A Temperate Sinorhizobium Phage, AP-16-3, Closely Related to Phage 16-3: Mosaic Genome and Prophage Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial and Phage Strains and Growth Conditions
2.2. Bacteriophage Isolation
2.3. DNA Isolation, Sequencing, and Annotation of the Bacteriophage Genome
2.4. The Search of Phage-like Sequences
2.5. Analysis of Homology, Codon Usage, tRNA, and Pairwise Comparison Analysis of Nucleotide and Amino Acid Sequences
2.6. Phylogenetic Analysis
2.7. Nucleotide Accessions
3. Results
3.1. Biological and Molecular Genetic Characteristics of Phage AP-16-3
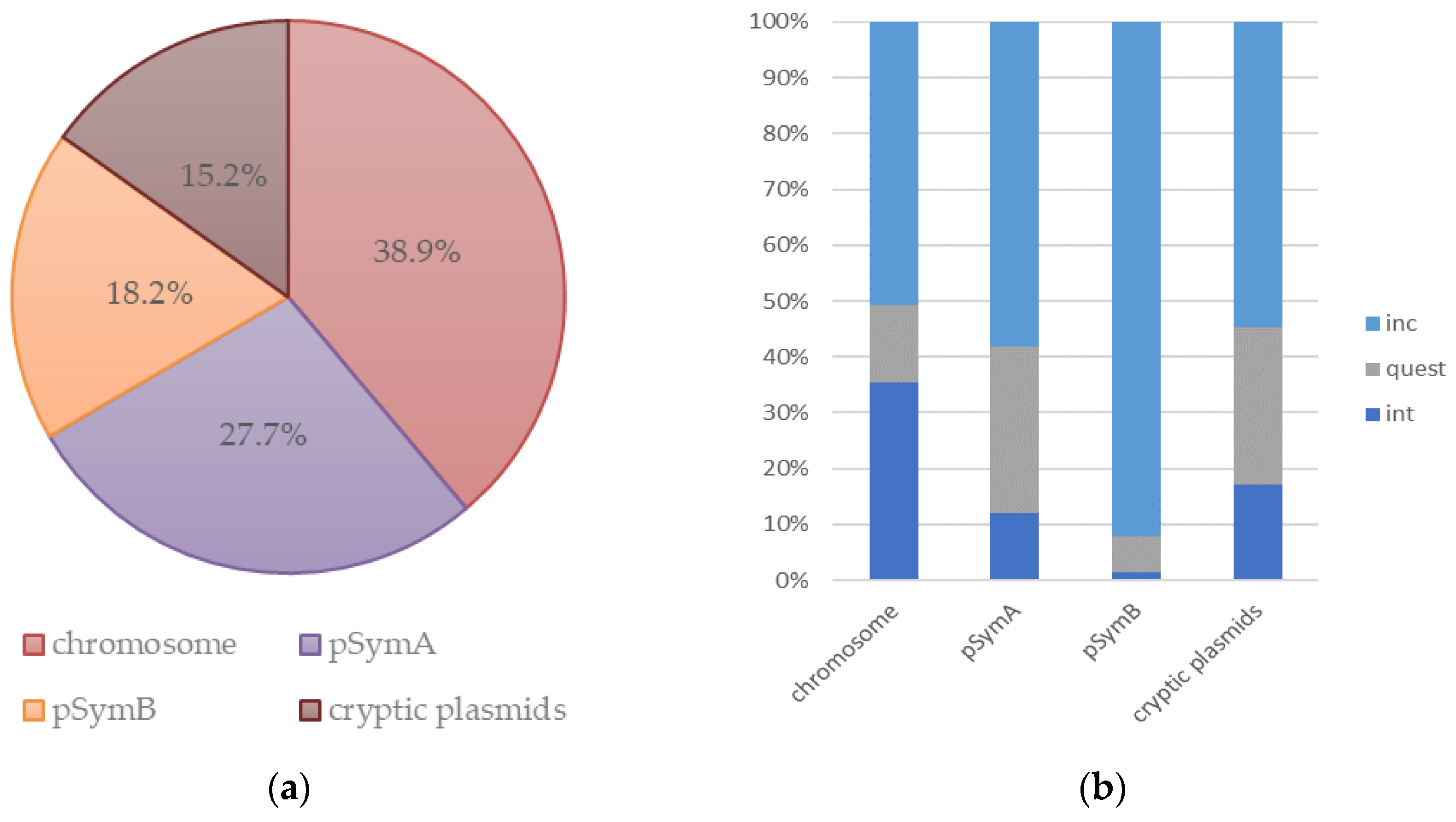
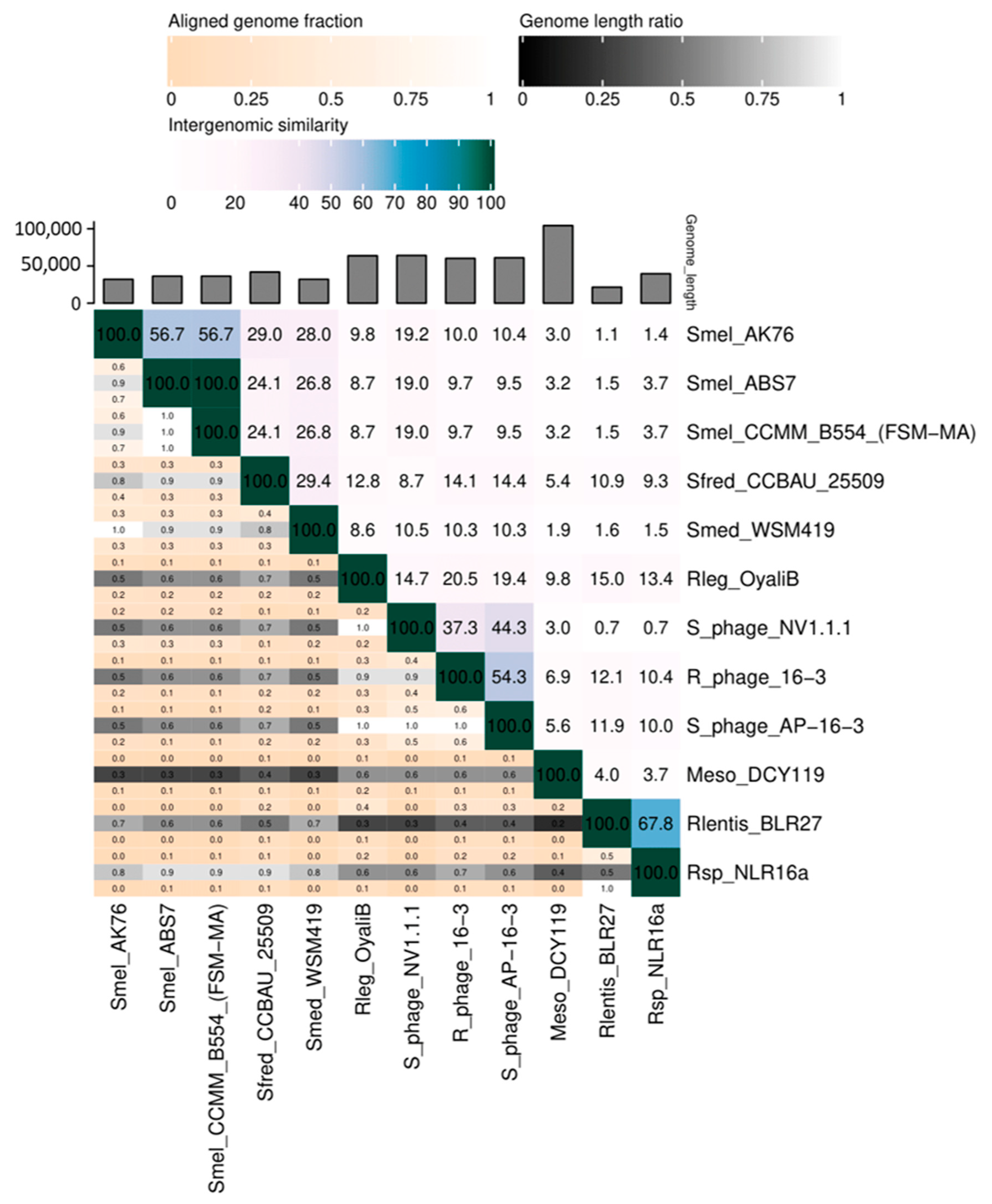
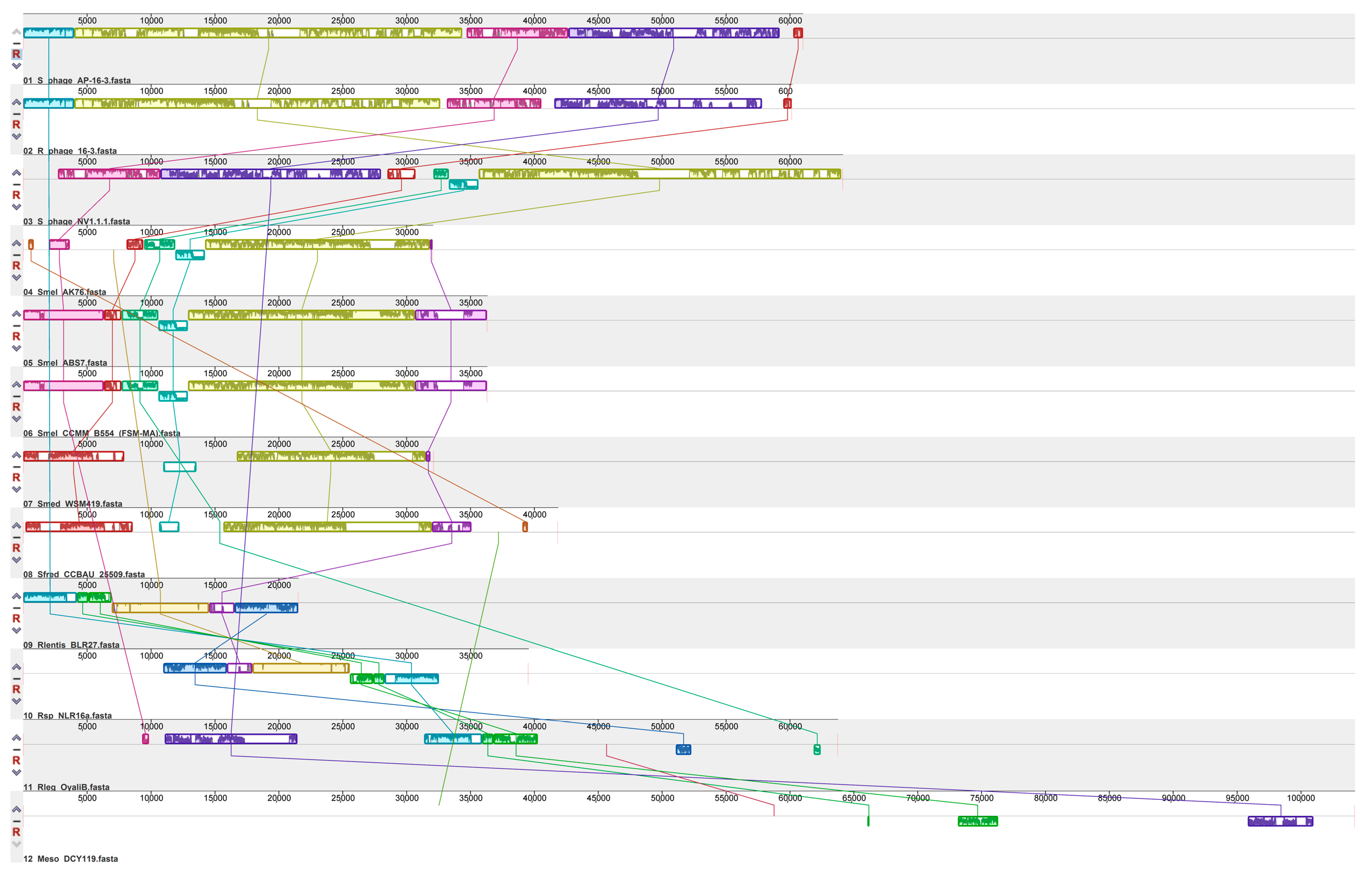
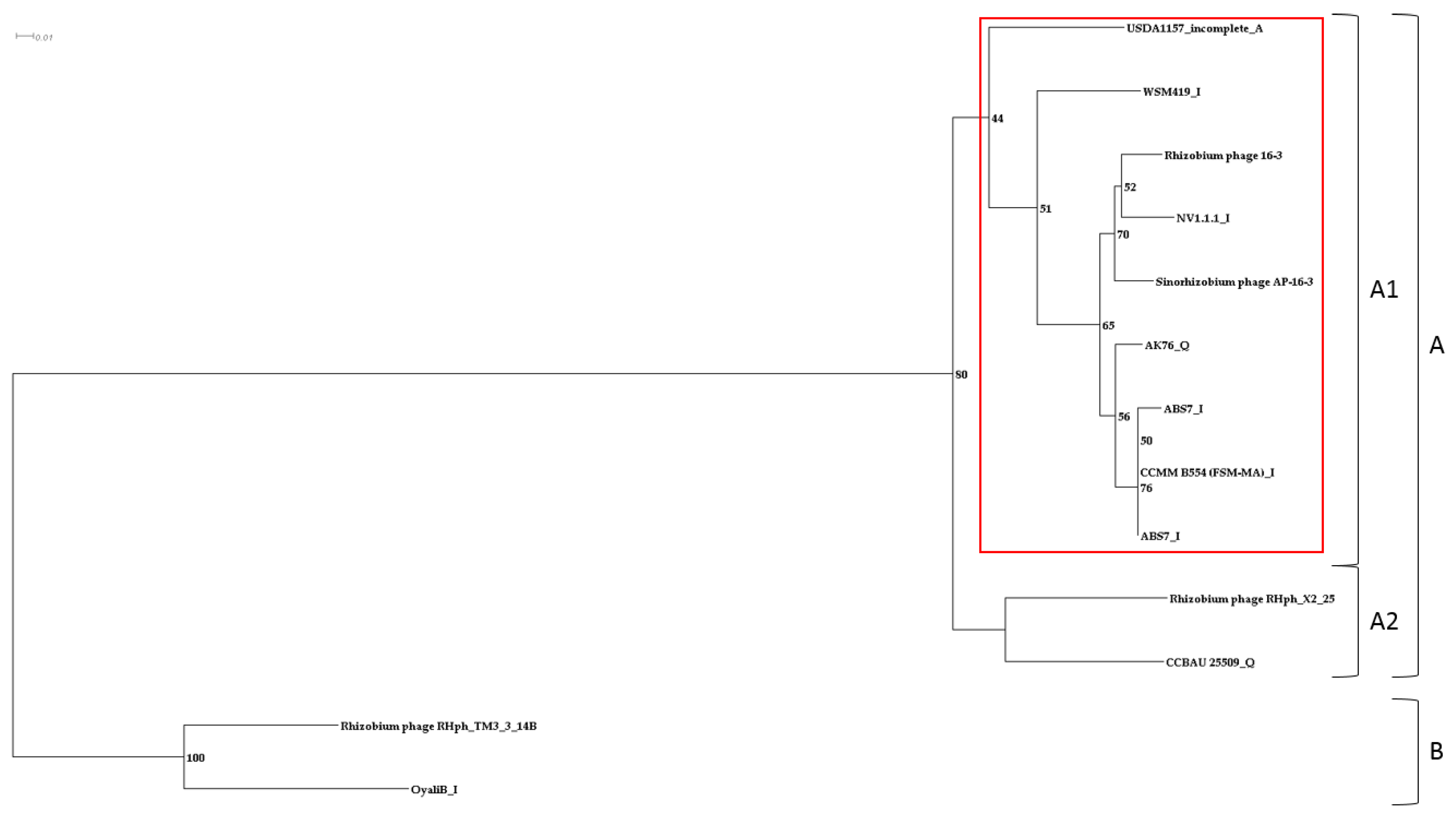
3.2. Prophages in Rhizobia Genomes
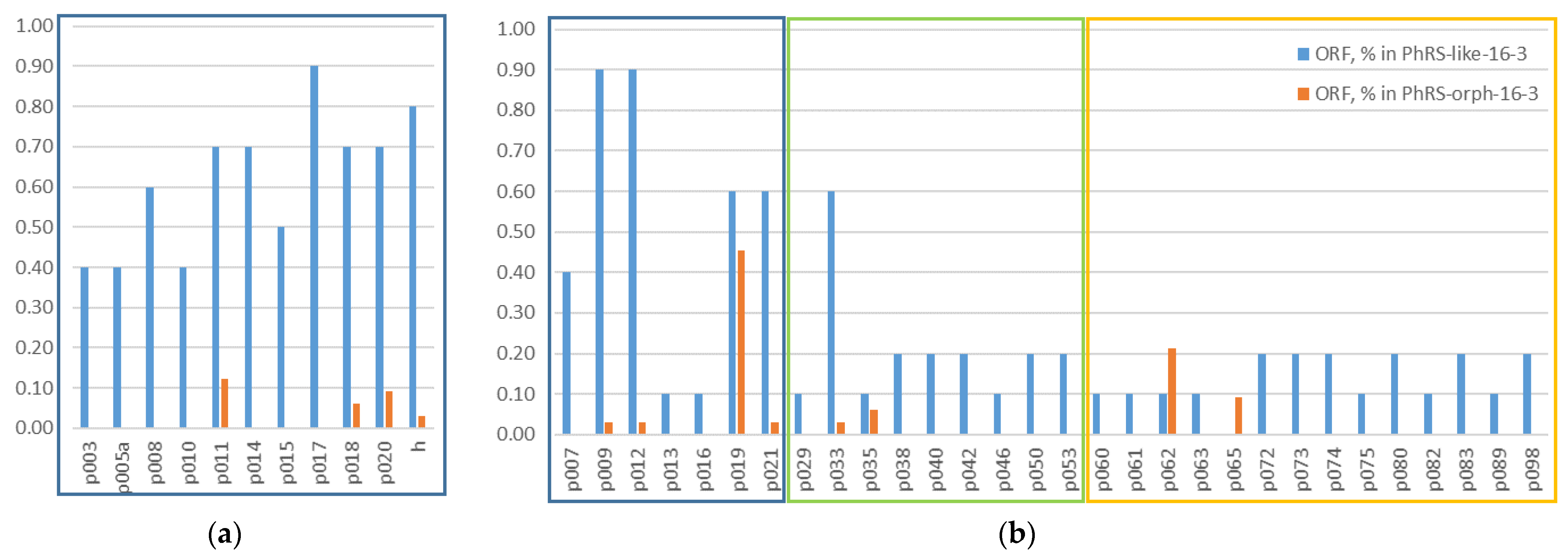
Prophages Containing ORFs Homologous to AP-16-3 and 16-3 Phages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graham, E.B.; Paez-Espino, D.; Brislawn, C.; Hofmockel, K.S.; Wu, R.; Kyrpides, N.C.; Jansson, J.K.; McDermott, J.E. Untapped Viral Diversity in Global Soil Metagenomes. bioRxiv 2019, 583997. [Google Scholar] [CrossRef]
- Semsey, S.; Papp, I.; Buzas, Z.; Patthy, A.; Orosz, L.; Papp, P.P. Identification of Site-Specific Recombination Genes int and xis of the Rhizobium Temperate Phage 16-3. J. Bacteriol. 1999, 181, 4185–4192. [Google Scholar] [CrossRef][Green Version]
- Csiszovszki, Z.; Buzás, Z.; Semsey, S.; Ponyi, T.; Papp, P.P.; Orosz, L. immX Immunity Region of Rhizobium Phage 16-3: Two Overlapping Cistrons of Repressor Function. J. Bacteriol. 2003, 185, 4382–4392. [Google Scholar] [CrossRef] [PubMed]
- Ferenczi, S.; Orosz, L.; Papp, P.P. Repressor of Phage 16-3 with Altered Binding Specificity Indicates Spatial Differences in Repressor-Operator Complexes. J. Bacteriol. 2006, 188, 1663–1666. [Google Scholar] [CrossRef]
- Halmillawewa, A. Isolation, Characterization, and Applications of Rhizobiophages. Ph.D. Thesis, University of Calgary, 2014. Available online: https://prism.ucalgary.ca/items/5b708b71-7841-4fc1-a7ff-2cc84ccabcdd (accessed on 1 July 2023). [CrossRef]
- Keen, E.C.; Bliskovsky, V.V.; Malagon, F.; Baker, J.D.; Prince, J.S.; Klaus, J.S.; Adhya, S.L. Novel “Superspreader” Bacteriophages Promote Horizontal Gene Transfer by Transformation. mBio 2017, 8, e02115-16. [Google Scholar] [CrossRef] [PubMed]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages Contribute to the Spread of Antibiotic Resistance Genes among Foodborne Pathogens of the Enterobacteriaceae Family—A Review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef] [PubMed]
- Subirats, J.; Sànchez-Melsió, A.; Borrego, C.M.; Balcázar, J.L.; Simonet, P. Metagenomic Analysis Reveals That Bacteriophages Are Reservoirs of Antibiotic Resistance Genes. Int. J. Antimicrob. Agents 2016, 48, 163–167. [Google Scholar] [CrossRef]
- Carey, J.N.; Mettert, E.L.; Fishman-Engel, D.R.; Roggiani, M.; Kiley, P.J.; Goulian, M. Phage Integration Alters the Respiratory Strategy of Its Host. eLife 2019, 8, e49081. [Google Scholar] [CrossRef]
- Forcone, K.; Coutinho, F.H.; Cavalcanti, G.S.; Silveira, C.B. Prophage Genomics and Ecology in the Family Rhodobacteraceae. Microorganisms 2021, 9, 1115. [Google Scholar] [CrossRef]
- Oliveira, P.H.; Touchon, M.; Rocha, E.P.C. The Interplay of Restriction-Modification Systems with Mobile Genetic Elements and Their Prokaryotic Hosts. Nucleic Acids Res. 2014, 42, 10618–10631. [Google Scholar] [CrossRef]
- Al-Shayeb, B.; Skopintsev, P.; Soczek, K.M.; Stahl, E.C.; Li, Z.; Groover, E.; Smock, D.; Eggers, A.R.; Pausch, P.; Cress, B.F.; et al. Diverse Virus-Encoded CRISPR-Cas Systems Include Streamlined Genome Editors. Cell 2022, 185, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Grose, J.H.; Casjens, S.R. Understanding the Enormous Diversity of Bacteriophages: The Tailed Phages That Infect the Bacterial Family Enterobacteriaceae. Virology 2014, 468–470, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Touchon, M.; Moura De Sousa, J.A.; Rocha, E.P. Embracing the Enemy: The Diversification of Microbial Gene Repertoires by Phage-Mediated Horizontal Gene Transfer. Curr. Opin. Microbiol. 2017, 38, 66–73. [Google Scholar] [CrossRef]
- Nanda, A.M.; Thormann, K.; Frunzke, J. Impact of Spontaneous Prophage Induction on the Fitness of Bacterial Populations and Host-Microbe Interactions. J. Bacteriol. 2015, 197, 410–419. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Jacobs-Sera, D.; Bustamante, C.A.G.; Garlena, R.A.; Mavrich, T.N.; Pope, W.H.; Reyes, J.C.C.; Russell, D.A.; Adair, T.; Alvey, R.; et al. Prophage-Mediated Defence against Viral Attack and Viral Counter-Defence. Nat. Microbiol. 2017, 2, 16251. [Google Scholar] [CrossRef]
- Chevallereau, A.; Pons, B.J.; Van Houte, S.; Westra, E.R. Interactions between Bacterial and Phage Communities in Natural Environments. Nat. Rev. Microbiol. 2022, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Asadulghani, M.; Ogura, Y.; Ooka, T.; Itoh, T.; Sawaguchi, A.; Iguchi, A.; Nakayama, K.; Hayashi, T. The Defective Prophage Pool of Escherichia coli O157: Prophage–Prophage Interactions Potentiate Horizontal Transfer of Virulence Determinants. PLoS Pathog. 2009, 5, e1000408. [Google Scholar] [CrossRef]
- Ooka, T.; Ogura, Y.; Asadulghani, M.; Ohnishi, M.; Nakayama, K.; Terajima, J.; Watanabe, H.; Hayashi, T. Inference of the Impact of Insertion Sequence (IS) Elements on Bacterial Genome Diversification through Analysis of Small-Size Structural Polymorphisms in Escherichia coli O157 Genomes. Genome Res. 2009, 19, 1809–1816. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Jaiswal, S.K.; Dakora, F.D. Identification and Characterization of Phages Parasitic on Bradyrhizobia Nodulating Groundnut (Arachis hypogaea L.) in South Africa. Appl. Soil Ecol. 2016, 108, 334–340. [Google Scholar] [CrossRef]
- Cubo, M.T.; Alías-Villegas, C.; Balsanelli, E.; Mesa, D.; De Souza, E.; Espuny, M.R. Diversity of Sinorhizobium (Ensifer) meliloti Bacteriophages in the Rhizosphere of Medicago marina: Myoviruses, Filamentous and N4-Like Podovirus. Front. Microbiol. 2020, 11, 22. [Google Scholar] [CrossRef]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; Van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of Morphology-Based Taxa and Change to Binomial Species Names: 2022 Taxonomy Update of the ICTV Bacterial Viruses Subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W. 5500 Phages Examined in the Electron Microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage Diversity, Genomics and Phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Johnson, M.C.; Sena-Velez, M.; Washburn, B.K.; Platt, G.N.; Lu, S.; Brewer, T.E.; Lynn, J.S.; Stroupe, M.E.; Jones, K.M. Structure, Proteome and Genome of Sinorhizobium meliloti Phage ΦM5: A Virus with LUZ24-like Morphology and a Highly Mosaic Genome. J. Struct. Biol. 2017, 200, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.; Moeskjær, S.; Young, P.; Santamaría, R.I.; Harrison, E. Introducing a Novel, Broad Host Range Temperate Phage Family Infecting Rhizobium leguminosarum and Beyond. Front. Microbiol. 2021, 12, 765271. [Google Scholar] [CrossRef] [PubMed]
- Finan, T.M.; Hartweig, E.; LeMieux, K.; Bergman, K.; Walker, G.C.; Signer, E.R. General Transduction in Rhizobium meliloti. J. Bacteriol. 1984, 159, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Fleagle, B.; Imamovic, A.; Toledo, S.; Couves, M.; Jensen, A.; Vang, M.; Steevens, A.; Young, N.D.; Sadowsky, M.J.; Martinez-Vaz, B.M. Complete Genome Sequence of Sinorhizobium meliloti Bacteriophage HMSP1-Susan. Genome Announc. 2018, 6, e01450-17. [Google Scholar] [CrossRef]
- Dziewit, L.; Oscik, K.; Bartosik, D.; Radlinska, M. Molecular Characterization of a Novel Temperate Sinorhizobium Bacteriophage, ФLM21, Encoding DNA Methyltransferase with CcrM-Like Specificity. J. Virol. 2014, 88, 13111–13124. [Google Scholar] [CrossRef]
- Ordogh, F.; Szende, K. Temperate Bacteriophages Isolated from Rhizobium meliloti. Acta Microbiol. Acad. Sci. Hung. 1961, 7, 65–71. [Google Scholar]
- Dudás, B.; Orosz, L. Correlation between map position and phenotype of cti mutants in the c cistron of Rhizobium meliloti phage 16-3. Genetics 1980, 96, 321–329. [Google Scholar] [CrossRef]
- Orosz, L. Methods for analysis of the c cistron of temperate phage 16–3 of Rhizobium meliloti. Genetics 1980, 94, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, G.; Papp, P.; Orosz, L. Related Repressor Specificity of Unrelated Phages. Nature 1987, 330, 398–401. [Google Scholar] [CrossRef]
- Putnoky, P.; Deák, V.; Békási, K.; Pálvölgyi, A.; Maász, A.; Palágyi, Z.; Hoffmann, G.; Kerepesi, I. H Protein of Bacteriophage 16-3 and RkpM Protein of Sinorhizobium meliloti 41 Are Involved in Phage Adsorption. J. Bacteriol. 2004, 186, 1591–1597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deák, V.; Lukács, R.; Buzás, Z.; Pálvölgyi, A.; Papp, P.P.; Orosz, L.; Putnoky, P. Identification of Tail Genes in the Temperate Phage 16-3 of Sinorhizobium meliloti 41. J. Bacteriol. 2010, 192, 1617–1623. [Google Scholar] [CrossRef]
- Orosz, L. Genetic Studies on Rhizobiophage 16-3. I. Genes and Functions on the Chromosome. Mol. Gen. Genet. 1973, 125, 341–350. [Google Scholar] [CrossRef]
- Dallmann, G.; Orosz, L.; Sain, B. Restriction Mapping of DNA of Temperate Rhizobium meliloti Phage 16-3: Comparison of Genetic and Physical Maps Indicates a Long, Genetically Silent Chromosomal Arm. Mol. Gen. Genet. MGG 1979, 176, 439–448. [Google Scholar] [CrossRef]
- Dorgai, L.; Olasz, F.; Berényi, M.; Dallmann, G.; Páy, A.; Orosz, L. Orientation of the Genetic and Physical Map of Rhizobium meliloti Temperate Phage 16-3. Mol. Gen. Genet. MGG 1981, 182, 321–325. [Google Scholar] [CrossRef]
- Skvortsov, T.; De Leeuwe, C.; Quinn, J.P.; McGrath, J.W.; Allen, C.C.R.; McElarney, Y.; Watson, C.; Arkhipova, K.; Lavigne, R.; Kulakov, L.A. Metagenomic Characterisation of the Viral Community of Lough Neagh, the Largest Freshwater Lake in Ireland. PLoS ONE 2016, 11, e0150361. [Google Scholar] [CrossRef]
- Costeira, R.; Doherty, R.; Allen, C.C.R.; Larkin, M.J.; Kulakov, L.A. Analysis of Viral and Bacterial Communities in Groundwater Associated with Contaminated Land. Sci. Total Environ. 2019, 656, 1413–1426. [Google Scholar] [CrossRef]
- Kowalski, M. Prophage Substitution in Rhizobium meliloti Strains. Microb. Genet. Bull. 1965, 22, 19. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; ISBN 978-0-87969-309-1. [Google Scholar]
- Roumiantseva, M.L.; Vladimirova, M.E.; Saksaganskaia, A.S.; Muntyan, V.S.; Kozlova, A.P.; Afonin, A.M.; Baturina, O.A.; Simarov, B.V. Ensifer meliloti L6-AK89, an Effective Inoculant of Medicago lupulina Varieties: Phenotypic and Deep-Genome Screening. Agronomy 2022, 12, 766. [Google Scholar] [CrossRef]
- Beck, N.K.; Callahan, K.; Nappier, S.P.; Kim, H.; Sobsey, M.D.; Meschke, J.S. Development of a spot-titer culture assay for quantifying bacteria and viral indicators. J. Rapid Methods Autom. Microbiol. 2009, 17, 455–464. [Google Scholar] [CrossRef]
- Mangieri, N.; Picozzi, C.; Cocuzzi, R.; Foschino, R. Evaluation of a Potential Bacteriophage Cocktail for the Control of Shiga-Toxin Producing Escherichia coli in Food. Front. Microbiol. 2020, 11, 1801. [Google Scholar] [CrossRef] [PubMed]
- Twest, R.; Kropinski, A.M. Bacteriophage Enrichment from Water and Soil. In Bacteriophages; Clokie, M.R.J., Kropinski, A.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 501, pp. 15–21. ISBN 978-1-58829-682-5. [Google Scholar]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of Bacteriophages by Double Agar Overlay Plaque Assay. In Bacteriophages; Clokie, M.R.J., Kropinski, A.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 1, pp. 69–76. ISBN 978-1-58829-682-5. [Google Scholar]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. EggNOG-Mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sun, H.-X.; Zhang, C.; Cheng, L.; Peng, Y.; Deng, Z.; Wang, D.; Wang, Y.; Hu, M.; Liu, W.; et al. Prophage Hunter: An Integrative Hunting Tool for Active Prophages. Nucleic Acids Res. 2019, 47, W74–W80. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Brinkman, F.S.L. Improved Genomic Island Predictions with IslandPath-DIMOB. Bioinformatics 2018, 34, 2161–2167. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Gene Predict. 2019, 1962, 1–14. [Google Scholar] [CrossRef]
- Stothard, P. The Sequence Manipulation Suite: JavaScript Programs for Analyzing and Formatting Protein and DNA Sequences. BioTechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC—A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A Genome Comparison Visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Talavera, G.; Castresana, J. Improvement of Phylogenies after Removing Divergent and Ambiguously Aligned Blocks from Protein Sequence Alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Scornavacca, C. Dendroscope 3: An Interactive Tool for Rooted Phylogenetic Trees and Networks. Syst. Biol. 2012, 61, 1061–1067. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Golec, P.; Dąbrowski, K.; Hejnowicz, M.S.; Gozdek, A.; Łoś, J.M.; Węgrzyn, G.; Łobocka, M.B.; Łoś, M. A Reliable Method for Storage of Tailed Phages. J. Microbiol. Methods 2011, 84, 486–489. [Google Scholar] [CrossRef]
- Dremel, S.E.; Didychuk, A.L. Better Late than Never: A Unique Strategy for Late Gene Transcription in the Beta- and Gammaherpesviruses. Semin. Cell Dev. Biol. 2023, 146, 57–69. [Google Scholar] [CrossRef]
- Pendleton, K.E.; Chen, B.; Liu, K.; Hunter, O.V.; Xie, Y.; Tu, B.P.; Conrad, N.K. The U6 SnRNA M6A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 2017, 169, 824–835.e14. [Google Scholar] [CrossRef]
- Brown, J.A.; Kinzig, C.G.; DeGregorio, S.J.; Steitz, J.A. Methyltransferase-like Protein 16 Binds the 3′-Terminal Triple Helix of MALAT1 Long Noncoding RNA. Proc. Natl. Acad. Sci. USA 2016, 113, 14013–14018. [Google Scholar] [CrossRef]
- Leiman, P.G.; Chipman, P.R.; Kostyuchenko, V.A.; Mesyanzhinov, V.V.; Rossmann, M.G. Three-Dimensional Rearrangement of Proteins in the Tail of Bacteriophage T4 on Infection of Its Host. Cell 2004, 118, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.M.I.; Prokhorov, N.S.; Guerrero-Ferreira, R.C.; Shneider, M.M.; Browning, C.; Goldie, K.N.; Stahlberg, H.; Leiman, P.G. Structure of the T4 Baseplate and Its Function in Triggering Sheath Contraction. Nature 2016, 533, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, J.; Santamaría, R.I.; Bustos, P.; Juárez, S.; Ducci, M.A.; Figueroa Fleming, T.; Etcheverry, A.V.; González, V. Spatial Patterns in Phage-Rhizobium Coevolutionary Interactions across Regions of Common Bean Domestication. ISME J. 2021, 15, 2092–2106. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, R.I.; Bustos, P.; Van Cauwenberghe, J.; González, V. Hidden Diversity of Double-Stranded DNA Phages in Symbiotic Rhizobium Species. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200468. [Google Scholar] [CrossRef] [PubMed]
- Ramisetty, B.C.M.; Sudhakari, P.A. Bacterial “Grounded” Prophages: Hotspots for Genetic Renovation and Innovation. Front. Genet. 2019, 10, 65. [Google Scholar] [CrossRef]
- Kozlova, A.; Muntyan, V.; Vladimirova, M.; Grudinin, M.; Roumiantseva, M. Comparative Genomics of the Sinorhizobial phiLM21-like Intact Prophages on Complete Chromosomes. In Proceedings of the 20th International Multidisciplinary Scientific GeoConference SGEM 2020; 2020; pp. 207–214. Available online: https://www.proquest.com/openview/21e63f3681637f8b183a91ec0558f758/1?pq-origsite=gscholar&cbl=1536338 (accessed on 1 July 2023). [CrossRef]
- Vargas-Palacios, A.G. Expanding the Genetic Resources of Lentils and Rhizobium to Increase Nitrogen Fixation (BNF) in the Lentil Crop. Ph.D. Thesis, University of Saskatchewan, 2018. Available online: https://hdl.handle.net/10388/13599 (accessed on 1 July 2023).
- Huo, Y.; Kang, J.P.; Ahn, J.C.; Kim, Y.J.; Piao, C.H.; Yang, D.U.; Yang, D.C. Siderophore-Producing Rhizobacteria Reduce Heavy Metal-Induced Oxidative Stress in Panax Ginseng Meyer. J. Ginseng Res. 2021, 45, 218–227. [Google Scholar] [CrossRef]
- Villalpando-Aguilar, J.L.; Matos-Pech, G.; López-Rosas, I.; Castelán-Sánchez, H.G.; Alatorre-Cobos, F. Phage Therapy for Crops: Concepts, Experimental and Bioinformatics Approaches to Direct Its Application. Int. J. Mol. Sci. 2022, 24, 325. [Google Scholar] [CrossRef]
- Ilyina, T.S. Filamentous Bacteriophages and Their Role in the Virulence and Evolution of Pathogenic Bacteria. Mol. Genet. Microbiol. Virol. 2015, 30, 1–9. [Google Scholar] [CrossRef]
- Crook, M.B. Modulators of Symbiotic Outcome in Sinorhizobium meliloti. Ph.D. Thesis, Brigham Young University, 2013. Available online: http://hdl.lib.byu.edu/1877/etd6113 (accessed on 1 July 2023).
- Brewer, T.E.; Washburn, B.K.; Lynn, J.S.; Jones, K.M. Complete Genome Sequence of Sinorhizobium Phage ΦM6, the First Terrestrial Phage of a Marine Phage Group. Microbiol. Resour. Announc. 2018, 7, e01143-18. [Google Scholar] [CrossRef]

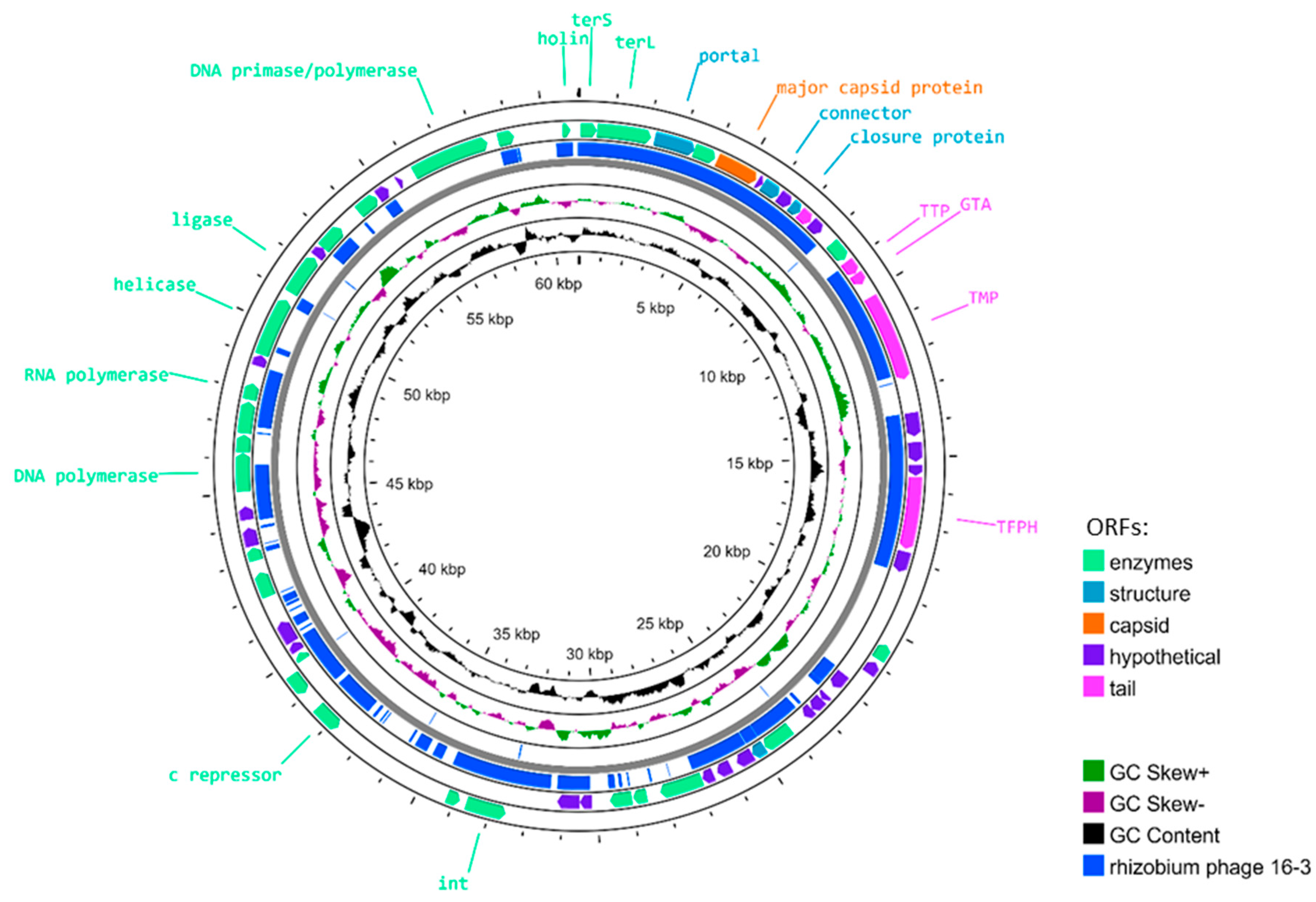


| Characteristics | Sinorhizobium Phage AP-16-3 | Rhizobium Phage 16-3 |
|---|---|---|
| Size, kbp | 61.0 | 60.2 |
| GC content, % | 59.22 | 58.95 |
| Number of ORFs | 62 | 62 * |
| Common ORFs (nucleotide/amino acid) | 42/45 | |
| Species | Strain 1 | Length, kb | GC, % | Completeness | Number of ORFs | |||
|---|---|---|---|---|---|---|---|---|
| Total | Phage Hit Proteins 2 | Identical to Rhizobium Phage 16-3 | ||||||
| Sinorhizobium sp. | S. meliloti | NV1.1.1 | 64.2 | 59.40 | Int 3 | 105 | 71 | 51 |
| AK76 | 32 | 58.58 | Quest 4 | 35 | 21 | 13 | ||
| CCMM B554 (FSM-MA) | 36.3 | 57.99 | int | 36 | 22 | 12 | ||
| ABS7 | 36.3 | 57.96 | int | 40 | 23 | 12 | ||
| S. medicae | WSM419 | 32.1 | 59.12 | int | 44 | 29 | 14 | |
| S. fredii | CCBAU 25509 | 41.8 | 58.14 | quest | 36 | 21 | 16 | |
| Rh. lentis | BLR27 | 21.5 | 60.40 | quest | 28 | 20 | 12 | |
| Rh. spp. | NLR16a | 39.5 | 58.76 | int | 42 | 24 | 12 | |
| Rh. leguminosarum | OyaliB | 63.7 | 58.69 | int | 100 | 63 | 41 | |
| Mesorhizobium spp. | DCY119 | 104.2 | 59.90 | int | 116 | 78 | 14 | |
| ORF | Product | Prophages in Genomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. meliloti | S. medicae WSM419 | S. fredii CCBAU 25509 | Rh lentis BLR27 | Rh sp. NLR16a | Rh leguminosarum OyaliB | Mesorhizo bium sp. DCY119 | |||||
| NV1.1.1 | AK76 | CCMM B554 (FSM-MA) | ABS7 | ||||||||
| p001 (terL) | terminase large subunit | - | - | - | - | - | - | + | + | + | - |
| p002 (terS) | terminase small subunit | - | - | - | - | - | - | + | + | + | - |
| p003 | portal protein | - | - | - | - | - | + | + | + | + | - |
| p004 | protease | - | - | - | - | - | + | + | + | + | - |
| p005a | major capsid protein | - | - | - | - | - | + | + | + | + | - |
| p007 | hp * | - | - | - | - | - | + | + | + | - | + |
| p008 | head–tail connector protein | - | - | - | - | + | + | + | + | + | + |
| p009 | hp * | + | + | + | + | + | + | + | + | + | - |
| p010 | head closure protein | + | - | + | - | - | + | - | - | + | - |
| p011 | tail protein | + | + | + | + | + | + | - | - | + | - |
| p012 | hp * | + | + | + | + | + | + | + | + | + | - |
| p013 | hp * | + | - | - | - | - | - | - | - | - | - |
| p014 | tail tube protein | + | + | + | + | + | + | - | - | + | - |
| p015 | gene transfer agent family protein | + | + | - | - | + | + | - | - | + | - |
| p016 | hp * | + | - | - | - | - | - | - | - | - | - |
| p017 | tail length tape measure family protein | + | + | + | + | + | + | + | + | + | - |
| p018 | tail protein | + | + | + | + | + | + | - | - | + | - |
| p019 | hp * | + | + | + | + | + | + | - | - | - | - |
| p020 | tail protein | + | + | + | + | + | + | - | - | + | - |
| p021 | hp * | + | + | + | + | + | - | - | - | + | - |
| h | tail fiber protein H ** | + | + | + | + | + | + | - | - | + | - |
| p023 | kinase | + | + | + | + | + | - | - | - | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlova, A.P.; Saksaganskaia, A.S.; Afonin, A.M.; Muntyan, V.S.; Vladimirova, M.E.; Dzyubenko, E.A.; Roumiantseva, M.L. A Temperate Sinorhizobium Phage, AP-16-3, Closely Related to Phage 16-3: Mosaic Genome and Prophage Analysis. Viruses 2023, 15, 1701. https://doi.org/10.3390/v15081701
Kozlova AP, Saksaganskaia AS, Afonin AM, Muntyan VS, Vladimirova ME, Dzyubenko EA, Roumiantseva ML. A Temperate Sinorhizobium Phage, AP-16-3, Closely Related to Phage 16-3: Mosaic Genome and Prophage Analysis. Viruses. 2023; 15(8):1701. https://doi.org/10.3390/v15081701
Chicago/Turabian StyleKozlova, Alexandra P., Alla S. Saksaganskaia, Alexey M. Afonin, Victoria S. Muntyan, Maria E. Vladimirova, Elena A. Dzyubenko, and Marina L. Roumiantseva. 2023. "A Temperate Sinorhizobium Phage, AP-16-3, Closely Related to Phage 16-3: Mosaic Genome and Prophage Analysis" Viruses 15, no. 8: 1701. https://doi.org/10.3390/v15081701
APA StyleKozlova, A. P., Saksaganskaia, A. S., Afonin, A. M., Muntyan, V. S., Vladimirova, M. E., Dzyubenko, E. A., & Roumiantseva, M. L. (2023). A Temperate Sinorhizobium Phage, AP-16-3, Closely Related to Phage 16-3: Mosaic Genome and Prophage Analysis. Viruses, 15(8), 1701. https://doi.org/10.3390/v15081701






