Treatment Outcomes of Sofosbuvir/Velpatasvir/Voxilaprevir in Direct-Acting Antiviral-Experienced Hepatitis C Virus Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Outcomes Assessed
2.5. Data Synthesis and Analysis
2.6. Quality Assessment
3. Results
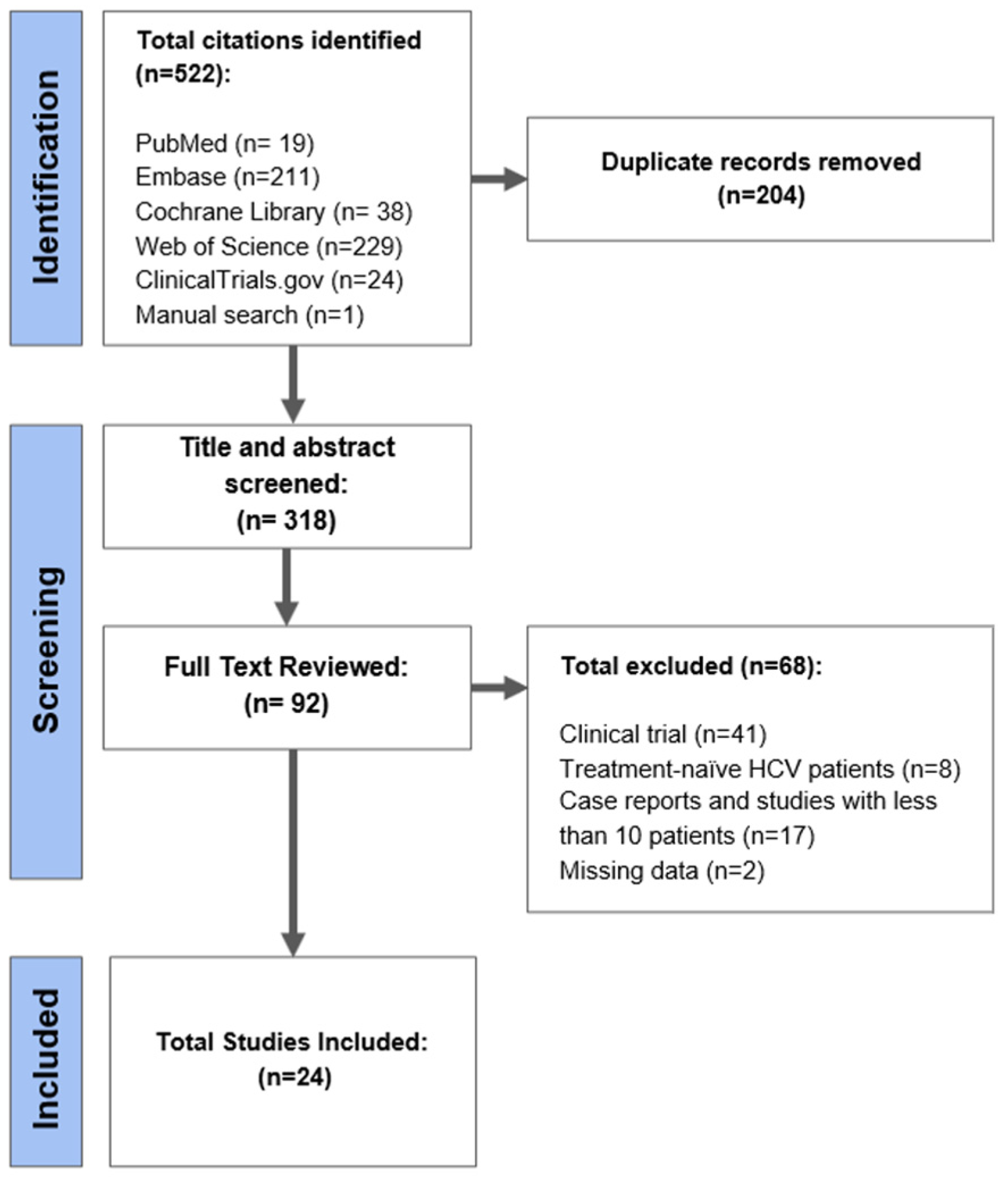
3.1. Search Results
3.2. Population Characteristics
3.3. Sustained Virological Response at 12 Weeks (SVR12)
3.3.1. HCV Genotype
3.3.2. Liver Cirrhosis
3.3.3. Prior Sofosbuvir/Velpatasvir Exposure
3.3.4. Hepatocellular Carcinoma
3.3.5. RAS Mutation
3.3.6. Ribavirin
3.4. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blach, S.; Terrault, N.A.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Löve, A. Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Gamkrelidze, I.; Pawlotsky, J.M.; Lazarus, J.V.; Feld, J.J.; Zeuzem, S.; Bao, Y.; dos Santos, A.G.P.; Gonzalez, Y.S.; Razavi, H. Progress towards hepatitis C virus elimination in high-income countries: An updated analysis. Liver Int. 2021, 41, 456–463. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, R.; Degasperi, E.; Anolli, M.P.; Fanetti, I.; Borghi, M.; Soffredini, R.; Iavarone, M.; Tosetti, G.; Perbellini, R.; Sangiovanni, A.; et al. Incidence of liver- and non-liver-related outcomes in patients with HCV-cirrhosis after SVR. J. Hepatol. 2022, 76, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.J.; Thurairajah, P.H.; Kumar, R.; Tan, J.; Fock, K.M.; Law, N.M.; Li, W.; Kwek, A.; Bin Tan, Y.; Koh, J.; et al. Efficacy and safety of sofosbuvir/velpatasvir in a real-world chronic hepatitis C genotype 3 cohort. J. Gastroenterol. Hepatol. 2021, 36, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.H.; Xu, W.X.F.; Low, J.T.; Tay, W.X.; Ang, L.S.; Tam, Y.C.; Thurairajah, P.H.; Kumar, R.; Wong, Y.J. Efficacy and safety of sofosbuvir/velpatasvir with or without ribavirin in hepatitis C genotype 3 compensated cirrhosis: A meta-analysis. World J. Hepatol. 2022, 14, 1248–1257. [Google Scholar] [CrossRef]
- Liu, C.H.; Peng, C.Y.; Liu, C.J.; Chen, C.Y.; Lo, C.C.; Tseng, K.C.; Su, P.Y.; Kao, W.Y.; Tsai, M.C.; Tung, H.D.; et al. Sofosbuvir/velpatasvir/voxilaprevir for patients with chronic hepatitis C virus infection previously treated with NS5A direct-acting antivirals: A real-world multicenter cohort in Taiwan. Hepatol. Int. 2023, 17, 291–302. [Google Scholar] [CrossRef]
- Ghany, M.G.; Morgan, T.R.; AASLD/IDSA HCV Guidance Panel. Recommendations for testing, managing, and treating hepatitis C. Updated August 27, 2020. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Bourlière, M.; Gordon, S.C.; Flamm, S.L.; Cooper, C.L.; Ramji, A.; Tong, M.; Ravendhran, N.; Vierling, J.M.; Tran, T.T.; Pianko, S.; et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N. Engl. J. Med. 2017, 376, 2134–2146. [Google Scholar] [CrossRef]
- Llaneras, J.; Riveiro-Barciela, M.; Lens, S.; Diago, M.; Cachero, A.; García-Samaniego, J.; Conde, I.; Arencibia, A.; Arenas, J.; Gea, F.; et al. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs. J. Hepatol. 2019, 71, 666–672. [Google Scholar] [CrossRef]
- Degasperi, E.; Spinetti, A.; Lombardi, A.; Landonio, S.; Rossi, M.C.; Pasulo, L.; Pozzoni, P.; Giorgini, A.; Fabris, P.; Romano, A.; et al. Real-life effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous DAA failure. J. Hepatol. 2019, 71, 1106–1115. [Google Scholar] [CrossRef]
- Pearlman, B.; Perrys, M.; Hinds, A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am. J. Gastroenterol. 2019, 114, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, K.; Yang, Q.; Ma, C.; Jin, Q.; Hu, A.; Jin, J.; Yan, D.; Lv, F.; Shi, Y.; et al. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir retreatment in hepatitis C patients with different genotypes with DAAS failure in East China. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Wong, Y.J.; Kumar, R.; Tan, J.; Liu, C.H.; Hui, V.K.; Tan, S.S.; Kao, J.H.; Wong, G.H.; Thurairajah, P.H. Treatment outcomes of sofosbuvir/velpatasvir/voxilaprevir among NS5A inhibitor-experienced patients with hepatitis C: Real-world data from a multicenter Asian registry. J. Gastroenterol. Hepatol. 2022, 37, 1642–1644. [Google Scholar] [CrossRef]
- Heo, J.; Ahn, S.H.; Kim, Y.J.; Lee, S.W.; Lim, Y.-S.; Lee, Y.-J.; Yoon, K.T.; Byun, K.-S.; Jung, Y.J.; Tak, W.Y.; et al. The efficacy and safety of sofosbuvir/velpatasvir and sofosbuvir/velpatasvir/voxilaprevir in HCV infected Korean patients. Hepatol. Int. 2022, 16 (Suppl. 1), S1–S503. [Google Scholar] [CrossRef]
- Gheorghe, L.; Preda, C.; Trifan, A.; Manuc, M.; Staniciu, C.; Istratescu, D.; Popescu, C.P.; Diculescu, M.M.; Tieranu, C.G.; Manuc, T.; et al. Real-World Efficacy and Safety of Sofosbuvir + Velpatasvir + Voxilaprevir in Romanian Patients with Genotype 1b HCV Infection Non-reponders to DAAs Therapy. J. Gastrointest. Liver Dis. 2022, 31, 437–443. [Google Scholar] [CrossRef]
- Gupta, N.; Manirambona, L.; Shumbusho, F.; Kabihizi, J.; Murangwa, A.; Serumondo, J.; Makuzam, J.D.; Nsanzimana, S.; Muvunyi, C.M.; Mukabatsinda, C.; et al. Safety and efficacy of sofosbuvir-velpatasvir-voxilaprevir for re-treatment of chronic hepatitis C virus infection in patients with previous direct-acting antiviral treatment failure in Rwanda (SHARED-3): A single-arm trial. Lancet Gastroenterol. Hepatol. 2022, 7, 542–551. [Google Scholar] [CrossRef]
- Shousha, H.I.; Abdelghafour, R.; Dabees, H.; AbdelRazek, W.; Said, M. Three regimens for re-treatment failure of Sofosbuvir-based therapy for chronic hepatitis-C genotype-4: A cohort study. Revista do Instituto de Medicina Tropical de São Paulo 2022, 64, e50. [Google Scholar] [CrossRef]
- Brown, P.; Demertzis, Z.; Jafri, S.-M. Tu1500—Single Center Experience with Sofosbuvir/Velpatasvir/Voxilaprevir (VOSEVI) Based Therapy for Chronic Hepatitis C Infection. Gastroenterology 2019, 156, S1344. [Google Scholar] [CrossRef]
- Graf, C.; Dietz, J.; Müllhaupt, B.; Buggisch, P.; Schattenberg, J.; Antoni, C.; Mauss, S.; Durmashkina, E.; Niederau, C.; Discher, T.; et al. Effectiveness of voxilaprevir/velpatasvir/sofosbuvir in hepatitis C patients previously treated with direct-acting antiviral agents (DAA). J. Hepatol. 2022, 77, S16. [Google Scholar] [CrossRef]
- Ruane, P.; Strasser, S.I.; Gane, E.J.; Hyland, R.H.; Shao, J.; Dvory-Sobol, H.; Tran, T.; Stamm, L.M.; Brainard, D.M.; Nyberg, L.; et al. Sofosbuvir/velpatasvir/voxilaprevir for patients with HCV who previously received a sofosbuvir/velpatasvir-containing regimen: Results from a retreatment study. J. Viral Hepat. 2019, 26, 770–773. [Google Scholar] [CrossRef]
- Hezode, C.; Guyader, D.; Nguyen-Khac, E.; Larrey, D.; Truchi, R.; Di Martino, V.; Calmus, Y.; Franza, A.M.; Giuily, N.; Mokhtari, S.; et al. THU-142-Sofosbuvir + velpatasvir + voxilaprevir in DAA failure patients with cirrhosis: Final results of the French compassionate use program. J. Hepatol. 2019, 70, e224. [Google Scholar] [CrossRef]
- Janjua, N.; Wilton, J.; Cook, D.; Wong, S.; Butt, Z.; Bartlett, S.; Pearce, M.; Ramji, A.; Yoshida, E.; Yu, A.; et al. Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir as a hepatitis C virus infection salvage treatment. J. Hepatol. 2020, 73, S356–S357. [Google Scholar] [CrossRef]
- Belperio, P.S.; Shahoumian, T.A.; Loomis, T.P.; Backus, L.I. Real- world effectiveness of sofosbuvir/velpatasvir/voxi-laprevir in 573 direct-acting antiviral experienced hepatitis C patients. J. Viral Hepat. 2019, 26, 980–990. [Google Scholar]
- Salazar, A.D.; Dietz, J.; di Maio, V.C.; Vermehren, J.; Paolucci, S.; Müllhaupt, B.; Coppola, N.; Cabezas, J.; Stauber, R.E.; Puoti, M.; et al. Prevalence of resistance-associated substitutions and retreatment of patients failing a glecaprevir/pibrentasvir regi- men. J. Antimicrob. Chemother. 2020, 75, 3349–3358. [Google Scholar] [CrossRef]
- Pisaturo, M.; Starace, M.; Minichini, C.; De Pascalis, S.; Occhiello, L.; Fraia, A.D.; Messina, V.; Sangiovanni, V.; Claar, E.; Coppola, N.; et al. Virological patterns of hepatitis C virus patients with failure to the current-generation direct-acting antivirals. Int. J. Antimicrob. Agents 2020, 56, 106067. [Google Scholar] [CrossRef]
- Vermehren, J.; Serfert, Y.; Cornberg, M.; Stoehr, A.; Klinker, H.; Simon, K.-G.; Teuber, G.; Deterding, K.; Wiesch, J.S.Z.; Jung, M.-C.; et al. Sofos-buvir, velpatasvir, and voxilaprevir for patients with failure of previous direct-acting antiviral therapy for chronic hepatitis C: Results from the German Hepatitis C-Registry (DHC-R). Zeitschrift für Gastroenterologie 2020, 58, 841–846. [Google Scholar]
- Da, B.L.; Lourdusamy, V.; Kushner, T.; Dieterich, D.; Saberi, B. Efficacy of sofosbuvir/velpatasvir/voxilaprevir in direct-acting antiviral experienced patients with hepatitis C virus. Eur. J. Gastroenterol. Hepatol. 2021, 33, 859–861. [Google Scholar] [CrossRef]
- Onofrio, F.Q.; Cooper, C.; Borgia, S.M.; Vachon, M.-L.; Ramji, A.; Lilly, L.B.; Wong, A.; Booth, J.; Sattar, I.; Morales, H.; et al. Salvage Therapy with Sofosbuvir/Velpatasvir/Voxilaprevir in DAA-experienced Patients: Results from a Prospective Canadian Registry. Clin. Infect. Dis. 2021, 72, e799–e805. [Google Scholar] [CrossRef] [PubMed]
- Papaluca, T.; Roberts, S.K.; Strasser, S.I.; Stuart, K.A.; Farrell, G.; MacQuillan, G.; Dore, G.J.; Wade, A.J.; George, J.; Hazeldine, S.; et al. Efficacy and Safety of Sofosbuvir/Velpatasvir/Voxilaprevir for Hepatitis C Virus (HCV) NS5A-Inhibitor Experienced Patients With Difficult to Cure Characteristics. Clin. Infect. Dis. 2020, 73, e3288–e3295. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Bradshaw, D.; Mbisa, J.L.; Manso, C.F.; Bibby, D.F.; Singer, J.B.; Thomson, E.C.; Filipe, A.D.S.; Aranday-Cortes, E.; Ansari, M.A.; et al. Real world SOF/VEL/VOX retreatment outcomes and viral resistance analysis for HCV patients with prior failure to DAA therapy. J. Viral Hepat. 2021, 28, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- El-Kassas, M.; Emadeldeen, M.; Hassany, M.; Esmat, G.; Gomaa, A.A.; El-Raey, F.; Congly, S.E.; Liu, H.; Lee, S.S. A randomized-controlled trial of SOF/VEL/VOX with or without ribavirin for retreatment of chronic hepatitis C. J. Hepatol. 2023. [Google Scholar] [CrossRef]
- Wong, Y.J.; Tran, S.; Huang, C.F.; Hsu, Y.-C.; Preda, C.; Toyoda, H.; Liu, J.; Jun, D.W.; Landis, C.; Huang, D.Q.; et al. Real-world treatment outcome with protease inhibitor versus non-PI based direct acting antiviral (DAA) in decompensated hepatitis C (HCV) cirrhosis: A REAL-C study with inverse probability of treatment weighting (IPTW). Hepatology 2022, 76, 48. [Google Scholar]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218, Erratum in J. Hepatol. 2023, 78, 452. [Google Scholar]



| No | Authors, Years | Study Design | Country | Sample Size (n) | Genotype n, (%) | Prior SOF/VEL (n/n) | Use with RBV (n) | RAS Testing at Baseline (%) | SVR12 (PP) | Cirrhosis (%) | Serious AE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Liu CH, 2023 [6] | Prospective, multicenter | Taiwan | 107 | GT1a: 1.9/GT1b: 37.4/GT2: 36.4/GT3: 6.5/GT6: 16.8/GT intermediate: 0.9 | 22/107 | 1/107 | 64/99 | 104/104 | 0 | 2/104 |
| 2 | Chen S, 2022 [15] | Multicenter cohort | China | 13 | GT3b: 46.2/GT6: 38.4/GT1b: 15.4 | 10/13 | 0/13 | NA | 13/13 | 0 | 0 |
| 3 | YJ Wong, 2022 [16] | Retrospective, multicenter | Singapore, Taiwan, Hong Kong, Malaysia | 25 | G1: 8/G2: 8/G3: 56/G6: 8/Indeterminate: 20 | 20/25 | 0/25 | NA | 22/25 | 16/25 | 0 |
| 4 | Heo, J, 2022 [17] | Prospective, multicenter trial | Korea | 33 | G1b: 97/GT2: 3 | 0/33 | 0/33 | 33/33 | 33/33 | 9/33 | 1/33 |
| 5 | Gheorghe L, 2022 [18] | Retrospective, multicenter | Romania | 143 | G1b: 100 | 0/143 | 0/143 | NA | 141/143 | 0 | 1/143 |
| 6 | EL Kassas M, 2023 [34] | RCT, multicenter | Egypt | 315 | NA | 0/281 | 140/281 | NA | 276/298 | 104/281 | 1/281 |
| 7 | Gupta N, 2022 [19] | Prospective, multicenter trial | Rwanda | 40 | GT3: 2.5/G4: 95/Unknown: 2.5 | 0/40 | 0/40 | 33/40 | 39/40 | NA | 4/40 |
| 8 | Shousha HI, 2022 [20] | Prospective, multicenter | Egypt | 45 | G4: 100 | 0/45 | 0/45 | NA | 44/45 | 21/45 | 0 |
| 9 | Brown, 2019 [21] | Retrospective, single center | USA | 22 | GT1: 81.8/GT3: 9.1/GT4: 9.1 | 0/22 | 0/22 | NA | 22/22 | NA | 3/22 |
| 10 | Hezode,2019 [24] | Multicenter cohort | France | 46 | GT1: 32.6/GT2: 8.7/GT3: 39.1/GT4: 17.4/GT5: 2.2 | 0/46 | 10/46 | 34/39 | 42/44 | 41/46 | 3/44 |
| 11 | Janjua, 2020 [25] | Multicenter cohort | Canada | 191 | GT1: 54.5/GT2: 8.9/GT3: 32.5/others: 4.2 | 27/191 | 38/191 | NA | 182/191 | NA | 0 |
| 12 | Belperio, 2019 [26] | Retrospective, multicenter | USA | 573 | GT1: 85.5/GT2: 10.5/GT3: 26.7/GT4: 6.3 | 49/573 | 0/573 | 0 | 501/551 | 198/573 | 0 |
| 13 | Llaneras, 2019 [9] | Prospective, multicenter | Spain | 137 | GT1: 59.9/GT2: 5.1/GT3: 21.9/GT4: 10.2/Others: 2.9 | 8/137 | 0/137 | 43/49 | 128/135 | 46/137 | 0 |
| 14 | Degasperi, 2019 [10] | Retrospective, multicenter | Italy | 179 | GT1: 57.5/GT2: 10/GT3: 23.5/GT4: 8.9 | 36/179 | 39/179 | 94/115 | 162/169 | 78/179 | 11/179 |
| 15 | Pearlman, 2019 [11] | Prospective, multicenter | USA | 31 | GT1: 41.9/GT3: 58.1 | 0/31 | 0/31 | 28/31 | 29/31 | 18/31 | 0 |
| 16 | Salazar, 2020 [27] | Prospective, multicenter | Germany, Italy, Spain | 56 | GT1: 36.7/GT2: 22.2/GT3: 40/GT4: 1.1 | 0/56 | 9/56 | 53/85 | 45/46 | 5/46 | 0 |
| 17 | Pisaturo, 2020 [28] | Prospective, single center | Italy | 21 | GT1: 90.5/GT3: 9.5 | 17/61 | 0/21 | 19/21 | 21/21 | 6/21 | 0 |
| 18 | Vermehren, 2020 [29] | Prospective, multicenter | Germany | 110 | GT1: 64.5/GT3: 30.9/GT4: 4.5 | 18/110 | 4/110 | NA | 100/102 | 30/110 | 6/110 |
| 19 | Da, 2021 [30] | Retrospective, single-center | USA | 18 | GT1: 77.7/GT2: 11.1/GT3: 11.1 | 4/18 | 4/18 | 8/14 | 18/18 | 6/18 | 0 |
| 20 | Onofrio, 2021 [31] | Prospective, multicenter | Canada | 128 | GT1: 60.2/GT2: 3.1/GT3: 30.5/GT4: 4.7/GT6: 10.8/mixed: 0.8 | 35/128 | 26/128 | 28/51 | 123/128 | 56/128 | 0 |
| 21 | Papaluca, 2021 [32] | Retrospective, multicenter | Australian | 97 | GT1: 23.7/GT3: 72.2/GT4: 1/GT6: 3 | 19/97 | 3/97 | 49/54 | 82/91 | 76/97 | 3/97 |
| 22 | Smith, 2021 [33] | Prospective, multicenter | England | 144 | GT1: 45.8/GT2: 2.1/GT3: 43/GT4: 6.9/GT6: 2 | 17/144 | 0/144 | 101/144 | 129/144 | 58/144 | 0 |
| 23 | Graf C, 2022 [22] | Prospective, multicenter | Germany, Austria, Switzerland, Belgium | 416 | GT1: 53.8/GT2: 1.9/GT3: 38.9/GT4: 6 | 0/416 | 0/416 | 16/416 | 401/416 | NA | 0 |
| 24 | Ruane, 2019 [23] | Open-label trial | USA | 31 | GT1: 61.3/GT2: 6.5/GT3: 25.8/GT4: 3.2/GT5: 3.2 | 31/31 | 0/31 | 31/31 | 31/31 | 15/31 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devan, P.; Tiong, K.L.A.; Neo, J.E.; Mohan, B.P.; Wijarnpreecha, K.; Tam, Y.C.S.; Coppola, N.; Preda, C.M.; Wong, Y.J. Treatment Outcomes of Sofosbuvir/Velpatasvir/Voxilaprevir in Direct-Acting Antiviral-Experienced Hepatitis C Virus Patients: A Systematic Review and Meta-Analysis. Viruses 2023, 15, 1489. https://doi.org/10.3390/v15071489
Devan P, Tiong KLA, Neo JE, Mohan BP, Wijarnpreecha K, Tam YCS, Coppola N, Preda CM, Wong YJ. Treatment Outcomes of Sofosbuvir/Velpatasvir/Voxilaprevir in Direct-Acting Antiviral-Experienced Hepatitis C Virus Patients: A Systematic Review and Meta-Analysis. Viruses. 2023; 15(7):1489. https://doi.org/10.3390/v15071489
Chicago/Turabian StyleDevan, Pooja, Kai Le Ashley Tiong, Jean Ee Neo, Babu P. Mohan, Karn Wijarnpreecha, Yew Chong Steve Tam, Nicola Coppola, Carmen Monica Preda, and Yu Jun Wong. 2023. "Treatment Outcomes of Sofosbuvir/Velpatasvir/Voxilaprevir in Direct-Acting Antiviral-Experienced Hepatitis C Virus Patients: A Systematic Review and Meta-Analysis" Viruses 15, no. 7: 1489. https://doi.org/10.3390/v15071489
APA StyleDevan, P., Tiong, K. L. A., Neo, J. E., Mohan, B. P., Wijarnpreecha, K., Tam, Y. C. S., Coppola, N., Preda, C. M., & Wong, Y. J. (2023). Treatment Outcomes of Sofosbuvir/Velpatasvir/Voxilaprevir in Direct-Acting Antiviral-Experienced Hepatitis C Virus Patients: A Systematic Review and Meta-Analysis. Viruses, 15(7), 1489. https://doi.org/10.3390/v15071489








