Unusual Canine Distemper Virus Infection in Captive Raccoons (Procyon lotor)
Abstract
1. Introduction
2. Material and Methods
2.1. History, Examination, and Preliminary Treatment
2.2. Detection of CDV by RT-qPCR
2.3. Necropsy
2.4. Toxicology
2.5. Microbiology
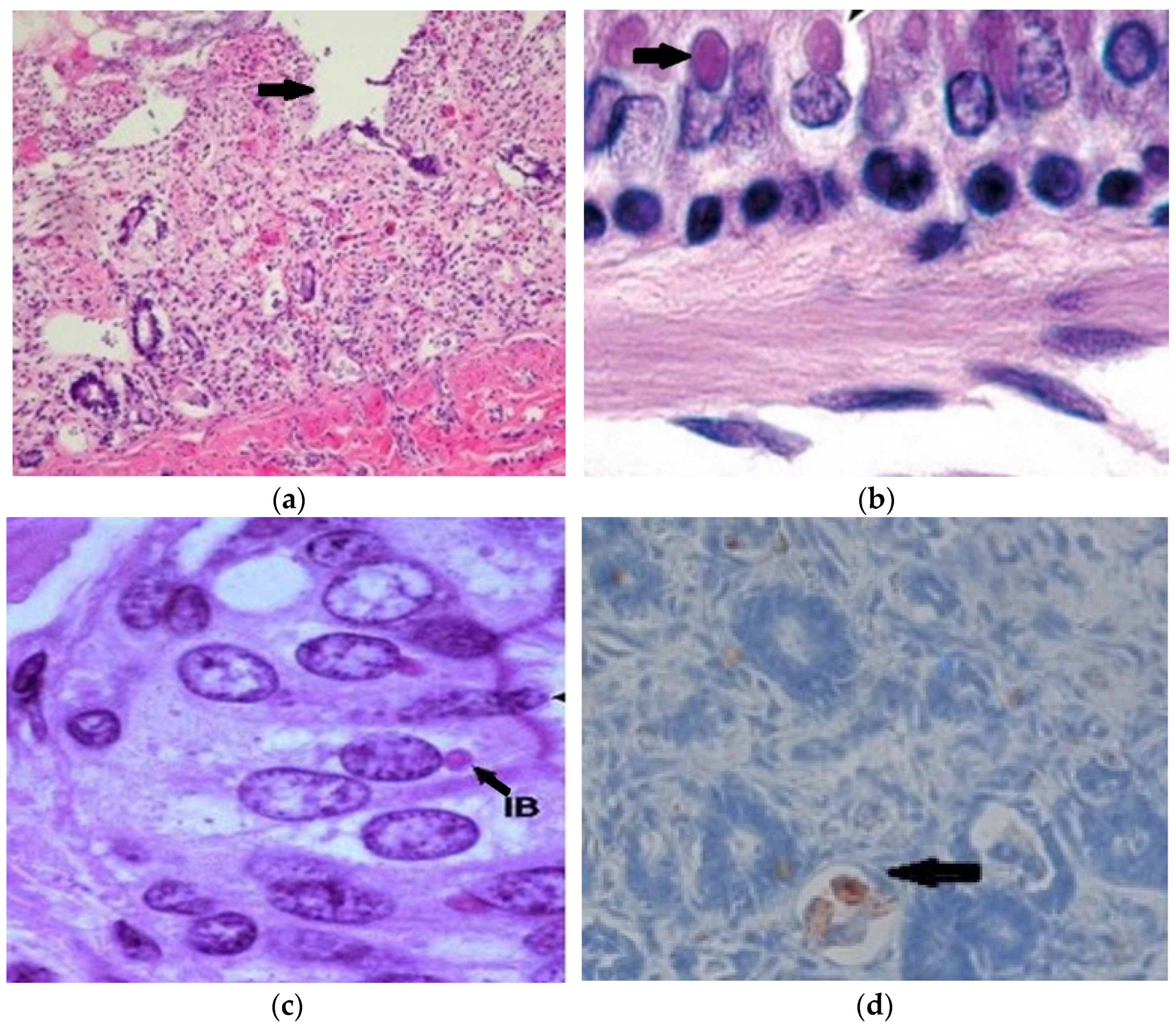
2.6. Histopathology and Immunohistochemistry
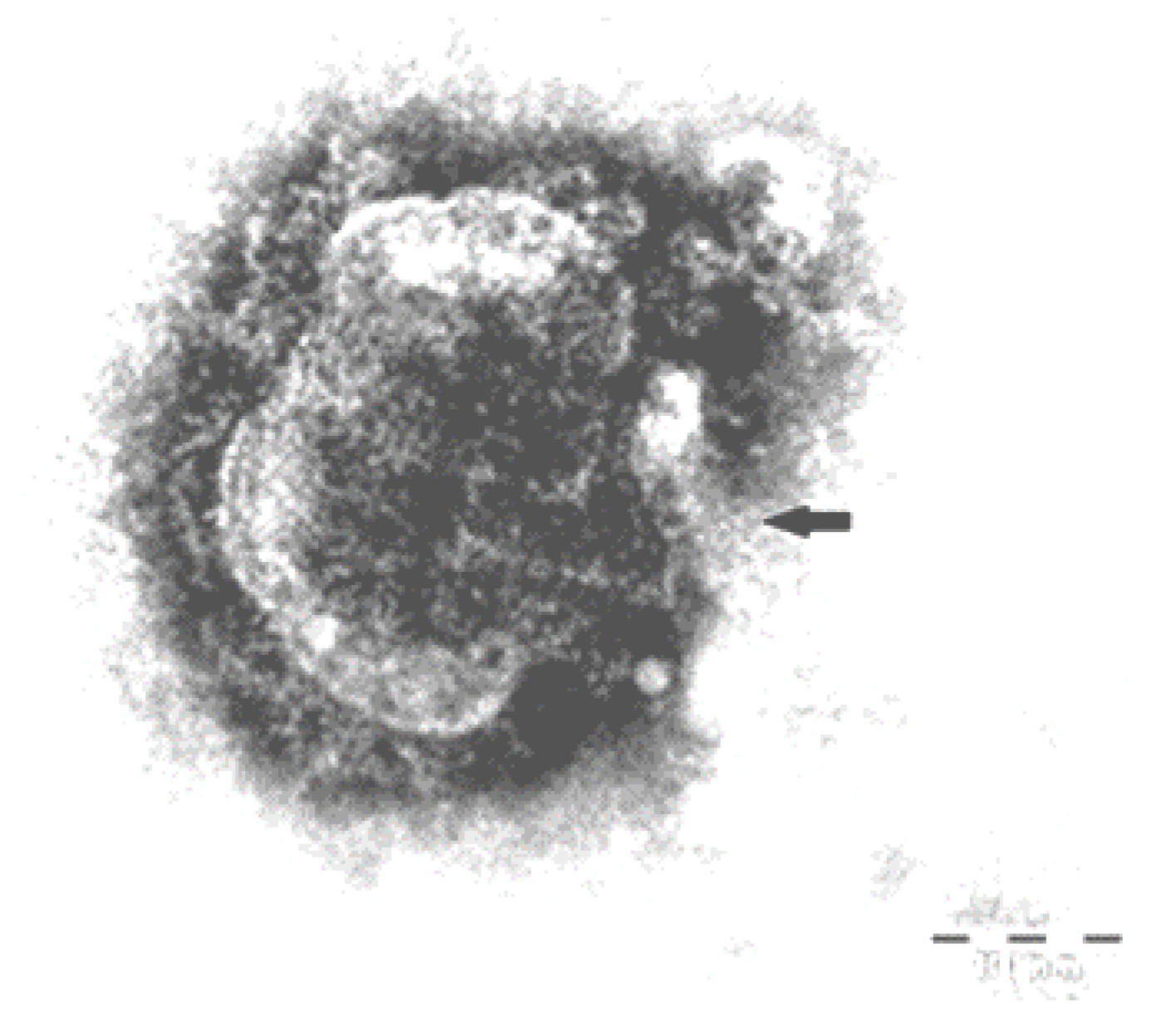
2.7. Immunohistochemistry and Electron Microscopy
3. Results
3.1. Clinical Outcome
3.2. Detection of CDV by RT-qPCR
3.3. Necropsy
3.4. Toxicology
3.5. Microbiology
3.6. Histopathology and Immunohistochemistry
3.7. Immunohistochemistry and Electron Microscope Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamir, A.N. Pathology of neurologic disorders of raccoons (Procyon lotor). J. Vet. Diagn. Investig. 2011, 23, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R.J. Canine distemper in terrestrial carnivores: A review. J. Zoo. Wildl. Med. 2000, 31, 441–451. [Google Scholar] [PubMed]
- Carré, H. Sur la maladie des jeuneschiens. CR Acad. Sci. 1905, 140, 1489–1491. [Google Scholar]
- Patel, J.R.; Heldens, J.G.; Bakonyi, T.; Rusvai, M. Important mammalian veterinary viral immunodiseases and their control. Vaccine 2012, 30, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, B.; Knotek, Z.; Farkas, J.; Ádám, É.; Dobay, O.; Nász, I. Adenovirus infection in cats. An epidemiological survey in the Czech Republic. Acta Vet. Brno. 1999, 68, 275–280. [Google Scholar] [CrossRef]
- Frölich, K.; Czupalla, O.; Haas, L.; Hentschke, J.; Dedek, J.; Fickel, J. Epizootiological investigations of canine distemper virus in free-ranging carnivores from Germany. Vet. Microbiol. 2000, 74, 283–292. [Google Scholar] [CrossRef]
- Munson, L. Feline Morbillivirus infection. In Infectious Diseases of Wild Mammals; Williams, E.S., Barker, I.K., Eds.; Blackwell Publishing: London, UK, 2001; pp. 59–62. [Google Scholar]
- Creevy, K.E.; Evans, J.B. Canine Distemper. In MDS Manual—Veterinary Manual; Merck & Co., Inc.: Rahway, NJ, USA, 2023. [Google Scholar]
- Shi, N.; Zhang, L.; Yu, X.; Zhu, X.; Zhang, S.; Zhang, D.; Duan, M. Insight into an Outbreak of Canine Distemper Virus Infection in Masked Palm Civets in China. Front. Vet. Sci. 2021, 8, 728238. [Google Scholar] [CrossRef]
- Appel, M.J.G. (Ed.) Canine distemper virus. In Virus Infections of Carnivores; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; pp. 133–159. [Google Scholar]
- Mochizuki, M.; Hashimoto, M.; Hagiwara, S.; Yoshida, Y.; Ishiguro, S. Genotypes of canine distemper virus determined by analysis of the hemagglutinin genes of recent isolates from dogs in Japan. J. Clin. Microbiol. 1999, 37, 2936–2942. [Google Scholar] [CrossRef]
- Pavlacik, L.; Celer, V.; Koubek, P.; Literak, I. Prevalence of canine distemper virus in wild mustelids in the Czech Republic and a case of canine distemper in young stone martens. Vet. Med.—Czech 2007, 52, 69–73. [Google Scholar] [CrossRef]
- Appel, M.J.G.; Summers, B. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet. Microbiol. 1995, 44, 187–191. [Google Scholar] [CrossRef]
- Harder, T.C.; Osterhaus, A.D.M.E. Canine distemper virus—A morbillivirus in search of new hosts? Trends Microbiol. 1997, 5, 120–124. [Google Scholar] [CrossRef]
- Lempp, C.; Spitzbarth, I.; Puff, C.; Cana, A.; Kegler, K.; Techangamsuwan, S.; Baumgärtner, W.; Seehusen, F. New Aspects of the Pathogenesis of Canine Distemper Leukoencephalitis. Viruses 2014, 6, 2571–2601. [Google Scholar] [CrossRef]
- Hoff, G.L.; Bigler, W.J.; Proctor, S.J.; Stallings, L.P. Epizootic of canine distemper virus infection among urban raccoons and gray foxes. J. Wildl. Dis. 1974, 10, 423–428. [Google Scholar] [CrossRef]
- Roscoe, D.E. Epizootiology of canine distemper in New Jersey raccoons. J. Wildl. Dis. 1993, 29, 390–395. [Google Scholar] [CrossRef]
- Schubert, C.A.; Barker, I.K.; Rosatte, R.C.; MacInnes, C.D.; Nudds, T.D. Effect of canine distemper on an urban raccoon population: An experiment. Ecol. Appl. 1998, 8, 379–387. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Dubach, J.; Kinsel, M.J.; Meehan, T.P.; Bocchetta, M.; Hungerford, L.L.; Sarich, N.A.; Witecki, K.E.; Braid, M.D.; Pedrak, C.; et al. Genetically distant American Canine distemper virus lineages have recently caused epizootics with somewhat different characteristics in raccoons living around a large suburban zoo in the USA. Virol. J. 2004, 1, 2. [Google Scholar] [CrossRef]
- Gutierrez, M.M.; Saenz, J.R. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar]
- Headley, S.A.; Oliveira, T.E.S.; Pereira, A.H.T.; Moreira, J.R.; Michelazzo, M.M.Z.; Pires, B.G.; Marutani, V.H.B.; Xavier, A.A.C.; Di Santis, G.W.; Garcia, J.L.; et al. Canine morbillivirus (canine distemper virus) with concomitant canine adenovirus, canine parvovirus-2, and Neospo-racaninum in puppies: A retrospective immunohistochemical study. Sci. Rep. 2018, 8, 13477. [Google Scholar] [CrossRef]
- Sykes, J.E. Canine Distemper Virus Infection. In Canine and Feline Infectious Diseases; W.B. Saunders: Saint Louis, MO, USA, 2014. [Google Scholar] [CrossRef]
- Jamison, R.K.; Lazar, E.C.; Binn, L.N.; Alexander, A.D. Survey for Antibodies to Canine Viruses in Selected Wild Mammals. J. Wild Dis. 1973, 9, 2–3. [Google Scholar] [CrossRef]
- Williams, E.S.; Barker, I.K. Infectious Diseases of Wild Mammals, 3rd ed.; Iowa State University Press: Ames, IA, USA, 2001; p. 43. [Google Scholar]
- Cranfield, M.R.; Barker, I.K.; Mehren, K.G.; Rapley, W.A. Canine Distemper in Wild Raccoons (Procyon lotor) at the Metropolitan Toronto Zoo. Can. Vet. J. 1984, 25, 63–66. [Google Scholar]
- Kameo, Y.; Nagao, Y.; Nishio, Y.; Shimoda, H.; Nakano, H.; Suzuki, K.; Une, K.; Sato, A.; Shimojima, M.; Maeda, K. Epizootic canine distemper virus infection among wild mammals. Vet. Microbiol. 2012, 154, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Nishio, Y.; Shiomoda, H.; Tamaru, S.; Shimojima, M.; Goto, M.; Une, Y.; Sato, A.; Ikebe, Y.; Maeda, K. An outbreak of canine distemper virus in tigers (Panthera tigris): Possible transmission from wild animals to zoo animals. J. Vet. Med. Sci. 2012, 74, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Guercio, A.; Mira, F.; Di Bella, S.; Gucciardi, F.; Lastra, A.; Purpari, G.; Castronovo, C.; Pennisi, M.; Di Marco Lo Presti, V.; Rizzo, M.; et al. Biomolecular Analysis of Canine Distemper Virus Strains in Two Domestic Ferrets (Mustela putorius furo). Vet. Sci. 2023, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.A.; Serge Lariviėre, S.; Messier, F. Survival and Body Condition of Raccoons at the Edge of the Range. J. Wildl. Manag. 2008, 72, 389–395. Available online: http://www.jstor.org/stable/25097551 (accessed on 15 March 2022). [CrossRef]
- Wang, J.; Wang, J.; Li, R.; Liu, L.; Yuan, W. Rapid and sensitive detection of canine distemper virus by real-time reverse transcription recombinase polymerase amplification. BMC Vet. Res. 2017, 13, 241. [Google Scholar] [CrossRef]
- Elia, G.; Decaro, N.; Martella, V.; Cirone, F.; Lucente, M.S.; Lorusso, E.; Di Trani, L.; Buonavoglia, C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 2006, 136, 171–176. [Google Scholar] [CrossRef]
- Habermann, R.T.; Herman, C.M.; Willams, F.P. Distemper in raccoons and foxes suspected of having rabies. J. Am. Vet. Med. Assoc. 1958, 132, 31–35. [Google Scholar]
- Espinal, M.A.; Díaz, F.J.; Ruiz-Saenz, J. Phylogenetic evidence of a new canine distemper virus lineage among domestic dogs in Colombia, South America. Vet. Microbiol. 2014, 172, 168–176. [Google Scholar] [CrossRef]
- Shabbir, M.Z.; Rabbani, M.; Ahmad, A.; Ahmed, A.; Muhammad, K.; Anwar, I. Comparative evaluation of clinical samples from naturally infected dogs for early detection of canine distemper virus. Turk. J. Vet. Anim. Sci. 2010, 34, 547–552. [Google Scholar] [CrossRef]
- Robinson, V.B.; Newberne, J.W.; Brooks, D.M. Distemper in the American raccoon (Procyon lotor). J. Am. Vet. Med. Assoc. 1957, 131, 276–278. [Google Scholar]
- Halecker, S.; Bock, S.; Beer, M.; Hoffmann, B.A. New Molecular Detection System for Canine Distemper Virus Based on a Double-Check Strategy. Viruses 2021, 13, 1632. [Google Scholar] [CrossRef]
- Maurer, K.E.; Nielsen, S.W. Neurologic disorders in the raccoon in northeastern United States. J. Am. Vet. Med. Assoc. 1981, 179, 1095–1098. [Google Scholar]
- Niederwerder, M.; Boyer, N. Canine Distemper: A Recent Uptick in the Number of Positive Raccoon Diagnostic Samples Underscores the Importance of Vaccination in Dogs. Available online: https://ksvdl.org/resources/news/diagnostic_insights_for_technicians/october2017/raccoons_CDV.html (accessed on 18 March 2022).
- Konjević, D.; Sabočanec, R.; Grabarević, Z.; Zurbriggen, A.; Bata, I.; Beck, A.; Kurilj, A.G.; Cvitković, D. Canine distemper in Siberian tiger cubs from Zagreb ZOO: Case report. Acta Vet. Brno 2011, 80, 47–50. [Google Scholar] [CrossRef]
- Alfano, F.; Fusco, G.; Mari, V.; Occhiogrosso, L.; Miletti, G.; Brunetti, R.; Galiero, G.; Desario, C.; Cirilli, M.; Decaro, N. Circulation of pantropic canine coronavirus in autochthonous and imported dogs, Italy. Transbound. Emerg. Dis. 2020, 67, 1991–1999. [Google Scholar] [CrossRef]
- Pratelli, A.; Martella, V.; Pistello, M.; Elia, G.; Decaro, N.; Buonavoglia, D.; Camero, M.; Tempesta, M.; Buonavoglia, C. Identification of coronaviruses in dogs that segregate separately from the canine coronavirus genotype. J. Virol. Methods 2003, 107, 213–222. [Google Scholar] [CrossRef]
- Linnè, T. Differences in the E3 regions of the canine adenovirus type 1 and type 2. Virus Res. 1992, 23, 119–133. [Google Scholar] [CrossRef]
- Decaro, N.; Amorisco, F.; Desario, C.; Lorusso, E.; Camero, M.; Bellacicco, A.L.; Sciarretta, R.; Lucente, M.S.; Martella, V.; Buonavoglia, C. Development and validation of a real-time PCR assay for specific and sensitive detection of canid herpesvirus 1. J. Virol. Methods 2010, 169, 176–180. [Google Scholar] [CrossRef]
- Martella, V.; Bányai, K.; Matthijnssens, J.; Buonavoglia, C.; Ciarlet, M. Zoonotic aspects of rotavirus. Vet. Microbiol. 2010, 140, 246–255. [Google Scholar] [CrossRef]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Di Trani, L.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet. Microbiol. 2005, 105, 19–28. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; Budaszewski, R.F.; CláudioWageck, C.C.; Ruiz-Saenzet, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef]
- Kazacos, K.R.; Wiriz, W.L.; Burger, P.P.; Chrisimas, C.S. Raccoon ascarid larvae as a cause of fatal central nervous system disease in subhuman primates. J. Am. Vet. Med. Assoc. 1981, 179, 1089–1094. [Google Scholar] [PubMed]
- Koutinas, A.F.; Baumgärtner, W.; Tontis, D.; Polizopoulou, Z.; Saridomichelakis, M.N.; Lekkas, S. Histopathology and immunohistochemistry of canine distemper virus-induced footpad hyperkeratosis (hard Pad disease) in dogs with natural canine distemper. Vet. Pathol. 2004, 41, 2–9. (accessed on 15 April 2022). [Google Scholar] [CrossRef] [PubMed]
- Haines, D.M.; Martin, K.M.; Chelack, B.J.; Sargent, R.A.; Outerbridge, C.A.; Clark, E.G. Immunohistochemical detection of canine distemper virus in haired skin, nasal mucosa, and footpad epithelium: A method for antemortem diagnosis of infection. J. Vet. Diagn. Investig. 1999, 11, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.G.; Karikalan, M.; Siddappa, C.M.; Mahendran, K.; Srivastava, G.; Rajak, K.K.; Bhardwaj, Y.; Varshney, R.; War, Z.A.; Singh, R.; et al. Molecular and pathological screening of canine distemper virus in Asiatic lions, tigers, leopards, snow leopards, clouded leopards, leopard cats, jungle cats, civet cats, fishing cat, and jaguar of different states, India. Infect. Genet. Evol. 2022, 98, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruíz-Sáenz, J. Evolution and Interspecies Transmission of Canine Distemper Virus—An Outlook of the Diverse Evolutionary Landscapes of a Multi-Host Virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Baumgärtner, W.; Wohlsein, P. Cross-species transmission of canine distemper virus—An update. One Health 2015, 1, 49–59. [Google Scholar] [CrossRef]
- Appel, M.J.; Yates, R.A.; Foley, G.L.; Bernstein, J.J.; Santinelli, S.; Spelman, L.H.; Miller, L.D.; Arp, L.H.; Anderson, M.; Barr, M.; et al. Canine distemper epizootic in lions, tigers, and leopards in North America. J. Vet. Diagn. Investig. 1994, 6, 277–288. [Google Scholar] [CrossRef]
- Alfano, F.; Lanave, G.; Lucibelli, M.G.; Miletti, G.; D’Alessio, N.; Gallo, A.; Auriemma, C.; Amoroso, M.G.; Lucente, M.S.; De Carlo, E.; et al. Canine Distemper Virus in Autochtonous and Imported Dogs, Southern Italy (2014–2021). Animals 2022, 12, 2852. [Google Scholar] [CrossRef]
- Alfano, F.; Dowgier, G.; Valentino, M.P.; Galiero, G.; Tinelli, A.; Decaro, N.; Fusco, G. Identification of Pantropic Canine Coronavirus in a Wolf (Canis lupus italicus) in Italy. J. Wildl. Dis. 2019, 55, 504–508. [Google Scholar]
- Sanjay, K.S.; Yeary, T.J. Canine Distemper Spillover in Domestic Dogs from Urban Wildlife. Vet. Clin. Small Anim. 2011, 41, 1069–1086. [Google Scholar] [CrossRef]
- Mee, A.P.; Dixon, J.A.; Hoyland, J.A.; Davies, M.; Selby, P.L.; Mawer, E.B. Detection of canine distemper virus in 100% of Paget’s disease samples by in situ-reverse transcriptase-polymerase chain reaction. Bone 1998, 23, 171–175. [Google Scholar] [CrossRef]
- Selby, P.L.; Davies, M.; Mee, A.P. Canine distemper virus induces human osteoclastogenesis through NF-kappaB and sequestosome 1/P62 activation. J. Bone Miner. Res. 2006, 21, 1750–1756. [Google Scholar] [CrossRef]
- Weckworth, J.K.; Davis, B.W.; Dubovi, E.; Fountain-Jones, N.; Packer, C.; Cleaveland, S.; Craft, M.E.; Eblate, E.; Schwartz, M.; Mills, L.S.; et al. Cross-species transmission and evolutionary dynamics of canine distemper virus during a spillover in African lions of Serengeti National Park. Molec. Ecol. 2020, 29, 4308–4321. [Google Scholar] [CrossRef]
- Stope, M.B. The Raccoon (Procyon lotor) as a Neozoon in Europe. Animals 2023, 13, 273. [Google Scholar] [CrossRef]
- Loots, A.K.; Mokgokong, P.S.; Mitchell, E.; Venter, E.H.; Kotze, A.; Dalton, D.L. Phylogenetic analysis of canine distemper virus in South African wildlife. PLoS ONE 2018, 13, e0199993. [Google Scholar] [CrossRef]

| Sample Code | Clinical Signs | Ct Value 1 | Results |
|---|---|---|---|
| 1 | Healthy, no clinical signs | 33.8 | positive |
| 2 | Healthy, no clinical signs | 31.4 | positive |
| 3 | Healthy, no clinical signs | 32.6 | positive |
| 4 | Healthy, no clinical signs | 34.5 | positive |
| 5 | Healthy, no clinical signs | 32.7 | positive |
| 6 | Clinical signs: diarrhea, hematemesis, death | 19.4 | positive |
| 7 | Clinical signs: severe diarrhea, hematemesis, dehydration, death | 21.2 | positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancu, A.C.; Voia, O.S.; Boldura, O.M.; Pasca, S.A.; Luca, I.; Hulea, A.S.; Ivan, O.R.; Dragoescu, A.A.; Lungu, B.C.; Hutu, I. Unusual Canine Distemper Virus Infection in Captive Raccoons (Procyon lotor). Viruses 2023, 15, 1536. https://doi.org/10.3390/v15071536
Stancu AC, Voia OS, Boldura OM, Pasca SA, Luca I, Hulea AS, Ivan OR, Dragoescu AA, Lungu BC, Hutu I. Unusual Canine Distemper Virus Infection in Captive Raccoons (Procyon lotor). Viruses. 2023; 15(7):1536. https://doi.org/10.3390/v15071536
Chicago/Turabian StyleStancu, Adrian Constantin, Octavian Sorin Voia, Oana Maria Boldura, Sorin Aurelian Pasca, Iasmina Luca, Anca Sofiana Hulea, Oana Roxana Ivan, Alina Andreea Dragoescu, Bianca Cornelia Lungu, and Ioan Hutu. 2023. "Unusual Canine Distemper Virus Infection in Captive Raccoons (Procyon lotor)" Viruses 15, no. 7: 1536. https://doi.org/10.3390/v15071536
APA StyleStancu, A. C., Voia, O. S., Boldura, O. M., Pasca, S. A., Luca, I., Hulea, A. S., Ivan, O. R., Dragoescu, A. A., Lungu, B. C., & Hutu, I. (2023). Unusual Canine Distemper Virus Infection in Captive Raccoons (Procyon lotor). Viruses, 15(7), 1536. https://doi.org/10.3390/v15071536








