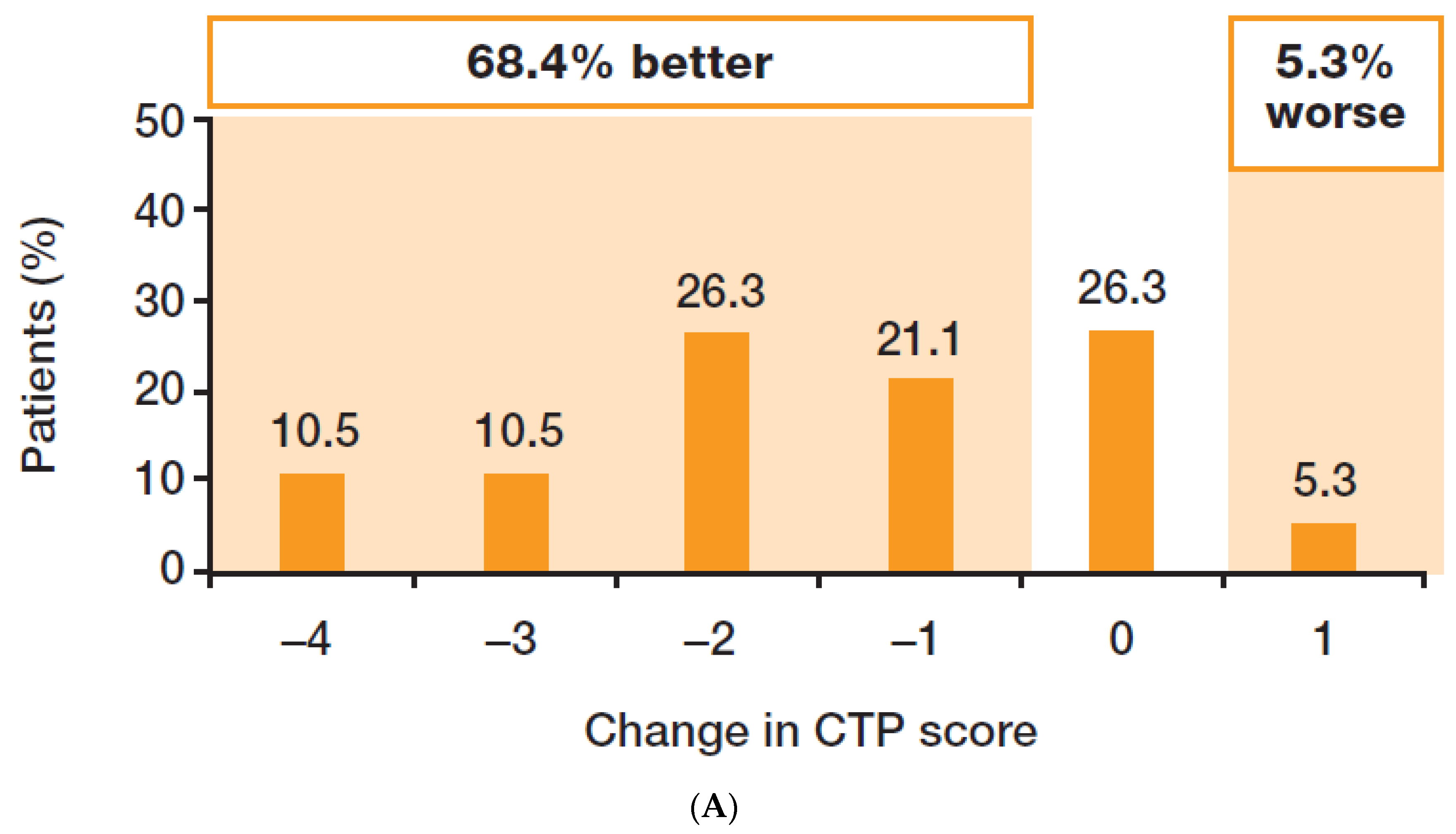
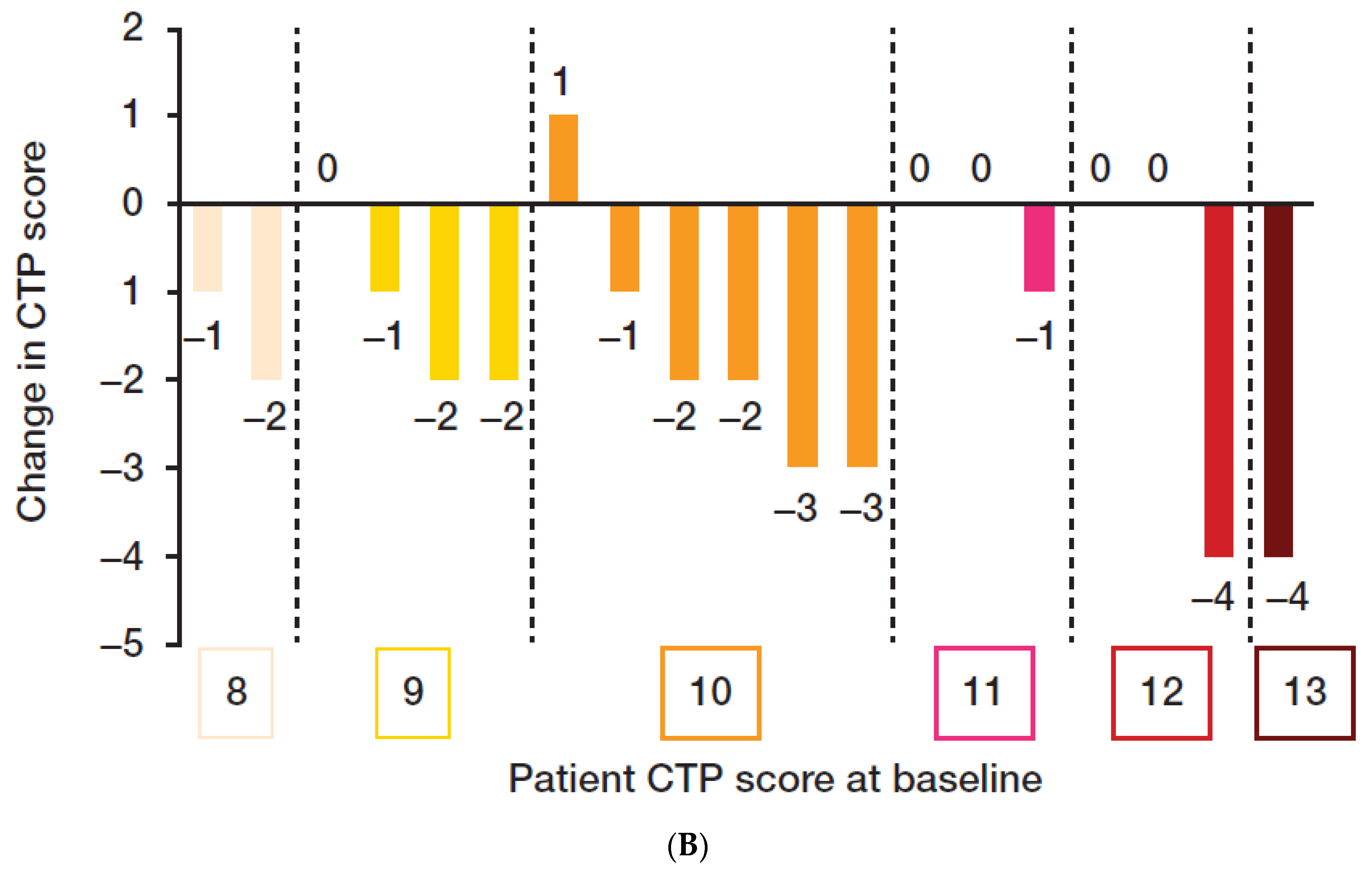
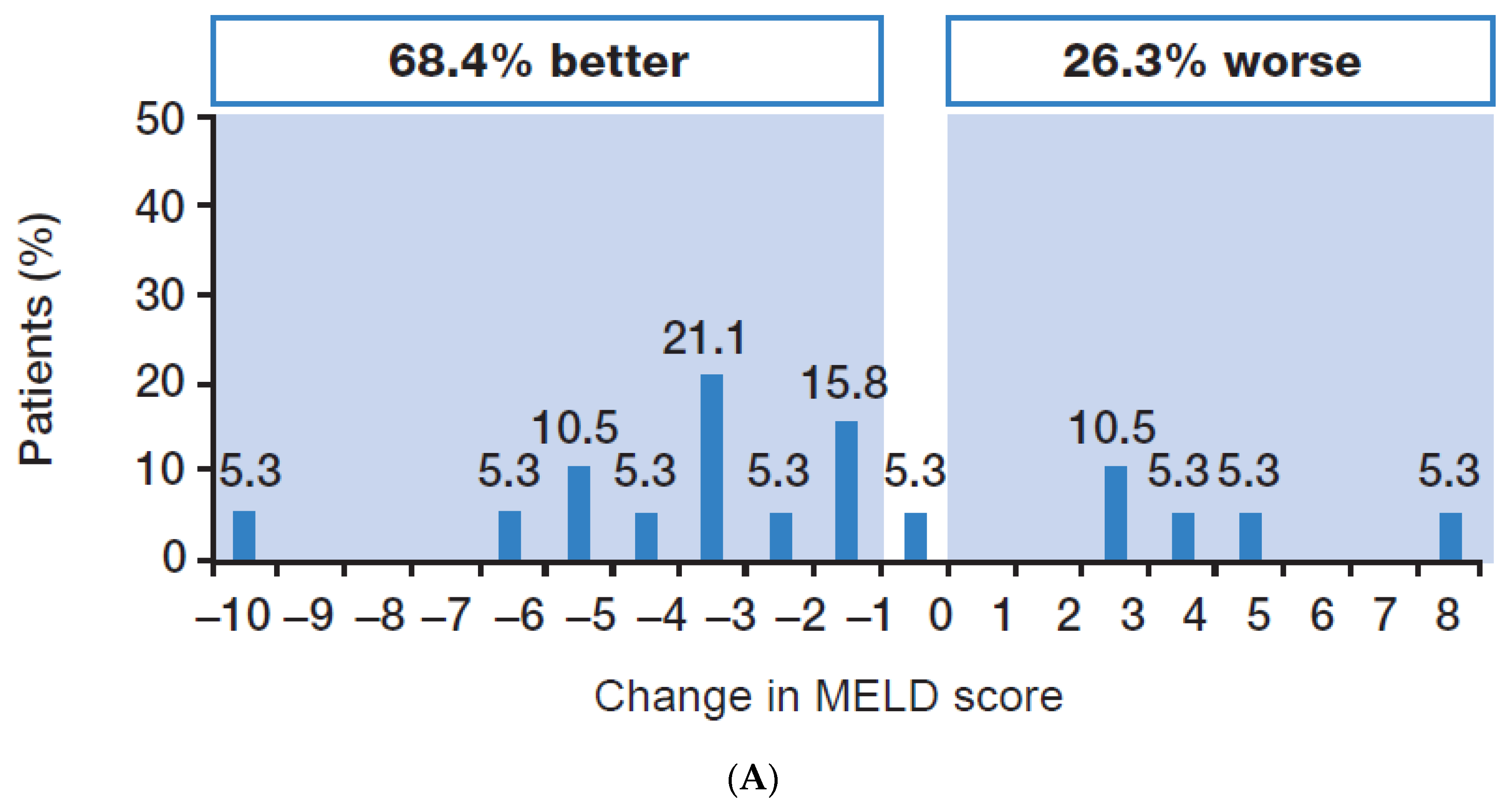
3.2. Efficacy
3.3. Impact on HRQoL
3.4. Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis C Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 25 July 2023).
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- American Association for the Study of Liver Diseases. HCV Guidance: Recommendations for Testing, Managing and Treating Hepatitis C. Available online: www.hcvguidelines.org (accessed on 25 July 2023).
- Curry, M.P.; O’leary, J.G.; Bzowej, N.; Muir, A.J.; Korenblat, K.M.; Fenkel, J.M.; Reddy, K.R.; Lawitz, E.; Flamm, S.L.; Schiano, T.; et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 2015, 373, 2618–2628. [Google Scholar] [CrossRef]
- Takehara, T.; Sakamoto, N.; Nishiguchi, S.; Ikeda, F.; Tatsumi, T.; Ueno, Y.; Yatsuhashi, H.; Takikawa, Y.; Kanda, T.; Sakamoto, M.; et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: An open-label Phase 3 trial. J. Gastroenterol. 2019, 54, 87–95. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chen, C.-Y.; Su, W.-W.; Liu, C.-J.; Lo, C.-C.; Huang, K.-J.; Chen, J.-J.; Tseng, K.-C.; Chang, C.-Y.; Peng, C.-Y.; et al. Sofosbuvir/velpatasvir plus ribavirin for Child-Pugh B and Child-Pugh C hepatitis C virus-related cirrhosis. Clin. Mol. Hepatol. 2021, 27, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-N.; Mo, L.-R.; Chen, C.-T.; Chen, C.-Y.; Huang, C.-F.; Kuo, H.-T.; Lo, C.-C.; Tseng, K.-C.; Huang, Y.-H.; Tai, C.-M.; et al. Sofosbuvir/velpatasvir for hepatitis C virus infection: Real-world effectiveness and safety from a nationwide registry in Taiwan. Infect. Dis. Ther. 2022, 11, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.; Milligan, S.; Khalili, M.; Fagiuoli, S.; Shafran, S.D.; Carrat, F.; Ouzan, D.; Papatheodoridis, G.; Ramji, A.; Borgia, S.M.; et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts. Liver Int. 2020, 40, 1841–1852. [Google Scholar] [CrossRef]
- Lucey, M.R.; Brown, K.A.; Everson, G.T.; Fung, J.J.; Gish, R.; Keeffe, E.B.; Kneteman, N.M.; Lake, J.R.; Martin, P.; McDiarmid, S.V.; et al. Minimal criteria for placement of adults on the liver transplant waiting list: A report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transplant. Surg. 1997, 3, 628–637. [Google Scholar] [CrossRef]
- Said, A.; Williams, J.; Holden, J.; Remington, P.; Gangnon, R.; Musat, A.; Lucey, M.R. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J. Hepatol. 2004, 40, 897–903. [Google Scholar] [CrossRef]
- Contopoulos-Ioannidis, D.G.; Karvouni, A.; Kouri, I.; Ioannidis, J.P.A. Reporting and interpretation of SF-36 outcomes in randomised trials: Systematic review. BMJ 2009, 338, a3006. [Google Scholar] [CrossRef] [PubMed]
- Escorpizo, R.; Bombardier, C.; Boonen, A.; Hazes, J.M.W.; Lacaille, D.; Strand, V.; Beaton, D. Worker productivity outcome measures in arthritis. J. Rheumatol. 2007, 34, 1372–1380. [Google Scholar] [PubMed]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Guyatt, G.; Kiwi, M.; Boparai, N.; King, D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999, 45, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; Bräu, N.; Gane, E.J.; Pianko, S.; Lawitz, E.; Thompson, A.; Shiffman, M.L.; Cooper, C.; et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Tang, S.; Liu, Y.; Du, X.; Qiu, L.; Liu, M.; Yu, H.; Pan, C.Q. Efficacy and safety of sofosbuvir-based regimens in hepatitis C patients with decompensated cirrhosis: A systematic review and meta-analysis. J. Clin. Transl. Hepatol. 2023, 11, 144–155. [Google Scholar] [CrossRef]
- Brok, J.; Gluud, L.L.; Gluud, C. Ribavirin monotherapy for chronic hepatitis C infection: A Cochrane Hepato-Biliary Group systematic review and meta-analysis of randomized trials. Am. J. Gastroenterol. 2006, 101, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chen, K.Y.; Lai, M.Y.; Chan, K.A. Meta-analysis: Ribavirin-induced haemolytic anaemia in patients with chronic hepatitis C. Aliment. Pharmacol. Ther. 2002, 16, 1623–1632. [Google Scholar] [CrossRef]
- Dusheiko, G.; Main, J.; Thomas, H.; Reichard, O.; Lee, C.; Dhillon, A.; Rassam, S.; Fryden, A.; Reesink, H.; Bassendine, M.; et al. Ribavirin treatment for patients with chronic hepatitis C: Results of a placebo-controlled study. J. Hepatol. 1996, 25, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.; Lawitz, E.; Gane, E.; Conway, B.; Ruane, P.; Abergel, A.; Mcnabb, B.; Osinusi, A.; Chen, F.; Dvory-Sobol, H.; et al. Long-term follow-up of patients with chronic HCV infection and compensated or decompensated cirrhosis following treatment with sofosbuvir-based regimens. J. Hepatol. 2018, 68, S67. [Google Scholar] [CrossRef]
- Verna, E.C.; Morelli, G.; Terrault, N.A.; Lok, A.S.; Lim, J.K.; Di Bisceglie, A.M.; Zeuzem, S.; Landis, C.S.; Kwo, P.; Hassan, M.; et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J. Hepatol. 2020, 7, 3540–3548. [Google Scholar] [CrossRef]
- Carrion, A.F.; Khaderi, S.A.; Sussman, N.L. Model for end-stage liver disease limbo, model for end-stage liver disease purgatory, and the dilemma of treating hepatitis C in patients awaiting liver transplantation. Liver Transplant. 2016, 22, 279–280. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Treat chronic hepatitis C virus infection in decompensated cirrhosis—Pre- or post-liver transplantation? The ironic conundrum in the era of effective and well-tolerated therapy. J. Viral Hepat. 2016, 23, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Belli, L.S.; Berenguer, M.; Cortesi, P.A.; Strazzabosco, M.; Rockenschaub, S.-R.; Martini, S.; Morelli, C.; Donato, F.; Volpes, R.; Pageaux, G.-P.; et al. on behalf of the European Liver and Intestine Association (ELITA). Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J. Hepatol. 2016, 65, 524–531. [Google Scholar] [CrossRef]
- Chhatwal, J.; Samur, S.; Kues, B.; Ayer, T.; Roberts, M.S.; Kanwal, F.; Hur, C.; Donnell, D.M.S.; Chung, R.T. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology 2017, 65, 777–788. [Google Scholar] [CrossRef]
- Terrault, N.A.; McCaughan, G.W.; Curry, M.P.; Gane, E.; Fagiuoli, S.; Fung, J.Y.Y.; Agarwal, K.; Lilly, L.; Strasser, S.I.; Brown, K.A.; et al. International Liver Transplantation Society consensus statement on hepatitis C management in liver transplant candidates. Transplantation 2017, 101, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Andraus, W. Barriers and limitations to access to liver transplantation in Latin America. Clin. Liver Dis. 2019, 13, 36–38. [Google Scholar] [CrossRef]
- Soyama, A.; Eguchi, S.; Egawa, H. Liver transplantation in Japan. Liver Transplant. 2016, 22, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- CalvCalvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 2018, 155, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Piñero, F.; Mendizabal, M.; Ridruejo, E.; Wolff, F.H.; Ameigeiras, B.; Anders, M.; Schinoni, M.I.; Reggiardo, V.; Palazzo, A.; Videla, M.; et al. Treatment with direct-acting antivirals for HCV decreases but does not eliminate the risk of hepatocellular carcinoma. Liver Int. 2019, 39, 1033–1043. [Google Scholar] [CrossRef]
- Krassenburg, L.A.; Maan, R.; Ramji, A.; Manns, M.P.; Cornberg, M.; Wedemeyer, H.; de Knegt, R.J.; Hansen, B.E.; Janssen, H.L.; de Man, R.A.; et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J. Hepatol. 2021, 74, 1053–1063. [Google Scholar] [CrossRef]
- Van Der Plas, S.M.; Hansen, B.E.; De Boer, J.B.; Stijnen, T.; Passchier, J.; De Man, R.A.; Schalm, S.W. Generic and disease-specific health related quality of life in non-cirrhotic, cirrhotic and transplanted liver patients: A cross-sectional study. BMC Gastroenterol. 2003, 3, 33. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Thacker, L.R.; Wade, J.B.; Sanyal, A.J.; Heuman, D.M.; Sterling, R.K.; Gibson, D.P.; Stravitz, R.T.; Puri, P.; Fuchs, M.; et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment. Pharmacol. Ther. 2011, 34, 1123–1132. [Google Scholar] [CrossRef] [PubMed]



| SOF/VEL + RBV 12 Weeks | ||
|---|---|---|
| ITT Analysis n = 32 | PP Analysis n = 25 | |
| Mean age, years (range) | 55 (39–77) | 54 (39–67) |
| Male, n (%) | 26 (81.3) | 19 (76.0) |
| Race, n (%) | ||
| White | 18 (56.3) | 14 (56.0) |
| Black | 6 (18.8) | 4 (16.0) |
| Other/not stated | 8 (25.0) | 7 (28.0) |
| Country of enrollment, n (%) | ||
| USA | 26 (81.3) | 19 (76.0) |
| France | 6 (18.8) | 6 (24.0) |
| HCV GT, n (%) | ||
| 1a 1b | 16 (50.0) 2 (6.3) | 11 (44.0) 2 (8.0) |
| 2 | 5 (15.6) | 4 (16.0) |
| 3 | 7 (21.9) | 6 (24.0) |
| Indeterminate * | 2 (6.3) | 2 (8.0) |
| CTP class, n (%) | ||
| B (7–9) | 9 (28.1) | 9 (36.0) |
| 7 | 0 (0.0) | 0 (0.0) |
| 8 9 | 2 (6.3) 7 (21.9) | 2 (8.0) 7 (28.0) |
| C (10–15) | 23 (71.9) | 16 (64.0) |
| 10 | 11 (34.4) | 9 (36.0) |
| 11 | 6 (18.8) | 3 (12.0) |
| 12 | 4 (12.5) | 3 (12.0) |
| 13 | 2 (6.3) | 1 (4.0) |
| 14 | 0 (0.0) | 0 (0.0) |
| 15 | 0 (0.0) | 0 (0.0) |
| MELD score, n (%) | ||
| 10–15 | 13 (40.6) | 10 (40.0) |
| 16–20 | 17 (53.1) | 14 (56.0) |
| 21–25 | 2 (6.3) | 1 (4.0) |
| Ascites, n (%) | ||
| None | 3 (9.4) | 3 (12.0) |
| Mild/moderate | 20 (62.5) | 17 (68.0) |
| Severe | 9 (28.1) | 5 (20.0) |
| Encephalopathy, n (%) | ||
| None | 5 (15.6) | 5 (20.0) |
| Medication-controlled | 27 (84.4) | 20 (80.0) |
| HCV RNA, log10 IU/mL mean (SD) | 5.2 (1.2) | 5.1 (1.2) |
| HCV RNA ≥ 800,000 IU/mL, n (%) | 9 (28.1) | 7 (28.0) |
| HCV treatment experienced, n (%) † | 4 (12.5) | 4 (16.0) |
| eGFRCG, mL/min, mean (SD) | 113.3 (41.2) | 111.0 (41.8) |
| Platelets, ×103/µL, mean (SD) | 89.1 (30.8) | 92.6 (30.8) |
| Albumin, g/dL, mean (SD) | 2.7 (0.5) | 2.8 (0.5) |
| INR, mean (SD) | 1.5 (0.3) | 1.5 (0.3) |
| Hemoglobin, g/dL, mean (SD) | 12.0 (1.3) | 12.0 (1.3) |
| Lymphocytes, ×103/µL (SD) | 1.6 (0.9) | 1.7 (0.9) |
| Bilirubin, mg/dL, mean (SD) | 3.4 (1.5) | 3.4 (1.5) |
| Event | SOF/VEL + RBV 12 Weeks n = 32 |
|---|---|
| Any AE, n (%) | 31 (96.9) |
| AEs seen in ≥5% of patients, n (%) | |
| Anemia * | 8 (25.0) |
| Nausea | 8 (25.0) |
| Asthenia | 6 (18.8) |
| Vomiting | 5 (15.6) |
| Grade 3–4 AEs, n (%) | 11 (34.4) † |
| Serious AEs, n (%) | 17 (53.1) † |
| SAEs seen in ≥5% of patients | |
| Hepatic hydrothorax | 2 (6.3) |
| Hepatocellular carcinoma | 3 (9.4) |
| Sepsis | 2 (6.3) |
| AE leading to discontinuation of all study drugs, n (%) | 2 (6.3) †‡ |
| Deaths, n (%) | 8 (25.0) † |
| Laboratory abnormalities seen in >1 patient, n (%) | |
| Grade 3 | 11 (34.4) |
| Low lymphocytes (350–<500/mm3) | 4 (12.5) |
| Low platelets (25,000–<50,000/mm3) | 4 (12.5) |
| Hyperbilirubinemia (>2.5–5.0 × ULN) | 6 (18.8) |
| Hyperuricemia (>12.0–15.0 mg/dL) | 5 (15.6) |
| Grade 4 | 7 (21.9) |
| Hyperbilirubinemia (>5.0 × ULN) | 5 (15.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flamm, S.; Lawitz, E.; Borg, B.; Charlton, M.; Landis, C.; Reddy, K.R.; Shiffman, M.; Alsina, A.; Chang, C.; Ravendhran, N.; et al. Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis. Viruses 2023, 15, 2026. https://doi.org/10.3390/v15102026
Flamm S, Lawitz E, Borg B, Charlton M, Landis C, Reddy KR, Shiffman M, Alsina A, Chang C, Ravendhran N, et al. Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis. Viruses. 2023; 15(10):2026. https://doi.org/10.3390/v15102026
Chicago/Turabian StyleFlamm, Steven, Eric Lawitz, Brian Borg, Michael Charlton, Charles Landis, K. Rajender Reddy, Mitchell Shiffman, Angel Alsina, Charissa Chang, Natarajan Ravendhran, and et al. 2023. "Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis" Viruses 15, no. 10: 2026. https://doi.org/10.3390/v15102026
APA StyleFlamm, S., Lawitz, E., Borg, B., Charlton, M., Landis, C., Reddy, K. R., Shiffman, M., Alsina, A., Chang, C., Ravendhran, N., Hernandez, C., Hézode, C., Scherbakovsky, S., Mercier, R.-C., & Samuel, D. (2023). Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis. Viruses, 15(10), 2026. https://doi.org/10.3390/v15102026







