Rapid Construction of an Infectious Clone of Fowl Adenovirus Serotype 4 Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid and Cell
2.2. Virus Isolation
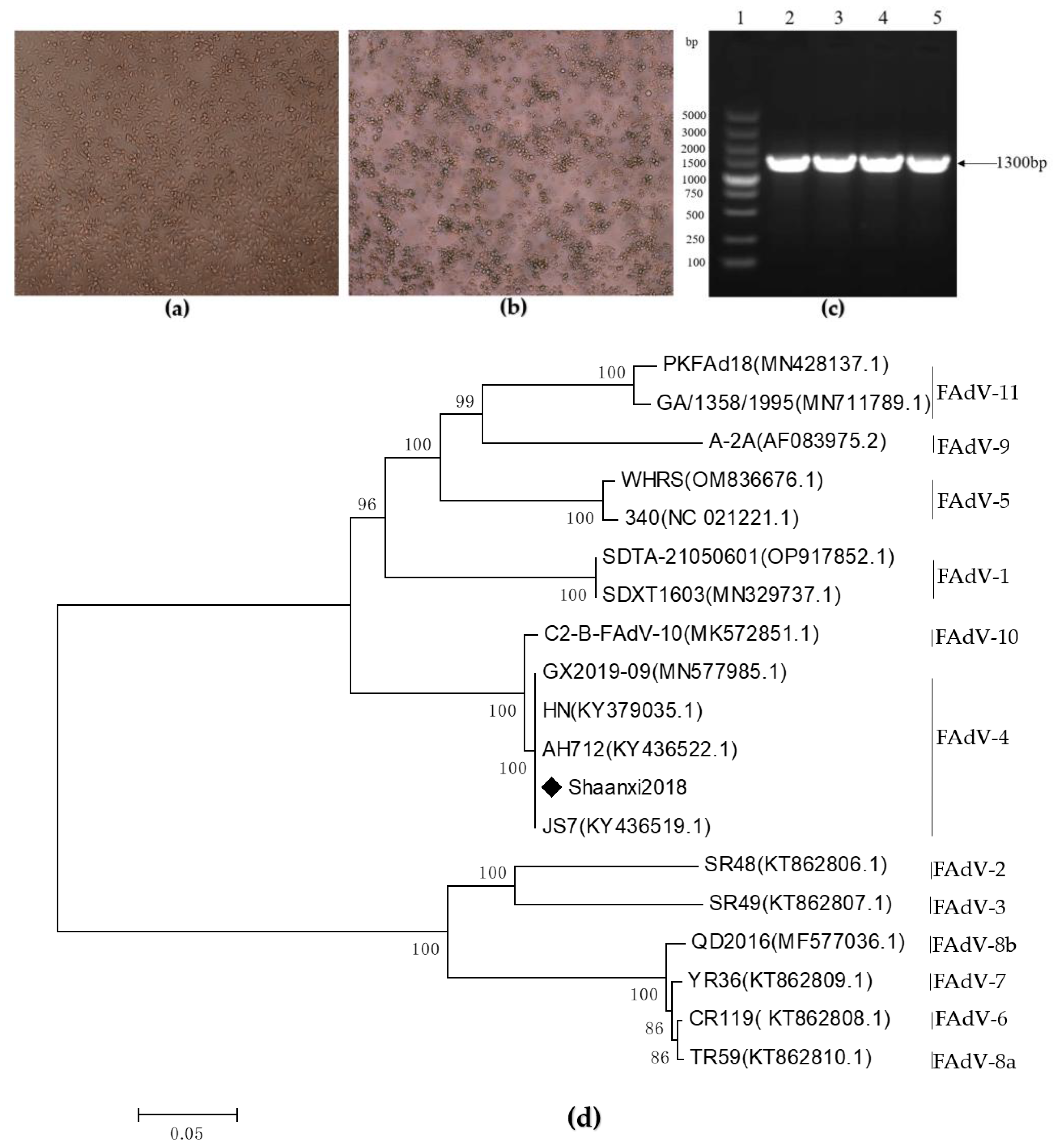
2.3. PCR Amplification and Sequencing of the Hexon Gene
2.4. Hexon Gene Sequence Analysis and Phylogenetic
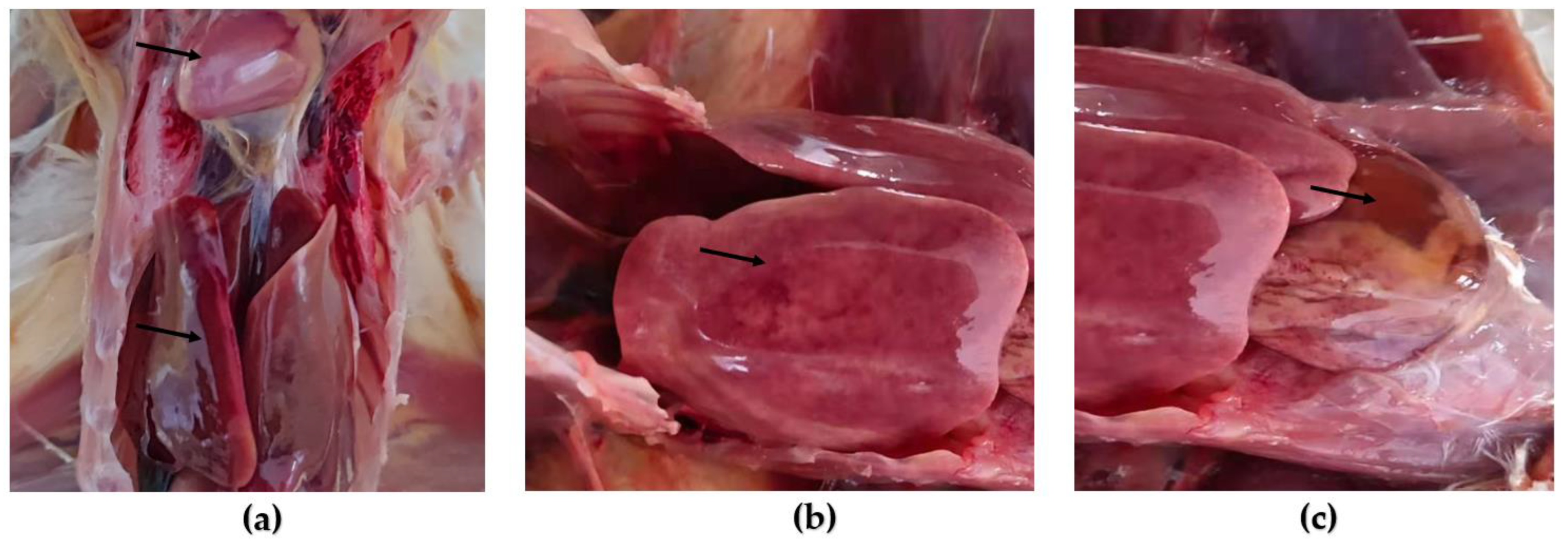
2.5. Animal Regression Test
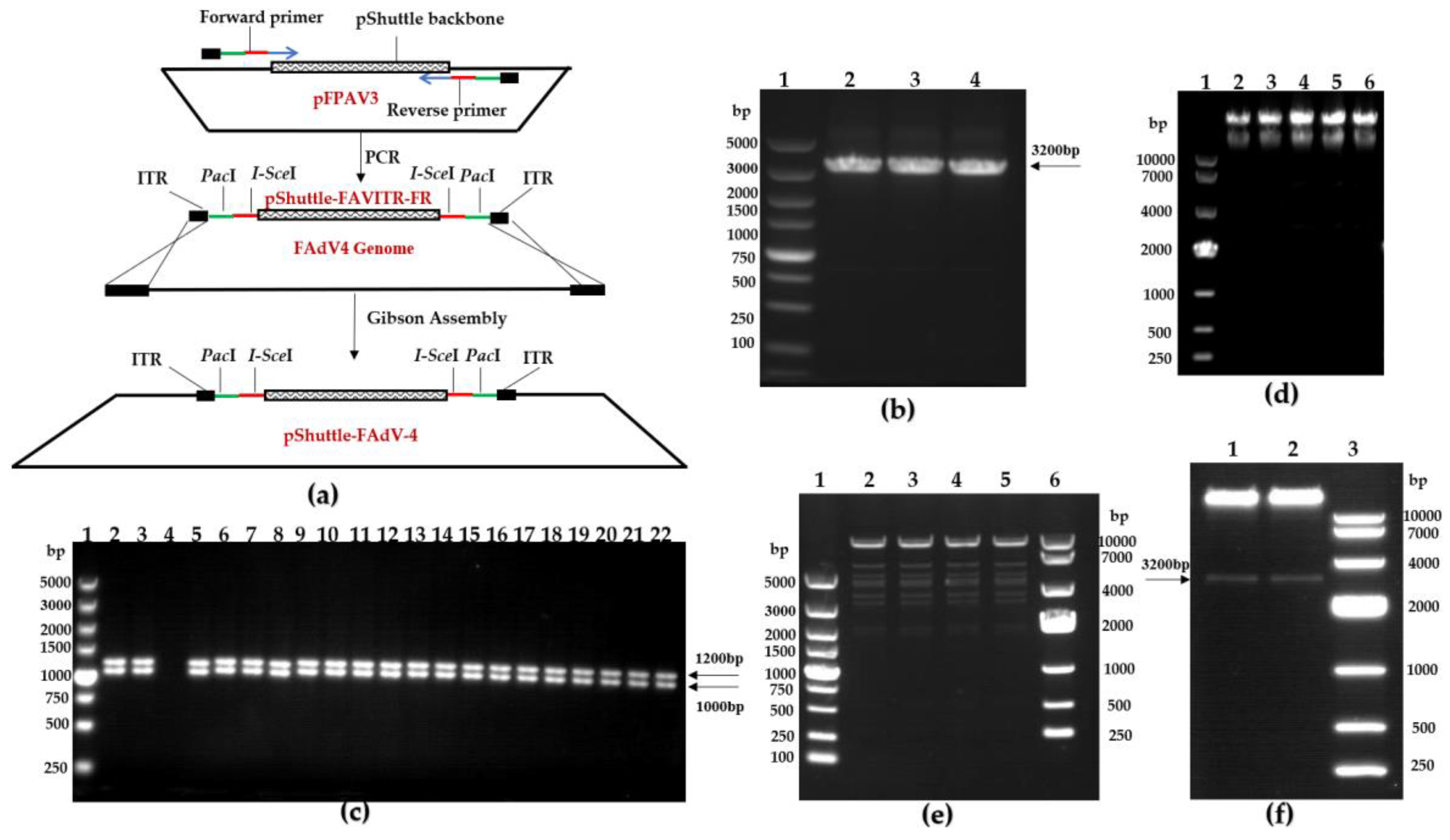
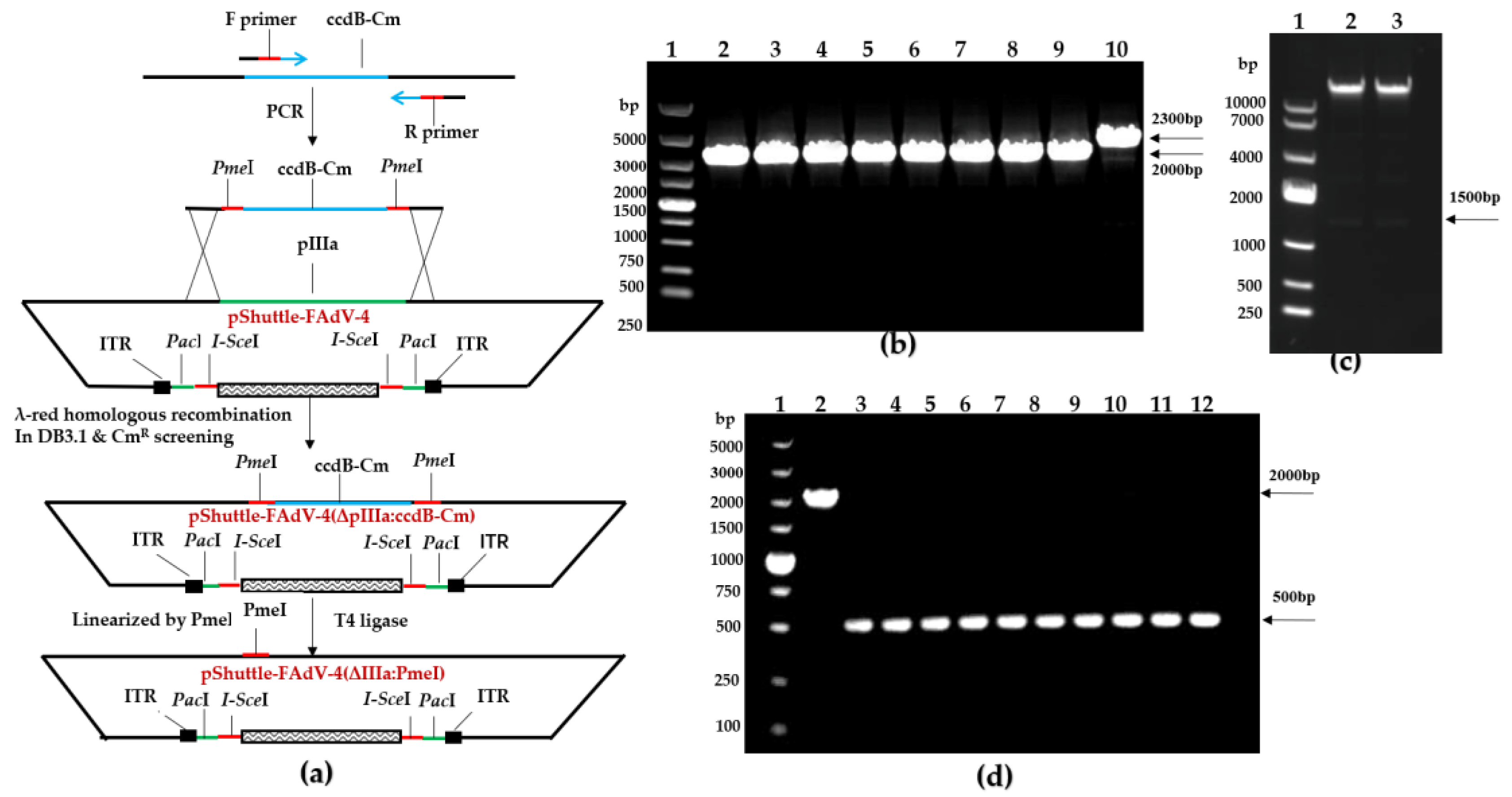
2.6. Construction of the Recombinant pShuttle-FAdV-4 Plasmid with the DNA Assembly Technique
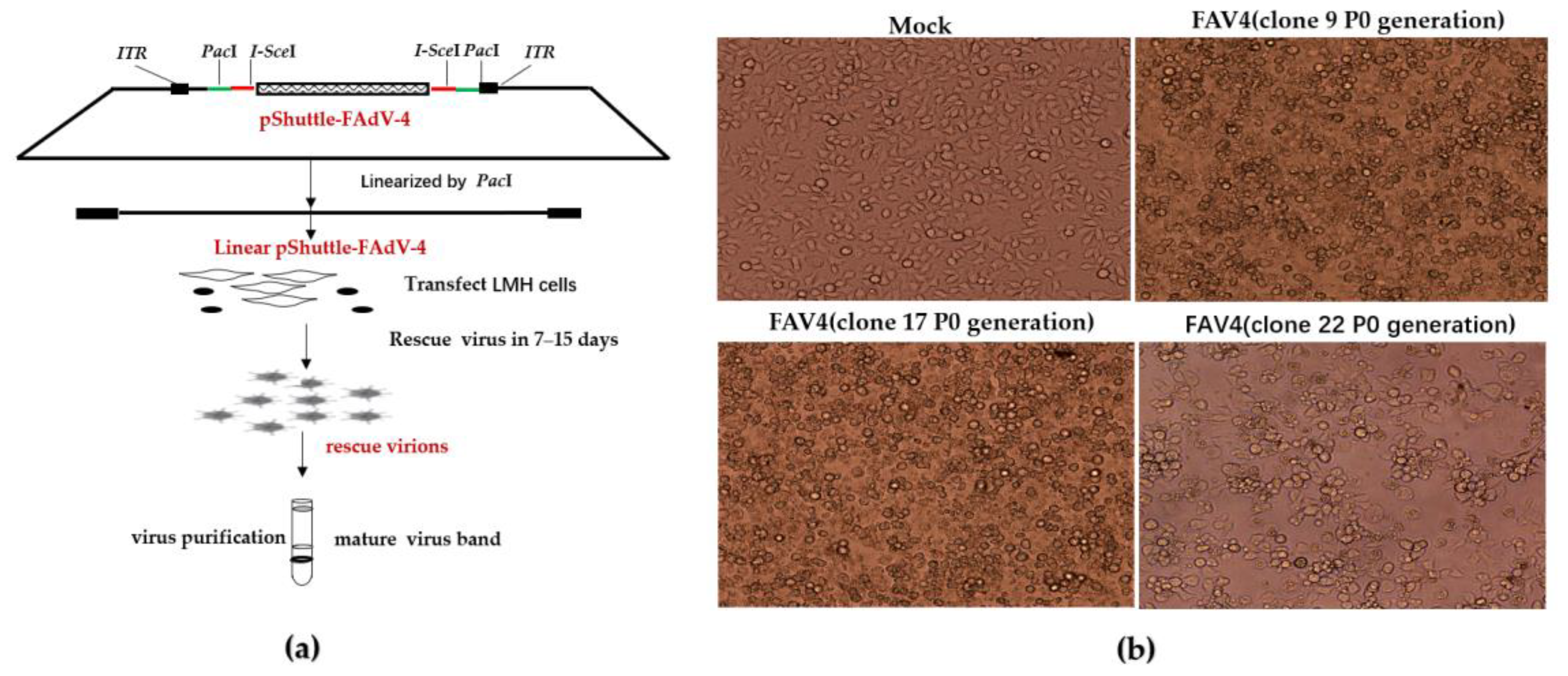
2.7. Rescue of FAdV-4 Infectious Clones
2.8. Construction of Replicating Single-Cycle FAdV-4 Vector
3. Results
3.1. Virus Isolation and Identification
3.2. Animal Regression Test
3.3. Construction of FAdV-4 Infectious Clones
3.4. Rescue of FAdV-4 Infectious Clones
3.5. Generation of a Replicating Single-Cycle Adenovirus Vector
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primer | Sequence (5′-3′) | PCR Product (bp) |
|---|---|---|
| hexon-F hexon-R | AACGTCAACCCCTTCAACCACC TTGCCTGTGGCGAAAGGCG | 1300 |
| pShuttle-PacI-ITR-F pShuttle-PacI-ITR-R | AAGACGCGGTTATATAAGATGATGTTAATTAAATTAC CCTGTTATCCCTAGAATTAATTCGATCCTGAATGGCG AAGACGCGGTTATATAAGATGATGTTAATTAAATTACC CTGTTATCCCTACATGCATGGATCCATATGCGG | 3200 |
| FAdV-4-23500-F FAdV-4-24501-R | TACCAACACGAGCACCACCAAAG GGGTTGACGTTGTCCATCTGGTC | 1000 |
| FAdV-4-ITR-F FAdV-4-1176-R | CATCATCTTATATAACCGCGTCTTTTGACACAC GAGCTCCGGTGTAAAGATTCGC | 1200 |
| IIIa-up-300-F IIIa-down-200-R | GTGCACGACGGTGGTCGAGC GACCGCCGCTAGAGGAACAG | 2300 2000 500 |
| FAdV-4 | CPE Time Point (d) | TCID50 | |
|---|---|---|---|
| 7 d | 10 d | ||
| rFAdV-4 (3) | 10 | 107.125 | 107.83 |
| rFAdV-4 (4) | 5 | 107 | 107.66 |
| rFAdV-4 (9) | 8 | 106.75 | 107.5 |
| rFAdV-4 (12) | 12 | 106.875 | 107.3 |
| rFAdV-4 (15) | 16 | 106.75 | 107.3 |
| rFAdV-4 (17) | 8 | 106.5 | 107.3 |
| rFAdV-4 (22) | 15 | 106.5 | 108 |
| rFAdV-4 (48) | 6 | 106.5 | 107.3 |
| rFAdV-4 (48) | 6 | 106.5 | 107.3 |
| rFAdV-4 (53) | 8 | 106.625 | 107.5 |
| rFAdV-4 (54) | 8 | 106.375 | 107.17 |
| rFAdV-4 (55) | 9 | 106.5 | 107.5 |
| rFAdV-4 (57) | 8 | 106.75 | 107.8 |
| rFAdV-4 (61) | 10 | 105.625 | 107.8 |
| rFAdV-4 (73) | 8 | 106.75 | 107.5 |
References
- Zsák, L.; Kisary, J. Grouping of fowl adenoviruses based upon the restriction patterns of DNA generated by BamHI and HindIII. Intervirol. 1984, 22, 110–114. [Google Scholar] [CrossRef]
- Steer, P.A.; Kirkpatrick, N.C.; O’Rourke, D.; Noormohammadi, A.H. Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. J. Clin. Microbiol. 2009, 47, 311–321. [Google Scholar] [CrossRef]
- Griffin, B.D.; Nagy, É. Coding potential and transcript analysis of fowl adenovirus 4: Insight into upstream ORFs as common sequence features in adenoviral transcripts. J. Gen. Virol. 2011, 92 Pt 6, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Benkő, M.; Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003, 84 Pt 11, 2895–2908. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Cuzange, A.; Ruigrok, R.W.; Chroboczek, J.; Jacrot, B. The avian adenovirus penton: Two fibres and one base. J. Mol. Biol. 1995, 252, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R.; Pache, L.; Reddy, V.; Stewart, P.L. Insights into adenovirus host cell interactions from structural studies. Virology 2009, 384, 380–388. [Google Scholar] [CrossRef]
- Ishag, H.Z.A.; Terab, A.M.A.; El Tigani-Asil, E.T.A.; Bensalah, O.K.; Khalil, N.A.H.; Khalafalla, A.I.; Al Hammadi, Z.; Shah, A.A.M.; Al Muhairi, S.S.M. Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE. Vet. Sci. 2022, 9, 154. [Google Scholar] [CrossRef]
- Dahiya, S.; Srivastava, R.N.; Hess, M.; Gulati, B.R. Fowl adenovirus serotype 4 associated with outbreaks of infectious hydropericardium in Haryana, India. Avian Dis. 2002, 46, 230–233. [Google Scholar] [CrossRef]
- Gomis, S.; Goodhope, A.R.; Ojkic, A.D.; Willson, P. Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Dis. 2006, 50, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.H.; Lee, H.J.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Youn, H.N.; Kim, M.S.; Youn, H.S.; Lee, J.B.; Park, S.Y.; et al. Identification and virulence characterization of fowl adenoviruses in Korea. Avian Dis. 2011, 55, 554–560. [Google Scholar] [CrossRef]
- Ye, J.; Liang, G.; Zhang, J.; Wang, W.; Song, N.; Wang, P.; Zheng, W.; Xie, Q.; Shao, H.; Wan, Z.; et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg. Microbes Infect. 2016, 5, e50. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Hou, L.; Wei, L.; Quan, R.; Wang, J.; Liu, H.; Liu, J. Fowl Adenovirus Serotype 4 Induces Hepatic Steatosis via Activation of Liver X Receptor-α. J. Virol. 2021, 95, e01938-20. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Zheng, P.P.; Zhang, T.F.; Wen, G.Y.; Shao, H.B.; Luo, Q.P. Fowl adenovirus serotype 4: Epidemiology, pathogenesis, diagnostic detection, and vaccine strategies. Poult. Sci. 2017, 96, 2630–2640. [Google Scholar] [CrossRef]
- Elkashif, A.; Alhashimi, M.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Adenoviral vector-based platforms for developing effective vaccines to combat respiratory viral infections. Clin. Transl. Immunol. 2021, 10, e1345. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, X.; Bian, A.; Li, H.; Chen, H.; Small, J.C.; Li, Y.; Giles-Davis, W.; Xiang, Z.; Ertl, H.C.J. An efficient method of directly cloning chimpanzee adenovirus as a vaccine vector. Nat. Protoc. 2010, 5, 1775–1785. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-CoV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Lévy, Y.; Lacabaratz, C.; Ellefsen-Lavoie, K.; Stöhr, W.; Lelièvre, J.D.; Bart, P.A.; Launay, O.; Weber, J.; Salzberger, B.; Wiedemann, A.; et al. Optimal priming of poxvirus vector (NYVAC)-based HIV vaccine regimens for T cell responses requires three DNA injections. Results of the randomized multicentre EV03/ANRS VAC20 Phase I/II Trial. PLoS Pathog. 2020, 16, e1008522. [Google Scholar] [CrossRef]
- Arnone, C.M.; Polito, V.A.; Mastronuzzi, A.; Carai, A.; Diomedi, F.C.; Antonucci, L.; Petrilli, L.L.; Vinci, M.; Ferrari, F.; Salviato, E.; et al. Oncolytic adenovirus and gene therapy with EphA2-BiTE for the treatment of pediatric high-grade gliomas. J. Immunother. Cancer 2021, 9, e001930. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Guo, X.; Deng, Y.; Chen, H.; Lan, J.; Wang, W.; Zou, X.; Hung, T.; Lu, Z.; Tan, W. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology 2015, 145, 476–484. [Google Scholar] [CrossRef]
- Zhu, F.C.; Wurie, A.H.; Hou, L.H.; Liang, Q.; Li, Y.H.; Russell, J.B.; Wu, S.P.; Li, J.X.; Hu, Y.M.; Guo, Q.; et al. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: A single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 389, 621–628. [Google Scholar] [CrossRef]
- Greenall, S.A.; Tyack, S.G.; Johnson, M.A.; Sapats, S.I. Antibody fragments, expressed by a fowl adenovirus vector, are able to neutralize infectious bursal disease virus. Avian Pathol. 2010, 39, 339–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pan, Q.; Zhang, Y.; Liu, A.; Cui, H.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Li, K.; Gao, L.; et al. Development of a Novel Avian Vaccine Vector Derived From the Emerging Fowl Adenovirus 4. Front. Microbiol. 2021, 12, 780978. [Google Scholar] [CrossRef] [PubMed]
- Ackford, J.G.; Corredor, J.C.; Pei, Y.; Krell, P.J.; Bédécarrats, G.; Nagy, É. Foreign gene expression and induction of antibody response by recombinant fowl adenovirus-9-based vectors with exogenous promoters. Vaccine 2017, 35, 4974–4982. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Corredor, J.C.; Griffin, B.D.; Krell, P.J.; Nagy, É. Fowl Adenovirus 4 (FAdV-4)-Based Infectious Clone for Vaccine Vector Development and Viral Gene Function Studies. Viruses 2018, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Corredor, J.C.; Nagy, E. The non-essential left end region of the fowl adenovirus 9 genome is suitable for foreign gene insertion/replacement. Virus Res. 2010, 149, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Griffin, B.; de Jong, J.; Krell, P.J.; Nagy, É. Rapid generation of fowl adenovirus 9 vectors. J. Virol. Methods 2015, 223, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Qin, X.; Krell, P.; Lu, R.; Sharif, S.; Nagy, É. Characterization and functional studies of fowl adenovirus 9 dUTPase. Virology 2016, 497, 251–261. [Google Scholar] [CrossRef]
- He, T.C.; Zhou, S.; da Costa, L.T.; Yu, J.; Kinzler, K.W.; Vogelstein, B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 1998, 95, 2509–2514. [Google Scholar] [CrossRef]
- Ng, P.; Parks, R.J.; Cummings, D.T.; Evelegh, C.M.; Graham, F.L. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum. Gene Ther. 2000, 11, 693–699. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., III; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yan, Y.; Zhang, J.; Zhao, S.; Feng, L.; Ou, J.; Cao, N.; Li, M.; Zhao, W.; Wan, C.; et al. Rapid Construction of a Replication-Competent Infectious Clone of Human Adenovirus Type 14 by Gibson Assembly. Viruses 2018, 10, 568. [Google Scholar] [CrossRef]
- Zou, X.H.; Bi, Z.X.; Guo, X.J.; Zhang, Z.; Zhao, Y.; Wang, M.; Zhu, Y.L.; Jie, H.Y.; Yu, Y.; Hung, T.; et al. (☆)DNA assembly technique simplifies the construction of infectious clone of fowl adenovirus. J. Virol. Methods 2018, 257, 85–92. [Google Scholar] [CrossRef]
- Crosby, C.M.; Barry, M.A. IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology 2014, 462–463, 158–165. [Google Scholar] [CrossRef]
- Crosby, C.M.; Nehete, P.; Sastry, K.J.; Barry, M.A. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J. Virol. 2015, 89, 669–675. [Google Scholar] [CrossRef]
- Le, C.T.; Gray, G.C.; Poddar, S.K. A modified rapid method of nucleic acid isolation from suspension of matured virus: Applied in restriction analysis of DNA from an adenovirus prototype strain and a patient isolate. J. Med. Microbiol. 2001, 50, 571–574. [Google Scholar] [CrossRef]
- Raue, R.; Hess, M. Hexon based PCRs combined with restriction enzyme analysis for rapid detection and differentiation of fowl adenoviruses and egg drop syndrome virus. J. Virol. Methods 1998, 73, 211–217. [Google Scholar] [CrossRef]
- Mase, M.; Mitake, H.; Inoue, T.; Imada, T. Identification of group I-III avian adenovirus by PCR coupled with direct sequencing of the hexon gene. J. Vet. Med. Sci. 2009, 71, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Niczyporuk, J.S. Phylogenetic and geographic analysis of fowl adenovirus field strains isolated from poultry in Poland. Arch. Virol. 2016, 161, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bijora, T.; Blawid, R.; Costa, D.K.T.; Aragão, F.J.L.; Souto, E.R.; Nagata, T. Construction of an agroinfectious clone of bean rugose mosaic virus using Gibson Assembly. Virus Genes. 2017, 53, 495–499. [Google Scholar] [CrossRef]
- Bordat, A.; Houvenaghel, M.C.; German-Retana, S. Gibson assembly: An easy way to clone potyviral full-length infectious cDNA clones expressing an ectopic VPg. Virol. J. 2015, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Blawid, R.; Nagata, T. Construction of an infectious clone of a plant RNA virus in a binary vector using one-step Gibson Assembly. J. Virol. Methods 2015, 222, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, W.; Kan, Q.; Mu, Y.; Zhang, W.; Chen, J.; Li, L.; Fu, H.; Li, T.; Wan, Z.; et al. FAdV-4 without Fiber-2 Is a Highly Attenuated and Protective Vaccine Candidate. Microbiol. Spectr. 2022, 10, e0143621. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Zhang, Y.; Chen, L.; Fang, L.; Chang, S.; Wang, Y.; Zhao, P. Construction of chicken infectious anemia virus infectious clone and study on its pathogenicity. Front. Microbiol. 2022, 13, 1016784. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.M.; Barry, M.A. Transgene Expression and Host Cell Responses to Replication-Defective, Single-Cycle, and Replication-Competent Adenovirus Vectors. Genes 2017, 8, 79. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, M.; Wang, Y.; Liu, S.; Li, B.; Du, E.; Gao, Y. Rapid Construction of an Infectious Clone of Fowl Adenovirus Serotype 4 Isolate. Viruses 2023, 15, 1657. https://doi.org/10.3390/v15081657
Gong M, Wang Y, Liu S, Li B, Du E, Gao Y. Rapid Construction of an Infectious Clone of Fowl Adenovirus Serotype 4 Isolate. Viruses. 2023; 15(8):1657. https://doi.org/10.3390/v15081657
Chicago/Turabian StyleGong, Minzhi, Yating Wang, Shijia Liu, Boshuo Li, Enqi Du, and Yupeng Gao. 2023. "Rapid Construction of an Infectious Clone of Fowl Adenovirus Serotype 4 Isolate" Viruses 15, no. 8: 1657. https://doi.org/10.3390/v15081657
APA StyleGong, M., Wang, Y., Liu, S., Li, B., Du, E., & Gao, Y. (2023). Rapid Construction of an Infectious Clone of Fowl Adenovirus Serotype 4 Isolate. Viruses, 15(8), 1657. https://doi.org/10.3390/v15081657




