Unveiling the Impact of the Omicron Variant: Insights from Genomic Surveillance in Mato Grosso do Sul, Midwest Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Molecular Diagnostic Assays
2.2. cDNA Synthesis and Whole-Genome Sequencing
2.3. Generation of Consensus Sequences
2.4. Phylogenetic Analysis
2.5. Epidamiological Data Assesment
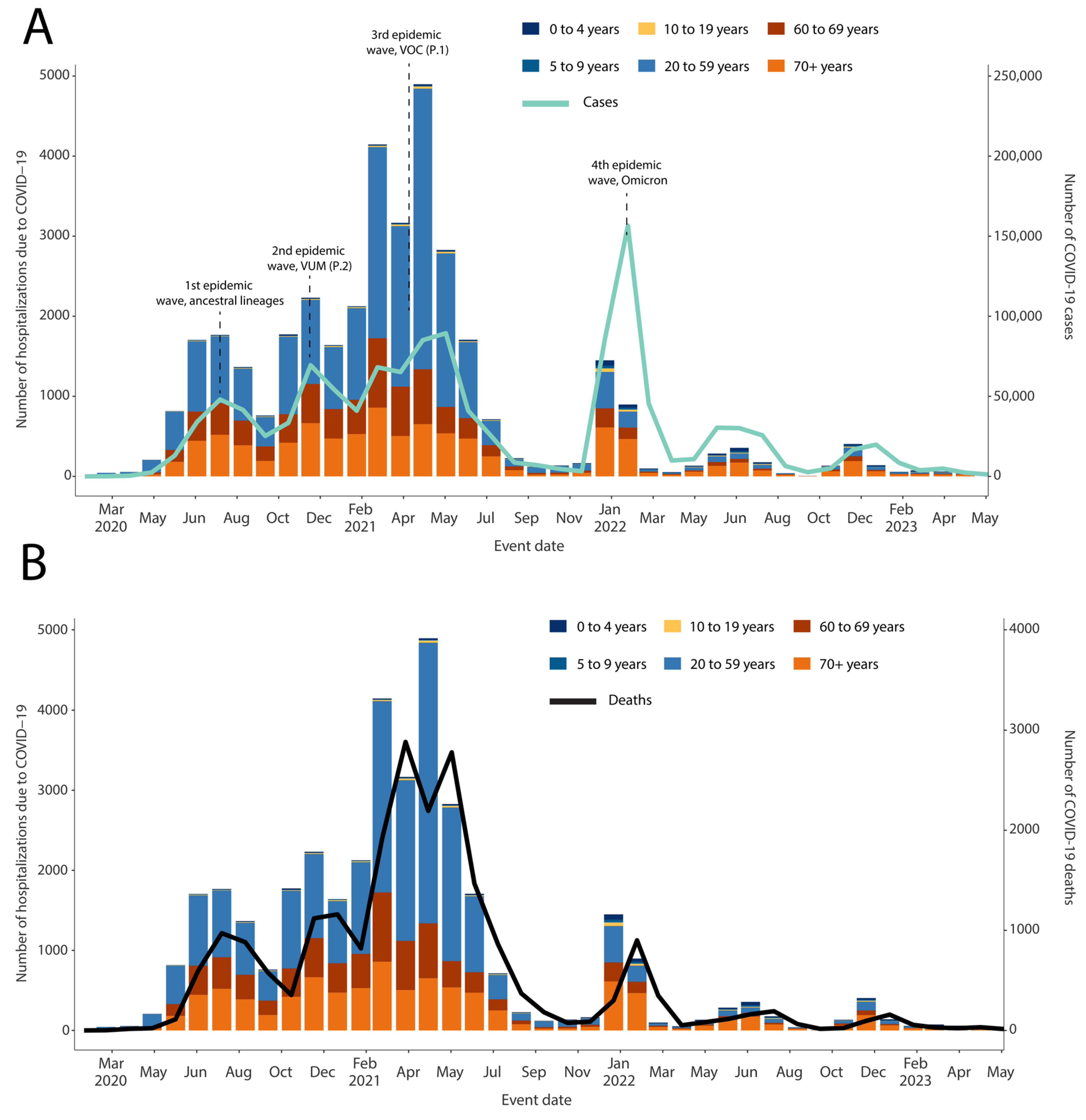
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Croda, J.H.R.; Garcia, L.P. Immediate Health Surveillance Response to COVID-19 Epidemic. Epidemiol. Serv. Saude 2020, 29, e2020002. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Slavov, S.N.; Fonseca, V.; Wilkinson, E.; Tegally, H.; PatanÉ, J.S.L.; Viala, V.L.; San, E.J.; Rodrigues, E.S.; Santos, E.V.; et al. Genomic epidemiology of the SARS-CoV-2 epidemic in Brazil. Nat. Microbiol. 2022, 7, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Estado de Saúde de Mato Grosso do Sul. Epidemiological Report from the State of Mato Grosso do Sul. SES MS. 2020. Available online: https://www.saude.ms.gov.br/ (accessed on 10 July 2023).
- Tosta, S.; Moreno, K.; Schuab, G.; Fonseca, V.; Segovia, F.M.C.; Kashima, S.; Elias, M.C.; Sampaio, S.C.; Ciccozzi, M.; Alcantara, L.C.J.; et al. Global SARS-CoV-2 genomic surveillance: What we have learned (so far). Infect. Genet. Evol. 2023, 108, 105405. [Google Scholar] [CrossRef] [PubMed]
- Vilsker, M.; Moosa, Y.; Nooij, S.; Fonseca, V.; Ghysens, Y.; Dumon, K.; Pauwels, R.; Alcantara, L.C.; Vanden Eynden, E.; Vandamme, A.-M.; et al. Genome Detective: An automated system for virus identification from high-throughput sequencing data. Bioinformatics 2019, 35, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Holmes, E.C.; O’Toole, Á.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534, Erratum in Mol. Biol. Evol. 2020, 37, 2461. [Google Scholar] [CrossRef] [PubMed]
- Sagulenko, P.; Puller, V.; Neher, R.A. TreeTime: Maximum-likelihood phylodynamic analysis. Virus Evol. 2018, 4, vex042. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Cella, E.; Benedetti, F.; Magalis, B.R.; Fonseca, V.; Fabris, S.; Campisi, G.; Ciccozzi, A.; Angeletti, S.; Borsetti, A.; et al. SARS-CoV-2 shifting transmission dynamics and hidden reservoirs potentially limit efficacy of public health interventions in Italy. Commun. Biol. 2021, 4, 489. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sasi, S.; Pillai, S.G.; Nag, A.; Shukla, D.; Singhal, R.; Phalke, S.; Velu, G.S.K. SARS-CoV-2 Mutations and Their Impact on Diagnostics, Therapeutics and Vaccines. Front. Med. 2022, 9, 815389. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, J.; Li, Y.; Zhang, H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit. Care 2021, 25, 244. [Google Scholar] [CrossRef] [PubMed]
- Resende, P.C.; Gräf, T.; Paixão, A.C.D.; Appolinario, L.; Lopes, R.S.; Mendonça, A.C.d.F.; da Rocha, A.S.B.; Motta, F.C.; Neto, L.G.L.; Khouri, R.; et al. A Potential SARS-CoV-2 Variant of Interest (VOI) Harboring Mutation E484K in the Spike Protein Was Identified within Lineage B.1.1.33 Circulating in Brazil. Viruses 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Nascimento, V.; de Souza, V.C.; Corado, A.d.L.; Nascimento, F.; Silva, G.; Costa, Á.; Duarte, D.; Pessoa, K.; Mejía, M.; et al. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat. Med. 2021, 27, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Leiva, G.D.C.; Dos Reis, D.S.; Filho, R.D.O. Estrutura urbana e mobilidade populacional: Implicações para o distanciamento social e disseminação da COVID-19. Rev. Bras. Estud. Popul. 2020, 37, e0118. [Google Scholar] [CrossRef]
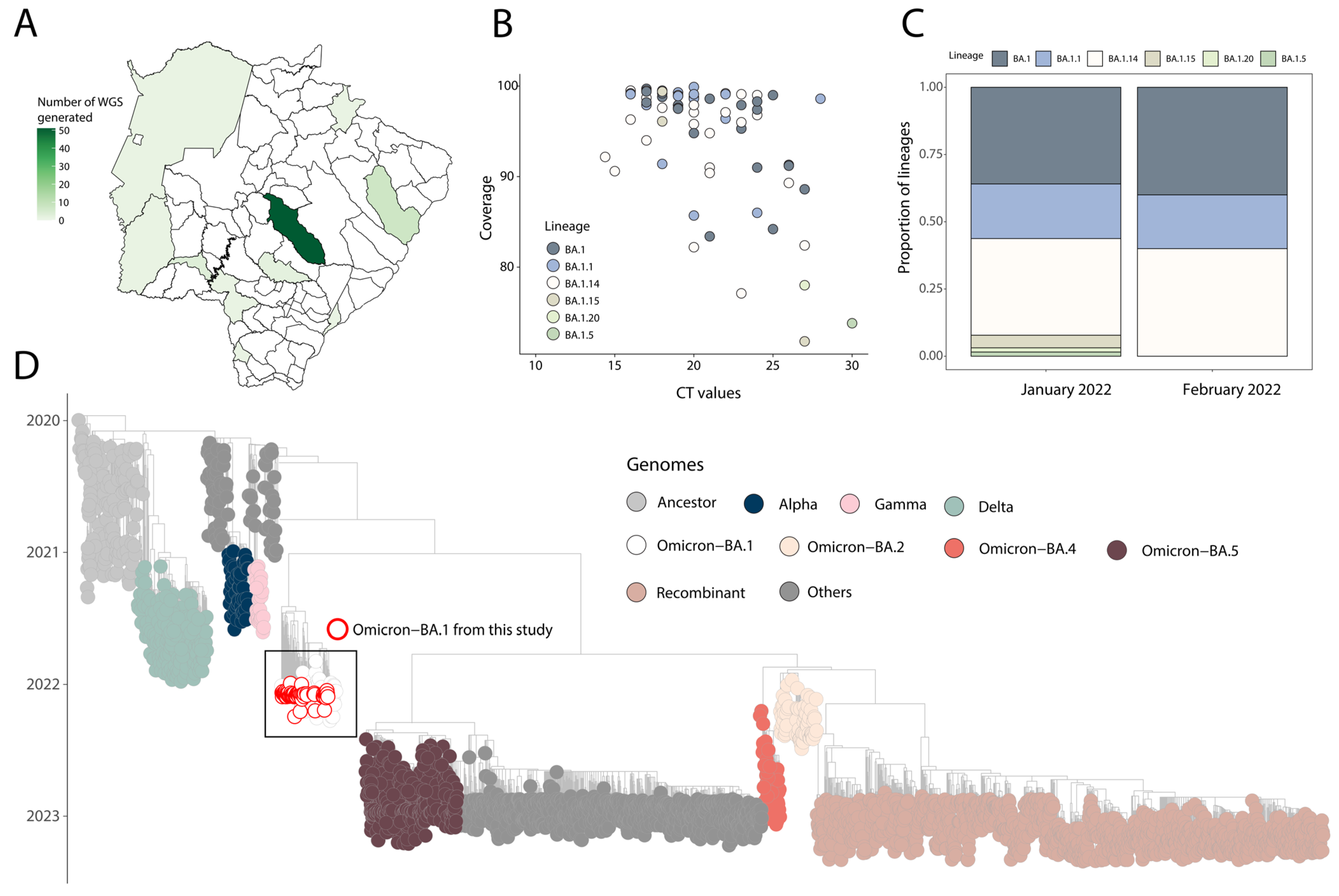
| ID | CT | State | Collection Date | Sex | Age | Reads | Coverage | Depth of Coverage | Lineage | Accession ID |
|---|---|---|---|---|---|---|---|---|---|---|
| LIBSARSMS2_barcode12|2022-01-24 | 23 | MS | 24 January 2022 | M | 48 | 60,759 | 99.1 | 1104 | BA.1.14 | EPI_ISL_17885390 |
| LIBSARSMS2_barcode16|2022-02-11 | 21 | MS | 28 January 2022 | F | 57 | 7159 | 91 | 141.7 | BA.1.14 | EPI_ISL_17885391 |
| LIBSARSMS2_barcode17|2022-01-27 | 25 | MS | 27 January 2022 | F | 43 | 91,596 | 99 | 1666.2 | BA.1 | EPI_ISL_17885392 |
| LIBSARSMS2_barcode19|2022-01-24 | 21 | MS | 25 January 2022 | F | 11 | 103,201 | 98.6 | 1885.8 | BA.1 | EPI_ISL_17885393 |
| LIBSARSMS2_barcode20|2022-01-25 | 27 | MS | 25 January 2022 | M | 13 | 3271 | 71.8 | 79.4 | BA.1.15 | EPI_ISL_17885394 |
| LIBSARSMS2_barcode21|2022-01-25 | 27 | MS | 25 January 2022 | M | 34 | 5427 | 82.4 | 119.8 | BA.1.14 | EPI_ISL_17885395 |
| LIBSARSMS2_barcode22|2022-01-25 | 18 | MS | 25 January 2022 | F | 18 | 138,324 | 98.8 | 2606.4 | BA.1 | EPI_ISL_17885396 |
| LIBSARSMS2_barcode23|2022-01-25 | 24 | MS | 25 January 2022 | F | 25 | 15,665 | 91 | 349.3 | BA.1 | EPI_ISL_17885397 |
| LIBSARSMS2_barcode25|2022-01-27 | 18 | MS | 26 January 2022 | F | 20 | 82,194 | 96.1 | 1525.3 | BA.1.15 | EPI_ISL_17885398 |
| LIBSARSMS2_barcode26|2022-01-26 | 23 | MS | 26 January 2022 | F | 74 | 54,957 | 95.3 | 1037 | BA.1 | EPI_ISL_17885399 |
| LIBSARSMS2_barcode27|2022-01-28 | 22 | MS | 27 January 2022 | M | 52 | 69,658 | 99.2 | 1261.8 | BA.1.14 | EPI_ISL_17885400 |
| LIBSARSMS2_barcode28|2021-12-29 | 24 | MS | 27 January 2022 | M | 54 | 63,882 | 99 | 1162.4 | BA.1.14 | EPI_ISL_17885401 |
| LIBSARSMS2_barcode29|2022-02-03 | 24 | MS | 26 January 2022 | F | 61 | 36,138 | 96.8 | 672.6 | BA.1.14 | EPI_ISL_17885402 |
| LIBSARSMS2_barcode30|2022-02-04 | 25 | MS | 26 January 2022 | F | 57 | 6383 | 84.2 | 135.5 | BA.1 | EPI_ISL_17885403 |
| LIBSARSMS2_barcode32|2022-02-04 | 27 | MS | 26 January 2022 | M | 37 | 8017 | 88.6 | 157.5 | BA.1 | EPI_ISL_17885404 |
| LIBSARSMS2_barcode34|2022-01-20 | 21 | MS | 26 January 2022 | M | 24 | 15,481 | 83.4 | 331.3 | BA.1 | EPI_ISL_17885405 |
| LIBSARSMS2_barcode35|2022-02-03 | 20 | MS | 26 January 2022 | M | 34 | 47,285 | 97.9 | 870.2 | BA.1 | EPI_ISL_17885406 |
| LIBSARSMS2_barcode36|2022-02-03 | 19 | MS | 26 January 2022 | M | 35 | 58,617 | 97.9 | 1082.5 | BA.1 | EPI_ISL_17885407 |
| LIBSARSMS2_barcode37|2022-02-03 | 17 | MS | 26 January 2022 | F | 37 | 199,034 | 99.7 | 3576.7 | BA.1 | EPI_ISL_17885408 |
| LIBSARSMS2_barcode38|2022-02-03 | 20 | MS | 26 January 2022 | M | 53 | 85,187 | 98.8 | 1561.2 | BA.1 | EPI_ISL_17885409 |
| LIBSARSMS2_barcode39|2022-01-31 | 27 | MS | 27 January 2022 | F | 20 | 2722 | 78 | 60.3 | BA.1.20 | EPI_ISL_17885410 |
| LIBSARSMS2_barcode41|2022-02-04 | 21 | MS | 27 January 2022 | F | 54 | 78,674 | 94.8 | 1483.9 | BA.1.14 | EPI_ISL_17885411 |
| LIBSARSMS2_barcode42|2022-02-04 | 17 | MS | 27 January 2022 | F | 37 | 77,568 | 97.9 | 1429.8 | BA.1.1 | EPI_ISL_17885412 |
| LIBSARSMS2_barcode43|2022-02-04 | 26 | MS | 27 January 2022 | F | 54 | 12,672 | 91.3 | 248.5 | BA.1 | EPI_ISL_17885413 |
| LIBSARSMS2_barcode44|2022-02-04 | 18 | MS | 27 January 2022 | M | 21 | 164,942 | 99.5 | 2957.3 | BA.1.1 | EPI_ISL_17885414 |
| LIBSARSMS2_barcode45|2022-02-04 | 17 | MS | 27 January 2022 | M | 19 | 141,215 | 99.5 | 2541.5 | BA.1.14 | EPI_ISL_17885415 |
| LIBSARSMS2_barcode46|2022-02-04 | 20 | MS | 27 January 2022 | M | 64 | 112,016 | 99.9 | 2027.6 | BA.1.1 | EPI_ISL_17885416 |
| LIBSARSMS2_barcode47|2022-03-17 | 20 | MS | 28 January 2022 | M | 57 | 23,799 | 95.8 | 446.7 | BA.1.14 | EPI_ISL_17885417 |
| LIBSARSMS2_barcode48|2022-01-29 | 19 | MS | 28 January 2022 | F | 19 | 166,009 | 99.3 | 2998.2 | BA.1.1 | EPI_ISL_17885418 |
| LIBSARSMS2_barcode49|2022-01-27 | 18 | MS | 27 January 2022 | F | 65 | 20,051 | 91.4 | 398.1 | BA.1.1 | EPI_ISL_17885419 |
| LIBSARSMS2_barcode50|2022-01-27 | 19 | MS | 27 January 2022 | F | 31 | 121,892 | 99 | 2209.4 | BA.1.14 | EPI_ISL_17885420 |
| LIBSARSMS2_barcode51|2022-01-27 | 17 | MS | 27 January 2022 | F | 54 | 188,893 | 98.7 | 3464.9 | BA.1.14 | EPI_ISL_17885421 |
| LIBSARSMS2_barcode52|2022-01-27 | 18 | MS | 27 January 2022 | M | 24 | 81,577 | 99.5 | 1477.2 | BA.1 | EPI_ISL_17885422 |
| LIBSARSMS2_barcode53|2022-01-27 | 18 | MS | 27 January 2022 | F | 36 | 160,723 | 99.2 | 2908.4 | BA.1 | EPI_ISL_17885423 |
| LIBSARSMS2_barcode55|2022-01-27 | 19 | MS | 27 January 2022 | M | 43 | 28,809 | 97.6 | 533.8 | BA.1 | EPI_ISL_17885424 |
| LIBSARSMS2_barcode56|2022-01-27 | 16 | MS | 27 January 2022 | M | 42 | 54,509 | 96.3 | 1005.8 | BA.1.14 | EPI_ISL_17885425 |
| LIBSARSMS2_barcode57|2022-01-27 | 19 | MS | 27 January 2022 | M | 35 | 64,896 | 97.5 | 1187.5 | BA.1 | EPI_ISL_17885426 |
| LIBSARSMS2_barcode58|2022-01-21 | 20 | MS | 28 January 2022 | F | 31 | 68,573 | 94.8 | 1309.9 | BA.1 | EPI_ISL_17885427 |
| LIBSARSMS2_barcode59|2022-01-28 | 20 | MS | 28 January 2022 | M | 37 | 77,921 | 97.1 | 1436.8 | BA.1.14 | EPI_ISL_17885428 |
| LIBSARSMS2_barcode60|2022-01-25 | 30 | MS | 25 January 2022 | F | 32 | 1494 | 73.8 | 33.8 | BA.1.5 | EPI_ISL_17885429 |
| LIBSARSMS2_barcode61|2022-01-31 | 18 | MS | 31 January 2022 | F | 65 | 180,831 | 99.4 | 3270.9 | BA.1.15 | EPI_ISL_17885430 |
| LIBSARSMS2_barcode62|2022-01-28 | 24 | MS | 28 January 2022 | F | 16 | 36,276 | 97.4 | 674.6 | BA.1 | EPI_ISL_17885431 |
| LIBSARSMS2_barcode63|2022-01-27 | 19 | MS | 27 January 2022 | F | 62 | 74,361 | 98.9 | 1364.4 | BA.1.1 | EPI_ISL_17885432 |
| LIBSARSMS2_barcode65|2022-01-27 | 28 | MS | 28 January 2022 | F | 60 | 198,829 | 98.6 | 3652.5 | BA.1.1 | EPI_ISL_17885433 |
| LIBSARSMS2_barcode66|2022-02-02 | 23 | MS | 2 February 2022 | F | 14 | 13,165 | 77.1 | 312.2 | BA.1.14 | EPI_ISL_17885434 |
| LIBSARSMS2_barcode67|2022-02-02 | 23 | MS | 2 February 2022 | M | 39 | 33,181 | 97.9 | 611.9 | BA.1 | EPI_ISL_17885435 |
| LIBSARSMS2_barcode68|2022-01-18 | 20 | MS | 31 January 2022 | F | 30 | 11,165 | 85.7 | 234.2 | BA.1.1 | EPI_ISL_17885436 |
| LIBSARSMS2_barcode69|2022-03-15 | 16 | MS | 30 January 2022 | M | 27 | 266,105 | 99.5 | 4833.1 | BA.1.14 | EPI_ISL_17885437 |
| LIBSARSMS2_barcode70|2022-03-13 | 20 | MS | 30 January 2022 | F | 26 | 112,571 | 98.6 | 2052.6 | BA.1.1 | EPI_ISL_17885438 |
| LIBSARSMS2_barcode71|2022-02-07 | 20 | MS | 31 January 2022 | F | 91 | 209,974 | 99.1 | 3941.7 | BA.1.1 | EPI_ISL_17885439 |
| LIBSARSMS2_barcode72|2022-03-31 | 15 | MS | 31 January 2022 | F | 91 | 35,836 | 90.6 | 712.6 | BA.1.14 | EPI_ISL_17885440 |
| LIBSARSMS2_barcode73|2022-01-31 | 24 | MS | 31 January 2022 | M | 79 | 27,752 | 86 | 578.3 | BA.1.1 | EPI_ISL_17885441 |
| LIBSARSMS2_barcode74|2022-01-31 | 17 | MS | 30 January 2022 | M | 23 | 76,270 | 94 | 1467 | BA.1.14 | EPI_ISL_17885442 |
| LIBSARSMS2_barcode76|2022-01-24 | 20 | MS | 1 February 2022 | F | 39 | 20,573 | 82.2 | 452.5 | BA.1.14 | EPI_ISL_17885443 |
| LIBSARSMS2_barcode78|2022-02-04 | 16 | MS | 1 February 2022 | F | 29 | 177,714 | 99.2 | 3249.3 | BA.1 | EPI_ISL_17885444 |
| LIBSARSMS2_barcode79|2022-02-04 | 22 | MS | 1 February 2022 | F | 23 | 14,615 | 96.4 | 272.6 | BA.1.1 | EPI_ISL_17885445 |
| LIBSARSMS2_barcode80|2022-01-29 | 21 | MS | 29 January 2022 | F | 52 | 11,173 | 90.4 | 221 | BA.1.14 | EPI_ISL_17885446 |
| LIBSARSMS2_barcode81|2022-01-29 | 24 | MS | 29 January 2022 | M | 62 | 41,745 | 98.3 | 763.3 | BA.1 | EPI_ISL_17885447 |
| LIBSARSMS2_barcode82|2022-01-29 | 26 | MS | 29 January 2022 | F | 63 | 13,046 | 89.3 | 264.1 | BA.1.14 | EPI_ISL_17885448 |
| LIBSARSMS2_barcode83|2022-01-29 | 22 | MS | 29 January 2022 | F | 49 | 35,076 | 99.1 | 631.7 | BA.1.1 | EPI_ISL_17885449 |
| LIBSARSMS2_barcode84|2022-01-29 | 17 | MS | 29 January 2022 | M | 34 | 218,985 | 98.2 | 3999.7 | BA.1 | EPI_ISL_17885450 |
| LIBSARSMS2_barcode86|2022-01-29 | 23 | MS | 29 January 2022 | F | 51 | 16,567 | 96 | 312.1 | BA.1.14 | EPI_ISL_17885451 |
| LIBSARSMS2_barcode87|2022-01-29 | 10 | MS | 29 January 2022 | M | 39 | 11,270 | 88.6 | 224.4 | BA.1.14 | EPI_ISL_17885452 |
| LIBSARSMS2_barcode88|2022-01-29 | 18 | MS | 29 January 2022 | M | 19 | 60,836 | 97.6 | 1112 | BA.1.14 | EPI_ISL_17885453 |
| LIBSARSMS2_barcode90|2022-01-29 | 17 | MS | 29 January 2022 | F | 22 | 225,902 | 99.4 | 4159.7 | BA.1 | EPI_ISL_17885454 |
| LIBSARSMS2_barcode91|2022-01-29 | 26 | MS | 29 January 2022 | F | 27 | 24,272 | 91.2 | 471.3 | BA.1 | EPI_ISL_17885455 |
| LIBSARSMS2_barcode93|2022-01-29 | 20 | MS | 29 January 2022 | M | 55 | 66,819 | 97.9 | 1331.5 | BA.1.14 | EPI_ISL_17885456 |
| LIBSARSMS2_barcode94|2022-01-18 | 16 | MS | 29 January 2022 | F | 30 | 203,956 | 99.1 | 4029.9 | BA.1.1 | EPI_ISL_17885457 |
| LIBSARSMS2_barcode95|2022-01-01 | 22 | MS | 29 January 2022 | F | 40 | 20,911 | 97.1 | 389.3 | BA.1.14 | EPI_ISL_17885458 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mello Almeida Maziero, L.; Giovanetti, M.; Fonseca, V.; Zardin, M.C.S.U.; de Castro Lichs, G.G.; de Rezende Romera, G.R.; Tsuha, D.H.; Frias, D.F.R.; Escandolhero, V.C.; Demarchi, L.H.; et al. Unveiling the Impact of the Omicron Variant: Insights from Genomic Surveillance in Mato Grosso do Sul, Midwest Brazil. Viruses 2023, 15, 1604. https://doi.org/10.3390/v15071604
de Mello Almeida Maziero L, Giovanetti M, Fonseca V, Zardin MCSU, de Castro Lichs GG, de Rezende Romera GR, Tsuha DH, Frias DFR, Escandolhero VC, Demarchi LH, et al. Unveiling the Impact of the Omicron Variant: Insights from Genomic Surveillance in Mato Grosso do Sul, Midwest Brazil. Viruses. 2023; 15(7):1604. https://doi.org/10.3390/v15071604
Chicago/Turabian Stylede Mello Almeida Maziero, Lívia, Marta Giovanetti, Vagner Fonseca, Marina Castilhos Souza Umaki Zardin, Gislene Garcia de Castro Lichs, Grazielli Rocha de Rezende Romera, Daniel Henrique Tsuha, Danila Fernanda Rodrigues Frias, Valdir Castanho Escandolhero, Luiz Henrique Demarchi, and et al. 2023. "Unveiling the Impact of the Omicron Variant: Insights from Genomic Surveillance in Mato Grosso do Sul, Midwest Brazil" Viruses 15, no. 7: 1604. https://doi.org/10.3390/v15071604
APA Stylede Mello Almeida Maziero, L., Giovanetti, M., Fonseca, V., Zardin, M. C. S. U., de Castro Lichs, G. G., de Rezende Romera, G. R., Tsuha, D. H., Frias, D. F. R., Escandolhero, V. C., Demarchi, L. H., Domingues Castilho, L., Barbosa, K. F., Tebet, D. G. M., Xavier, J., Fritsch, H., Lima, M., de Oliveira, C., Santos, E. V., Kashima, S., ... Cavalheiro Maymone Gonçalves, C. (2023). Unveiling the Impact of the Omicron Variant: Insights from Genomic Surveillance in Mato Grosso do Sul, Midwest Brazil. Viruses, 15(7), 1604. https://doi.org/10.3390/v15071604











