Application of High-Pressure Processing (or High Hydrostatic Pressure) for the Inactivation of Human Norovirus in Korean Traditionally Preserved Raw Crab
Abstract
:1. Introduction
2. Materials and Methods
2.1. HuNoV Stock Preparation
2.2. Preparation of Sample and Virus Inoculation
2.3. HPP Treatment of HuNoV in Raw Crabs
2.4. Propidium Monoazide and Sodium Lauroyl Sarcosinate Treatment
2.5. Virus Isolation and RNA Extraction
2.6. Analysis of HuNoV Using RT-qPCR
2.7. Measurement of pH, Sensory Quality, and Hunter Color of Raw Crabs
2.8. Statistical Analysis
3. Results
3.1. Effect of HPP on HuNoV GII.4 in Raw Crab Using Non-PMA/RT-qPCR and PMA + Sarkosyl/RT-qPCR
3.2. Changes in the pH, Sensory Analysis, and Hunter Color with HPP Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, M.-S.; Jeon, E.B.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Park, S.Y. Virucidal Effects of Dielectric Barrier Discharge Plasma on Human Norovirus Infectivity in Fresh Oysters (Crassostrea gigas). Foods 2020, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Trainor, E.; Nakagomi, T.; Cunliffe, N.A.; Nakagomi, O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: Global distribution of genogroups, genotypes and GII.4 variants. J. Clin. Virol. 2013, 56, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Graaf, M.; van Beek, J.; Koopmans, M.P.G. Human norovirus transmission and evolution in a changing world. Nat. Rev. Genet. 2016, 14, 421–433. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), 2023. Norovirus Burden and Trends. Available online: https://www.cdc.gov/norovirus/burden (accessed on 7 July 2023).
- Campos, C.J.A.; Lees, D.N. Environmental Transmission of Human Noroviruses in Shellfish Waters. Appl. Environ. Microbiol. 2014, 80, 3552–3561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parshionikar, S.; Laseke, I.; Fout, G.S. Use of Propidium Monoazide in Reverse Transcriptase PCR To Distinguish between Infectious and Noninfectious Enteric Viruses in Water Samples. Appl. Environ. Microbiol. 2010, 76, 4318–4326. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Ko, G. Using propidium monoazide to distinguish between viable and nonviable bacteria, MS2 and murine norovirus. Lett. Appl. Microbiol. 2012, 55, 182–188. [Google Scholar] [CrossRef]
- Coudray-Meunier, C.; Fraisse, A.; Martin-Latil, S.; Guillier, L.; Perelle, S. Discrimination of infectious hepatitis A virus and rotavirus by combining dyes and surfactants with RT-qPCR. BMC Microbiol. 2013, 13, 216. [Google Scholar] [CrossRef] [Green Version]
- Jeon, E.B.; Choi, M.-S.; Kim, J.Y.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Lee, H.J.; Jung, Y.J.; Ha, J.-H.; Park, S.Y. Characterizing the effects of thermal treatment on human norovirus GII.4 viability using propidium monoazide combined with RT-qPCR and quality assessments in mussels. Food Control 2019, 109, 106954. [Google Scholar] [CrossRef]
- Lee, H.-W.; Lee, H.-M.; Yoon, S.-R.; Kim, S.H.; Ha, J.-H. Pretreatment with propidium monoazide/sodium lauroyl sarcosinate improves discrimination of infectious waterborne virus by RT-qPCR combined with magnetic separation. Environ. Pollut. 2017, 233, 306–314. [Google Scholar] [CrossRef]
- Song, M.G.; Kim, J.Y.; Jeon, E.B.; Kim, S.H.; Heu, M.S.; Lee, J.S.; Kim, J.S.; Park, S.Y. Antiviral Efficacy of Dielectric Barrier Discharge Plasma against Hepatitis A Virus in Fresh Oyster Using PMA/RT-qPCR. Appl. Sci. 2023, 13, 3513. [Google Scholar] [CrossRef]
- Wang, H.; Gill, C.O.; Yang, X.Q. Use of sodium lauroyl sarcosinate (sarkosyl) in viable real-time PCR for enumeration of Escherichia coli. J. Microbiol. Methods 2014, 98, 89–93. [Google Scholar] [CrossRef]
- Fuster, N.; Pinto, R.M.; Fuentes, C.; Beguiristain, N.; Bosch, A.; Guix, S. Propidium monoazide RTqPCR assays for the assessment of hepatitis A inactivation and for a better estimation of the health risk of contaminated waters. Water Res. 2016, 101, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCaprio, E.; Ye, M.; Chen, H.Q.; Li, J.R. Inactivation of Human Norovirus and Tulane Virus by High Pressure Processing in Simple Mediums and Strawberry Puree. Front. Sustain. Food Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Leon, J.S.; Kingsley, D.H.; Montes, J.S.; Richards, G.P.; Lyon, G.M.; Abdulhafid, G.M.; Seitz, S.R.; Fernandez, M.L.; Teunis, P.F.; Flick, G.J.; et al. Randomized, Double-Blinded Clinical Trial for Human Norovirus Inactivation in Oysters by High Hydrostatic Pressure Processing. Appl. Environ. Microbiol. 2011, 77, 5476–5482. [Google Scholar] [CrossRef] [Green Version]
- Li, X.H.; Ye, M.; Neetoo, H.; Golovan, S.; Chen, H.Q. Pressure inactivation of Tulane virus, a candidate surrogate for human norovirus and its potential application in food industry. Int. J. Food Microbiol. 2013, 162, 37–42. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeon, E.B.; Song, M.G.; Ha, K.S.; Jeong, S.H.; Jung, Y.J.; Park, S.Y. Combination of ultrasonic waves and dielectric barrier discharge plasma for the viable reduction in human norovirus while retaining the quality of raw sea squirt. J. Food Process Eng. 2021, 44, e13847. [Google Scholar] [CrossRef]
- Jeon, E.B.; Choi, M.S.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Park, S.Y. Assessment of potential infectivity of human norovirus in the traditional Korean salted clam product “Jogaejeotgal” by floating electrode-dielectric barrier discharge plasma. Food Res. Int. 2021, 141, 110107. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.I.; Park, S.Y.; Ha, S.D. Thermal inactivation of human norovirus on spinach using propidium or ethidium monoazide combined with real-time quantitative reverse transcription-polymerase chain reaction. Food Control 2017, 78, 79–84. [Google Scholar] [CrossRef]
- Lee, H.W.; Yoon, S.R.; Lee, H.M.; Lee, J.Y.; Kim, S.H.; Ha, J.H. Use of RT-qPCR with combined intercalating dye and sodium lauroyl sarcosinate pretreatment to evaluate the virucidal activity of halophyte extracts against norovirus. Food Control 2018, 98, 100–106. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D.G.; Kingsley, D.H. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J. Food Prot. 2005, 68, 2389–2394. [Google Scholar] [CrossRef]
- Grove, S.F.; Forsyth, S.; Wan, J.; Coventry, J.; Cole, M.; Stewart, C.M.; Lewis, T.; Ross, T.; Lee, A. Inactivation of hepatitis A virus, poliovirus and a norovirus surrogate by high pressure processing. Innov. Food Sci. Emerg. Technol. 2008, 9, 206–210. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E. Susceptibility of human rotavirus to ozone, high pressure, and pulsed electric field. J. Food Prot. 2002, 65, 1441–1446. [Google Scholar] [CrossRef]
- Lou, F.F.; Neetoo, H.; Chen, H.Q.; Li, J.R. Inactivation of a Human Norovirus Surrogate by High-Pressure Processing: Effectiveness, Mechanism, and Potential Application in the Fresh Produce Industry. Appl. Environ. Microbiol. 2011, 77, 1862–1871. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, D.H.; Chen, H.Q. Influence of pH, salt, and temperature on pressure inactivation of hepatitis A virus. Int. J. Food Microbiol. 2009, 130, 61–64. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Hoover, D.G.; Papafragkou, E.; Richards, G.P. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J. Food Prot. 2002, 65, 1605–1609. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Chen, H.; Hoover, D.G. Inactivation of selected picornaviruses by high hydrostatic pressure. Virus Res. 2004, 102, 221–224. [Google Scholar] [CrossRef]
- Song, M.G.; Kim, S.H.; Jeon, E.B.; Ha, K.S.; Cho, S.R.; Jung, Y.J.; Choi, E.H.; Lim, J.S.; Choi, J.; Park, S.Y. Inactivation of Human Norovirus GII. 4 and Vibrio parahaemolyticus in the Sea Squirt (Halocynthia roretzi) by Floating Electrode-Dielectric Barrier Discharge Plasma. Foods 2023, 12, 1030. [Google Scholar] [CrossRef]
- Baert, L.; Debevere, J.; Uyttendaele, M. The efficacy of preservation methods to inactivate foodborne viruses. Int. J. Food Microbiol. 2009, 131, 83–94. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D.G. Pressure inactivation kinetics of Yersinia enterocolitica ATCC 35669. Int. J. Food Microbiol. 2003, 87, 161–171. [Google Scholar] [CrossRef]
- Tang, Q.J.; Li, D.; Xu, J.; Wang, J.F.; Zhao, Y.R.; Li, Z.J.; Xue, C.H. Mechanism of inactivation of murine norovirus-1 by high pressure processing. Int. J. Food Microbiol. 2010, 137, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Aznar, R.; Martinez, A.; Rodrigo, D. Inactivation of Human and Murine Norovirus by High-Pressure Processing. Foodborne Pathog. Dis. 2011, 8, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Baert, L.; Uyttendaele, M.; Vermeersch, M.; Van Coillie, E.; Debevere, J. Survival and transfer of murine norovirus 1, a surrogate for human noroviruses, during the production process of deep-frozen onions and spinach. J. Food Prot. 2008, 71, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.H.; Sair, A.; Williams, K.; Papafragkou, E.; Jean, J.; Moore, C.; Jaykus, L. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int. J. Food Microbiol. 2006, 108, 84–91. [Google Scholar] [CrossRef]
- Rzezutka, A.; Cook, N. Survival of human enteric viruses in the environment and food. FEMS Microbiol. Rev. 2004, 28, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Butot, S.; Putallaz, T.; Sanchez, G. Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. Int. J. Food Microbiol. 2008, 126, 30–35. [Google Scholar] [CrossRef]
- Kingsley, D.H. High pressure processing of bivalve shellfish and HPP’s use as a virus intervention. Foods 2014, 3, 336–350. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.K.; Jeon, E.B.; Park, S.Y. Effects of nonthermal dielectric barrier discharge plasma against Listeria monocytogenes and quality of smoked salmon fillets. J. Food Saf. 2022, 42, e13012. [Google Scholar] [CrossRef]
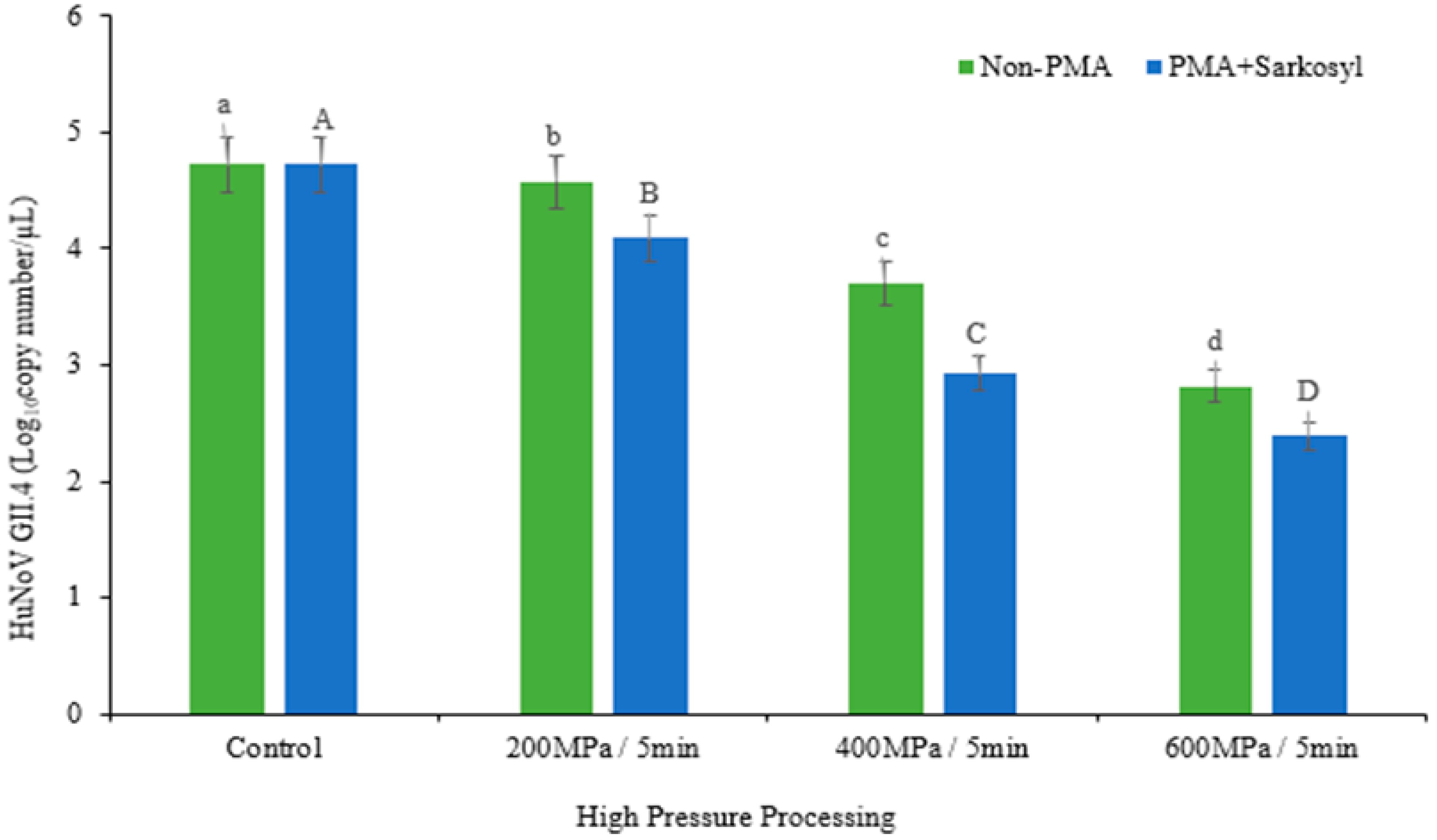
| Treatment | Non-PMA | PMA + Sarkosyl |
|---|---|---|
| log10copy Number/µL | log10copy Number/µL | |
| Control | 4.73 ± 0.06 a | 4.73 ± 0.06 a |
| 200 MPa | 4.58 ± 0.08 b | 4.09 ± 0.01 e |
| 400 MPa | 3.71 ± 0.05 c | 2.93 ± 0.00 f |
| 600 MPa | 2.82 ± 0.03 d | 2.39 ± 0.00 g |
| Treatment | High-Pressure Processing | |||
|---|---|---|---|---|
| Control | 200 MPa | 400 Mpa | 600 Mpa | |
| pH | 7.48 ± 0.02 a | 7.42 ± 0.05 ab | 7.38 ± 0.03 b | 7.28 ± 0.02 c |
| Treatment | Sensory Evaluation | ||||
|---|---|---|---|---|---|
| Color | Smell | Taste | Appearance | Overall Acceptability | |
| Control | 5.30 ± 0.60 | 5.30 ± 1.10 | 5.30 ± 0.80 | 5.60 ± 0.60 | 5.50 ± 1.50 |
| 200 Mpa | 5.20 ± 0.80 | 5.40 ± 0.60 | 5.20 ± 0.60 | 5.50 ± 0.80 | 5.20 ± 1.00 |
| 400 Mpa | 5.00 ± 0.70 | 5.40 ± 0.90 | 5.20 ± 0.60 | 5.50 ± 0.90 | 5.10 ± 0.70 |
| 600 Mpa | 4.70 ± 0.60 | 5.10 ± 0.50 | 5.10 ± 1.60 | 5.20 ± 1.05 | 4.90 ± 1.40 |
| Treatment | Hunter Colors | ||
|---|---|---|---|
| ‘L’ Value | ‘a’ Value | ‘b’ Value | |
| Control | 22.30 ± 0.22 d | 8.85 ± 0.02 d | 13.92 ± 0.06 a |
| 200 Mpa | 28.91 ± 0.03 c | 12.69 ± 0.03 c | 10.89 ± 0.06 b |
| 400 Mpa | 40.54 ± 0.12 b | 15.49 ± 0.05 b | 8.81 ± 0.10 c |
| 600 Mpa | 41.85 ± 0.05 a | 16.67 ± 0.03 a | 7.78 ± 0.04 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.K.; Jeon, E.B.; Kim, J.Y.; Park, S.Y. Application of High-Pressure Processing (or High Hydrostatic Pressure) for the Inactivation of Human Norovirus in Korean Traditionally Preserved Raw Crab. Viruses 2023, 15, 1599. https://doi.org/10.3390/v15071599
Roy PK, Jeon EB, Kim JY, Park SY. Application of High-Pressure Processing (or High Hydrostatic Pressure) for the Inactivation of Human Norovirus in Korean Traditionally Preserved Raw Crab. Viruses. 2023; 15(7):1599. https://doi.org/10.3390/v15071599
Chicago/Turabian StyleRoy, Pantu Kumar, Eun Bi Jeon, Ji Yoon Kim, and Shin Young Park. 2023. "Application of High-Pressure Processing (or High Hydrostatic Pressure) for the Inactivation of Human Norovirus in Korean Traditionally Preserved Raw Crab" Viruses 15, no. 7: 1599. https://doi.org/10.3390/v15071599
APA StyleRoy, P. K., Jeon, E. B., Kim, J. Y., & Park, S. Y. (2023). Application of High-Pressure Processing (or High Hydrostatic Pressure) for the Inactivation of Human Norovirus in Korean Traditionally Preserved Raw Crab. Viruses, 15(7), 1599. https://doi.org/10.3390/v15071599







