3.1. The Multi-Conformer Hexapeptide Library
A total of 810 hexapeptides were generated from the high-occurrence amino acids in the consensus sequence over all cleavage sites derived from Ullrich and Nitsche [
10]. Using the information from Ullrich and Nitsche [
10], octapeptides were initially considered, which would have resulted in 61,000 possible substrates. That many substrates would have required computational power and resources beyond the feasibility of our study. Hexapeptides, on the other hand, provided the appropriate computational manageability and intensity to study the substrate-binding profiles of SARS-CoV-2 M
pro. Since only the P3-P1’ residues are required for recognition, specificity, and cleavage, working with hexapeptides (the P3-P3′) provided the largest set of substrates that was still computationally manageable. The resulting library consisted of peptide chains demonstrating physicochemical diversity that allowed for broad-spectrum characterization of M
pro–substrate interaction (
Table 1). The combinations of high-occurrence amino acids used to create the multi-conformer library allowed for the assessment and study of potential/promising substrates that are not necessarily naturally occurring within the SARS-CoV-2 genome yet still adhere to the substrate recognition and specificity of M
pro; most importantly, they possessed desirable properties that could serve as a rational basis for drug design and discovery. By doing so, this study incorporated wide-range characterization of M
pro functionality (keeping in mind its genomic and structural conservation across variants and species), and in turn, provides insight into the protein’s behavior in the presence of mutant and homologous forms of the SARS-CoV genome and polyprotein substrates. Such information could serve as a basis for the development of broad-spectrum chemotherapies that retain efficacy in spite of emerging mutations.
Following the construction of each hexapeptide, terminal capping was performed to enhance the structural stability of the peptide substrates, particularly in dynamic environments. The subsequent conformational search provided an opportunity to identify the most favorable geometries prior to binding. This could have future use in the design of peptidomimetics displaying potent antiviral activity. Ultimately, docking studies were performed with 81,000 systems, comprising 100 conformers for each of the 810 hexapeptides.
3.2. Virtual High-Throughput Screening of the Hexapeptides
A quality crystal structure of SARS-CoV-2 M
pro was retrieved from the RCSB PDB (PDB ID: 6XHM) showing good resolution of 1.41 Å and no mutations or missing residues. The protein was reported as encoded using the SARS-CoV-2 MN908947.3 genome and synthesized via
Escherichia coli expression [
24]. Moreover, the crystal structure provided three rotamers of M
pro and this study utilized the rotamer designated “A”.
The high-throughput screening of the hexapeptides was performed on protomer B targeting the active site pocket, after preliminary docking studies assessing both protomers (
Supplementary Figure S14). Overall, the best docked poses (or docked pose with minimum energy) registered binding free energies ranging between −8.7 and −7.0 kcal.mol
−1 across all 810 substrates. The mean binding free energies of the hexapeptides—grouped by P3-P1 residues—alongside their respective ligand efficiencies are reported in
Table 2. These docking results were arguably good and alluded to high-affinity binding of the substrates onto the active site of SARS-CoV-2 M
pro, as to be expected. The ligand efficiencies indicated that all of the P3-P1 residue combinations displayed similar goodness of interaction with the receptor and thus showed similar potential improvement in binding affinity for structural- and efficiency-driven drug design [
25,
26,
27]. Therefore, all of these P3-P1 residue combinations suggested a viable basis for characterization of the active site for rational antiviral drug design, given their ligand efficiency (
Table 2).
Further evaluation and validation of the docking results revealed the reproducibility of the best docking pose together with conducive binding interactions with the active site residues for these particular poses. The visualization of the best poses of conformers per hexapeptide showed overlap in the backbone (α-carbons) of the peptide substrates when superimposed, with only a few substrates displaying alternative poses with minimum energy (
Supplementary Figure S1). This was an impressive feat considering the flexible nature of the peptide macromolecules.
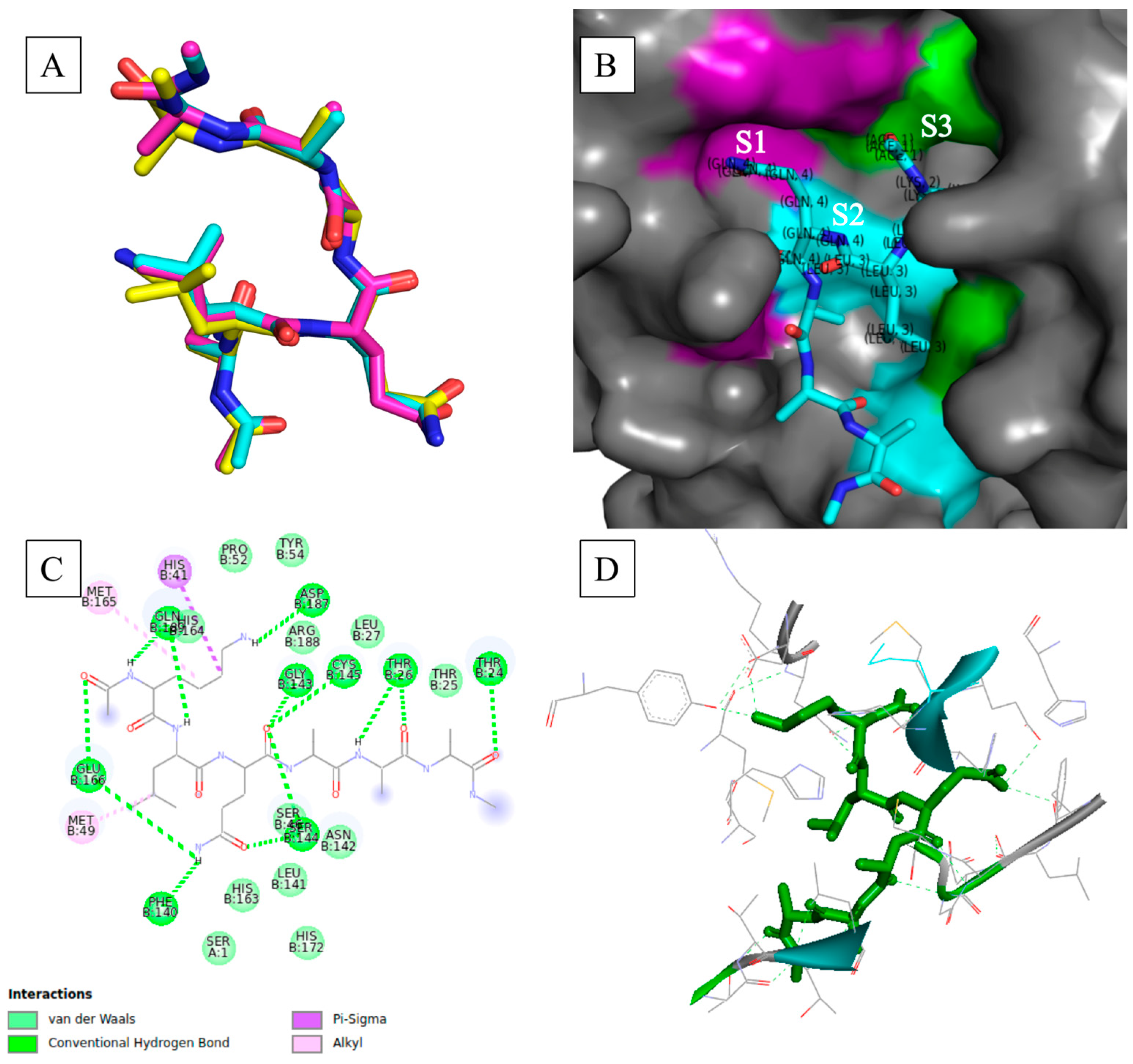
Figure 1A shows the superimposed poses with minimum binding free energy occupying the same docking/binding space. The use of a high degree of search exhaustiveness (of 480) substantially increased the probability of finding plausible poses that were reproducible.
Mapping of the substrates in the binding pocket of M
pro was used to visually assess the substrate recognition in accordance with the nomenclature of Schechter and Berger [
28] and Ullrich and Nitsche [
10]. Ullrich and Nitsche [
10] stipulated that SARS-CoV-2 M
pro mainly recognizes substrate residues P4-P1′; however, substrate specificity is determined by residues P2-P1′, as demonstrated by the highest degree of conservation across the pp1a/ab cleavage sites. The substrate residues were recognized and anchored within the binding pocket by specific active site residues that comprised subsites.
Table 3 lists these subsites as well as their constituent active site residues. These subsites surrounded the catalytic dyad consisting of His41 and Cys145, which mediates digestion of the polyproteins [
10]. The key active site residues that essentially mediate substrate binding and processing include His41, Met49, Gly143, Ser144, His163, His164, Met165, Glu166, Leu167, Asp187, Arg188, Gln189, Thr190, Ala191, and Gln192 [
11].
Figure 1B shows the binding mode of the hexapeptide within the binding pocket with respect to the active site subsites [
11,
29,
30]. The P3-P1 residues were mounted desirably onto the active site, with each residue being anchored onto its appropriate subsite. The desired binding modes and adherence of the hexapeptide binding modes to the nomenclature of Schechter and Berger [
28] were prevalent across the best docked poses. Violation of the nomenclature of Schechter and Berger [
28] was only apparent in some of the top docked poses. Hexapeptides such as RLQATF and RLQSTF—the top poses (−8.7 kcal.mol
−1)—showed the side chain of P3 anchored in S1, whereas the side chains of P2 and P1 were anchored in S3 and S2, respectively. Others, such as RLQAAN, showed S1 anchoring the side chains of P1 and P3, whilst S2 rightfully anchored P2 (
Supplementary Figures S2 and S3). Nonetheless, most binding modes showed that the M
pro crystal structure followed the substrate recognition commonly associated with SARS-CoV-2 M
pro. The side chains of the hexapeptides were appropriately accommodated in the subsites of the active site, regardless of significant differences in docking scores. Furthermore, these modes confirmed that these hexapeptides, as informed by the literature, were recognized by M
pro and appropriately bound to the receptor protein. Thus, substrate recognition by M
pro towards the generated hexapeptides was confirmed. Ultimately, the establishment of this recognition for the hexapeptides will assist in efforts to design potent and therapeutic peptidomimetics.
Resolution of the intermolecular interactions at the binding interface showed the high prevalence of hydrogen bonds and van der Waals interactions forming across all hexapeptides and active site residues, as shown in
Figure 1C. The formed hydrogen bonds included conventional hydrogen bonds, carbon–hydrogen bonds, and Pi-donor hydrogen bonds. Hydrogen bonds, especially conventional hydrogen bonds, are the fundamental stabilizing force in biomolecular structures that underpin structure, function, and conformational dynamics [
31,
32], and they also regulate complementarity and stability in protein–ligand complexes at the binding interface [
33,
34]. Therefore, the prevalence of hydrogen bond formation at the active site indicated shape and electrostatic complementarities between M
pro and the hexapeptides, and it also pointed to high affinity for the peptides, as evidently shown by the binding free energies and desirable binding modes (
Figure 1B–D;
Supplementary Figures S2–S7). Furthermore, the stabilizing effect of hydrogen bonds would prove to be integral in maintaining the complex structures in dynamic processes.
Notably, the key active site residues (including the catalytic dyad) frequently participated in hydrogen bonds and van der Waals interactions with the hexapeptides. Cys145 typically formed conventional hydrogen bonds with the oxygen atoms of the carboxyl group of P1, which in turn placed the catalytic residue in close proximity to the scissile peptide bond. Key residues 138 to 146, which constitute the oxyanion loop that confers substrate stability during the proteolytic process, participated in key stabilizing forces (hydrogen bonds and van der Waals forces of attraction) that promoted the formation of stable complexes. Other key residues, such as Met49, His163, His164, Met165, Glu166, Leu167, Asp187, Arg188, Gln189, Thr190, Ala191, and Gln192, which underpinned the subsites and accommodated the appropriate binding of the substrate residues via side-chain rearrangement [
11,
35,
36,
37], also formed the aforementioned stabilizing interactions with the hexapeptides.
Figure 1D shows that the entirety of the hexapeptide was anchored by various hydrogen-bonding interactions formed with active site residues. Conclusively, stable M
pro–hexapeptide complexed structures were constructed as shown by many stabilizing interactions at the binding interface. Moreover, the prevalent formation of hydrogen bonds at the binding interface pointed to the substrate specificity that a coronavirus main protease would typically exhibit towards peptide structures with the Leu-Gln↓(Ala/Ser) cleavage sequence.
3.3. Global Stability of the Mpro Systems
The 20 ns MD simulations were performed on 131 Lys-Leu-Gln*** (KLQ***)–M
pro complexed structures and an unbound M
pro (
apo-) structure. The exclusive use of KLQ*** complexed structures for molecular dynamics was to preserve comparability between systems—maintaining that the ligands shared identical P3-P1 residues and that they held similar ligand efficiencies with other P3-P1 ligand groups in this study. The global stability of the M
pro systems was assessed through the calculation of root mean square deviation (RMSD) and radius of gyration (Rg) values for the protein backbone (α-carbons) of M
pro based on the 20 ns MD simulation trajectories, which were plotted as violin plots to highlight significant changes in conformation and stability (
Figure 2 and
Figure 3).
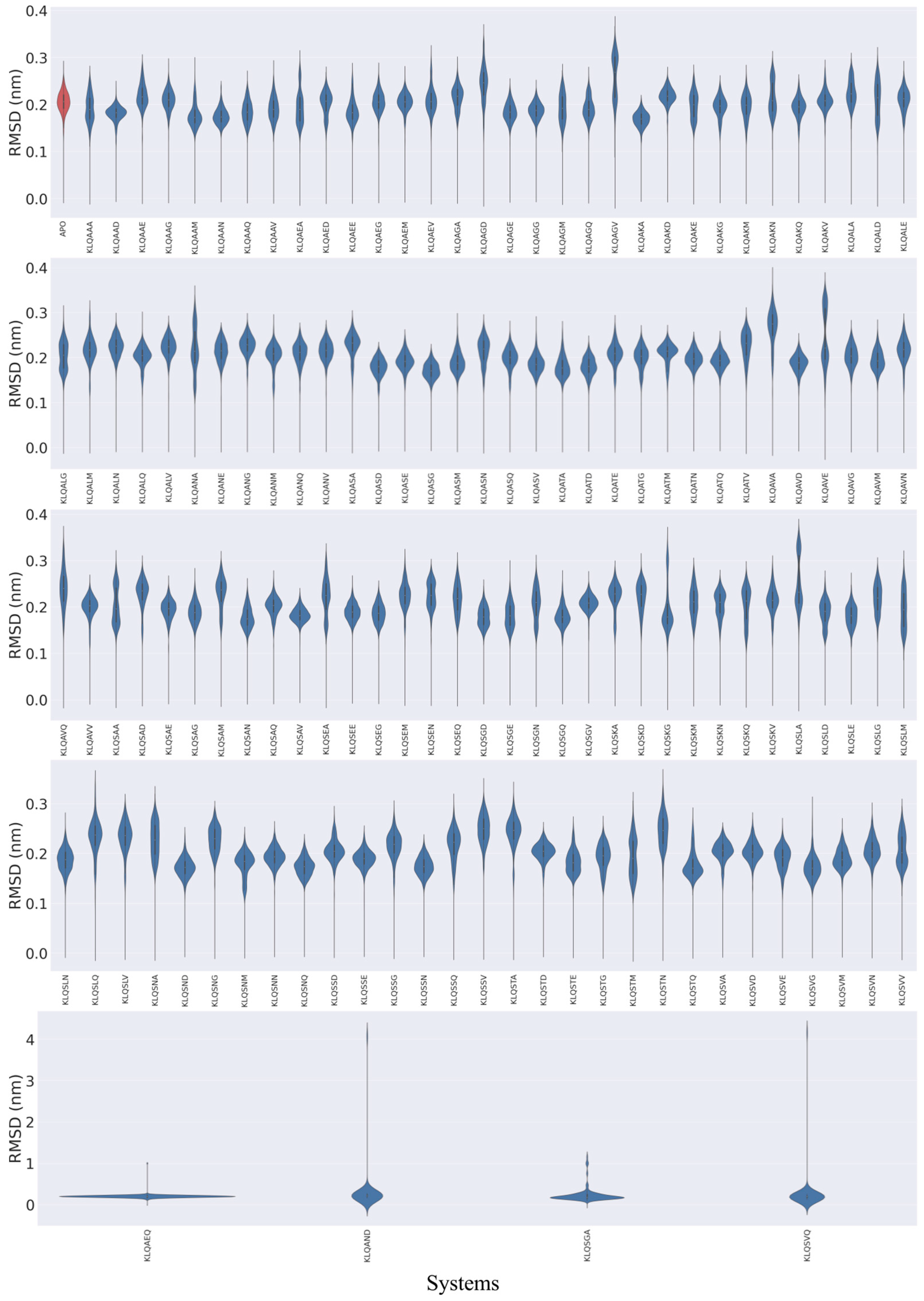
The RMSD plots (
Figure 2) showed the various behaviors of the protein across the systems and mainly displayed three distinct trends in structural stability listed in the following order of prevalence: substantial fluctuation, stable conformation, and significant changes in location (dissociation). Varying degrees of backbone fluctuation and conformational shifts were prevalent among the KLQ*** systems, as illustrated by bimodal and multimodal clustering and clustering at high RMSD values (>0.2 nm). Bimodal clustering, as clearly depicted in the KLQAGV, KLQAVE, KLQSAA, and KLQSLA systems, suggested that the protein converged on two alternative stable conformations during the simulation—an indication of reduced overall stability. Multimodal clustering (KLQAAA, KLQAEA, KLQAGM, KLQAGQ, KLQAKE, KLQAKN, etc.) and clustering at high values (KLQAED, KLQAGA, KLQAGD, KLQSKA, and KLQSKD) indicated substantial fluctuation of the backbone as the protein backbone struggled to achieve favorable conformations with the ligands, thus leading to overall conformational instability.
The apo-system and several other KLQ*** complexed systems displayed stable conformations, to varying degrees, as shown by the unimodal distribution of RMSD values. The unimodal distribution indicated a single dominant conformation for each Mpro system. The apo-system together with the KLQAAG, KLQAEG, KLQAEM, and KLQAKQ systems and others achieved moderate stability throughout the simulation. The plots suggested that these systems attained equilibration around 0.2 nm, as indicated by clustering, with a mean RMSD value of around 0.2 nm. The best stability of the Mpro backbone was demonstrated by the KLQAAD, KLQAKA, KLQASG, KLQAVD, KLQSAV, KLQSND, and KLQSNQ systems, all which achieved unimodal clustering at values less than 0.2 nm. In essence, these systems achieved favorable conformations with their ligands with low backbone fluctuations.
Conversely, significant changes in conformation and/or location in the Mpro structure were shown in the KLQAEQ, KLQAND, KLQSGA, and KLQSVQ systems, as they registered significantly steep changes in RMSD values during their respective simulations. The plots for the KLQAEQ and KLQSGA systems indicated the dissociation of the hexapeptide from the binding pocket, whereas the plots for the KLQAND and KLQSVQ systems showed the dissociation of the Mpro protomers.
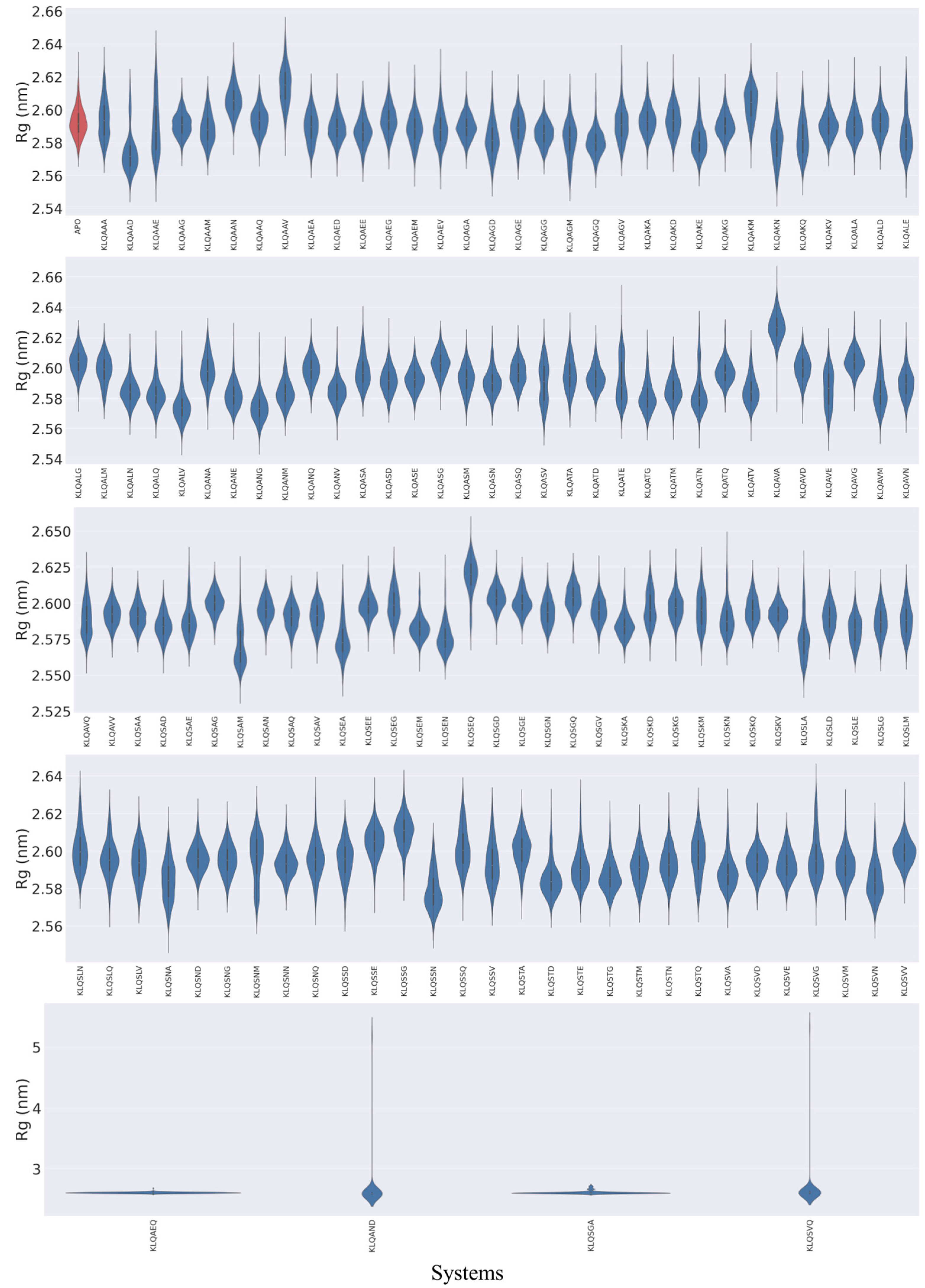
The behaviors of M
pro in the context of compactness and folding followed similar trends as the backbone fluctuations (RMSD values) for Rg values (
Figure 3). However, stable compactness was more prevalent than structural unfolding and/or stretching from the protein’s center of mass. Unimodal distribution of the Rg values was achieved by the majority of M
pro systems, wherein stable compactness was typically attained roughly around 2.56 to 2.60 nm. The KLQAAD system attained the lowest stable compactness, around 2.57 nm. Other stable systems included the unbound (
apo), KLQAEE, KLQAGA, KLQAKA, KLQAKG, KLQAKV, KLQASG, KLQATD, KLQAVG, KLQSAG, KLQSAN, KLQSEE, KLQSEM, KLQSGE, KLQSKA, KLQSND, KLQSNN, KLQSTG, KLQSVA, KLQSVD, KLQSVE, and KLQSVM, all of which maintained a stable state below 2.60 nm.
The unfolding and/or stretching of the protein backbone during the simulations were shown to varying degrees, which were indicated in the Rg plots as bimodal clustering, stretched bimodal clustering, and little to no clustering (stretched distribution) (
Figure 3). Similar to the RMSD values, the M
pro systems such as KLQASV, KLQATE, and KLQSAM registered bimodal clustering, suggesting that the protein backbone converged into two stable states. The stretched bimodal clustering in the Rg plots indicated that the M
pro backbone converged into multiple conformations but briefly attained two stable states, as depicted in the KLQAAV, KLQAED, KLQAGV, KLQALD, KLQASD, KLQSAA, KLQSAQ, KLQSEG, KLQSKD, and KLQSKV systems. The absence of clustering in other systems, such as KLQAAA, KLQAAE, KLQATE, KLQASV, KLQAVE, KLQAVQ, KLQSKM, KLQSNA, and KLQSNM, was indicative of steady increases in Rg values as a consequence of gradual stretching of the M
pro backbone during the simulation. This lack of clustering could indicate minor unfolding of the protein in the presence of the respective hexapeptides. However, as previously noted, the stretching of the M
pro backbone was gradual and not attributed to drastic denaturing of the M
pro structure. Lastly, the KLQAEQ, KLQAND, KLQSGA and KLQSVQ systems registered steep hikes in Rg values similar to the RMSD values. These steep changes in Rg values were due to the aforementioned dissociation events displayed in the RMSD plots.
Supplementary Tables S2–S4 provide the exact values for RMSD and Rg in terms of mean and standard deviation for all of the molecular dynamics simulations.
3.4. Local Stability of the Mpro Systems
Local chain fluctuations of M
pro were measured by computing the root mean square fluctuation (RMSF) and assessed using heatmaps. Heatmaps allowed the identification of high-flexibility regions, and their subsequent mapping on the M
pro crystal structure revealed the positions of these regions within the 3D protein structure (
Figure 4,
Figure 5 and
Figure 6). Across all M
pro systems, the RMSF values of both protomers of the protein approximated each other, with protomer A registering slightly higher RMSF values despite having no bound hexapeptide (
Figure 4A,B;
Figure 5A,B;
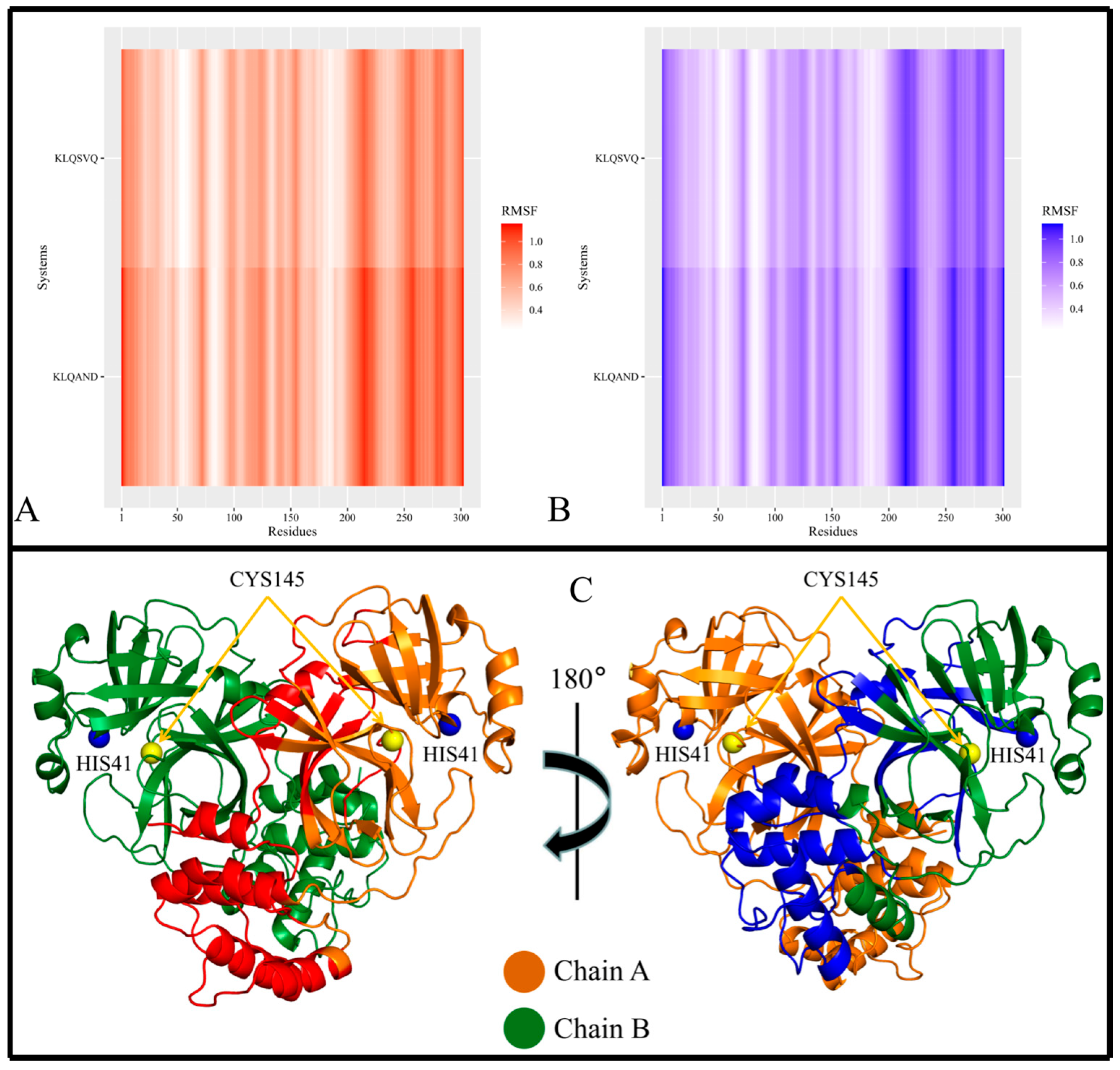
Figure 6A,B). Protomer B is where the bound hexapeptide resided. Overall, the highest RMSF values were obtained in the KLQAND and KLQSVQ systems (
Figure 6) due to the dissociation of the protomers. Since these values were disproportionately higher than those of the rest of the systems, the data were separated to optimize visualization as follows: (i) systems with KLQ*** substrates with Ala at P1′ (
Figure 4); (ii) systems KLQ*** substrates with Ser at P1′ (
Figure 5); and (iii) KLQAND and KLQSVQ systems (
Figure 6).
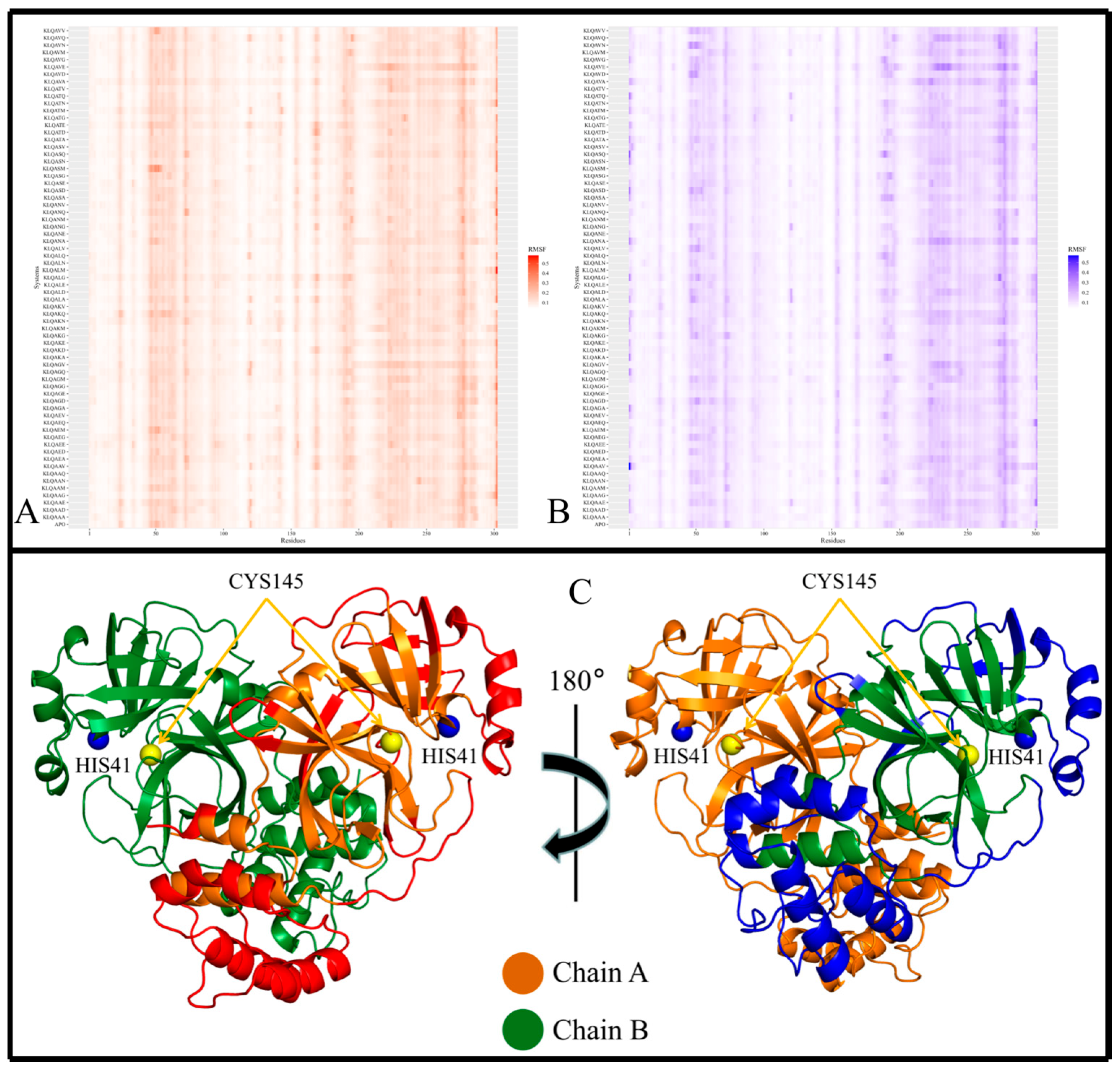
Figure 4 shows the heatmaps for both protomers of the Lys-Leu-Gln-Ala** (KLQA**) systems alongside
apo-M
pro. While the RMSF values slightly differed from one system to the next, both protomers of M
pro displayed high flexibility in the same regions in all systems (
Figure 4A,B). Overall, the RMSF values for the KLQA** systems ranged between 0.0384 and 0.5805 nm for protomer A and 0.0394–0.5698 nm for protomer B. High flexibility was observed in residues 21–26, 44–80, 92–97, 118–127, 141–144, 152–156, 167–171, 188–198, 215–288, and 298–302 in protomer A (
Figure 4A) and residues 1–4, 22–24, 44–80, 92–96, 118–125, 153–156, 168–171, 188–197, 212–288, and 297–301 in protomer B (
Figure 4B), respectively. The Lys-Leu-Gln-Ser** (KLQS**) systems showed similar trends as the KLQA** systems in terms of RMSF values and the localization of flexible residues (
Figure 5A,B). The overall range of RMSF values was between 0.0399 and 0.4223 nm for protomer A and 0.0372–0.3726 nm for protomer B. Similarly, flexibility was demonstrated in residues 21–26, 32–35, 44–80, 92–97, 119–123, 139–143, 153–156, 167–171, 187–197, 212–288, and 297–302 in protomer A and residues 1–5, 21–26, 33–35, 44–66, 70–80, 92–98, 152–156, 167–170, 186–197, 212–238, 241–286, and 297–301 for protomer B.
The majority of the highly fluctuating residues constituted loop regions in both protomers of M
pro, as shown (as red, and blue) in
Figure 4C and
Figure 5C. This high fluctuation could be attributed the flexible nature of loop structures. However, of all the flexible loop regions, residues 1–4 displayed flexibility exclusively in protomer B. Residues 1–9 comprise the N-finger terminal region, which plays a crucial role in dimer formation through interactions with Domain II of protomer A [
38]. Semi-flexibility in β-sheets (Domains I and II) was displayed on either ends of the structure in both protomers—connecting to or from loop regions. In addition, α-helices displayed semi-flexibility in residues 44–80 (domain I) and 212–288 (domain III). Of note, residues 44–80 are part of the catalytic domain responsible for catalysis and M
pro autocleavage [
30]. Sequentially, this α-helix comes after catalytic His41 and consists of key binding residues such as Met49, which contributes to substrate stabilization. While the RMSD and Rg values did not indicate overall destabilization of the M
pro structure, this apparent semi-flexibility could be attributed to functional flexibility and the intrinsic side-chain rearrangement mechanisms that accommodate the hexapeptide in the binding pocket. This point was further supported by the absence of hexapeptide ejection from the binding pocket. The α-helices of Domain III (
Figure 4C and
Figure 5C; the lower region of protein) consistently displayed high flexibility in both protomers. The high fluctuation could be attributed to their connection to long loop regions, as α-helices typically demonstrate restricted protein motion but can confer great flexibility, which is essential to protein function [
39].
In the context of the final two systems, KLQAND and KLQSVQ—for which the residue fluctuations were the highest among all systems due to dimer dissociation—RMSF values ranging between 0.2261 and 1.1565 nm for protomer A and 0.2174–1.1333 nm for protomer B were registered. However, the flexible residues were similar to those in the KLQA** and KLQS** systems, including residues 1–17, 69–73, 96–100, 111–127, 138–144, 152–157, 202–209, 210–223, 224–234, 236–237, 242–254, 255–259, 260–276, 277–285, 286–298, and 299–302 in protomer A and residues 1–19, 24–29, 69–74, 95–100, 111–128, 138–143, 151–157, 170–173, 199–206, 207–223, 224–227, 247–288, 291–299, and 300–301 in protomer B. Notably, these were the only instances where the N-finger terminal residues displayed flexibility in both protomers (
Figure 6), as expected, since dimer dissociation occurred in both of these systems.
Furthermore, the localization of the flexible residues showed greater residue fluctuation in Domain II involving entire β-sheet structures (
Figure 6C). This was not evident in the other systems, as fluctuations in β-sheets were only registered for their terminal ends connecting to loop regions. The semi-flexibility in α-helices (residues 44–63) surrounding the catalytic dyad was absent in these two systems, pointing to reduced activity and/or inactivity of the active site residues. Moreover, the α-helix residues constituting Domain III demonstrated much greater flexibility than any of the other systems, further indicating destabilization of the M
pro structure.
In conclusion, the RMSF results supported that the Mpro residues typically displayed similar fluctuation patterns in the presence of the KLQ*** substrates, with the exception of the KLQAND and KLQSVQ systems. The RMSF values of both protomers approximated one another in magnitude, and the locations of the high-fluctuation regions on the Mpro protomers were similar. Interestingly, the KLQAEQ and KLQSGA systems did not register high RMSF values like the KLQAND and KLQSVQ systems, but instead behaved similar to the majority of complexed systems. Therefore the unbinding of the hexapeptides did not cause significant changes to the motions and conformations of Mpro in these two systems (KLQAEQ and KLQSGA). Consequently, the steep hikes in RMSD and Rg values were only attributed to the fact the MD program interpreted the hexapeptide chain as a third chain in the overall protein system.
3.5. Principal Component Analysis and Protein Motion Classification
The prominent structural motions and conformational changes of the M
pro backbone during the 20 ns MD simulations were assessed using PCA calculations. PCA divided the overall protein motions of the trajectories into principal components that described the essential functional protein motions during the simulation. Since the first two principal components, PC1 and PC2, retained the majority of the variance of the original data, they could be used to provide a meaningful description of the protein motions throughout the course of the simulations. Thus, 2D projections of these principal components were plotted using the Cartesian coordinates of all backbone atoms to visualize and examine these conformational changes (
Supplementary Figure S8). The direction of change for the movement of M
pro against time was unique to each system. For example, some systems displayed clockwise changes in PCA, while others showed anti-clockwise changes in PCA. Seemingly, almost all systems retained steady conformational changes throughout the simulations as the distribution of the PCA coordinates was generally compact/clustered. Without regard to their pattern of progression, the typical range of the coordinates was between −5 and 5 for both PC1 and PC2. The KLQAEQ, KLQAND, KLQSGA, and KLQSVQ systems displayed the most drastic changes in protein motions due to the aforementioned changes in stability, structure, and conformation.
In an attempt to classify these PCA data, a custom pairwise comparison of the M
pro systems was performed as detailed in
Section 2.4.
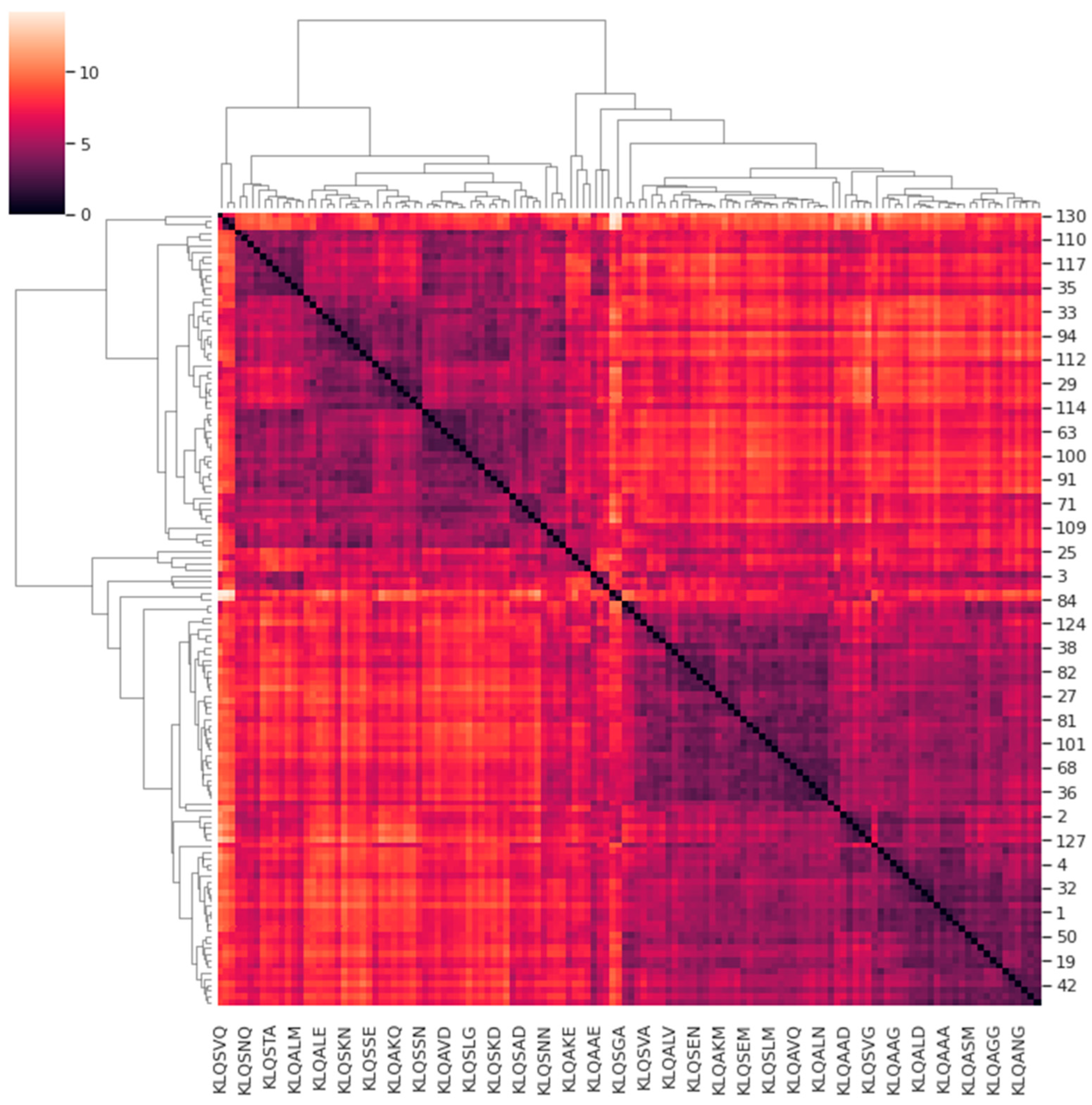
Figure 7 shows the cluster map illustrating the correlations of the protein motions for all systems with respect to one another (bearing in mind the underlying assumption). In the cluster map, systems sharing similarities in protein motions were registered in hues between black and violet, whereas systems with dissimilarities were registered in hues between white and orange. The accompanying dendrogram illustrates the similarities or correlations among the prominent protein motions of the clustered M
pro systems. Hierarchical clustering yielded four main classifications of correlation among all the systems that depicted similarity in proteins motions that describe the trajectories of the M
pro systems. The four classifications of similarity in the M
pro systems are listed in
Table 4.
Although three classifications of the systems shared varying similarities within and between them, the Group 1 PCA data (comprising the KLQSVQ, KLQAEQ, and KLQAND systems) demonstrated the highest dissimilarity to the rest of the PCA groups, as expected, considering their patterns in RMSD, Rg, and even RMSF values (for the KLQAND and KLQSVQ systems). The PCA of the KLQAND and KLQAEQ systems showed high similarity, as opposed to that of the KLQSVQ system. The KLQAND and KLQSVQ systems both showed dimer dissociation, although not similarly, during their simulations. Nevertheless, the clustering was successful in identifying highly dissimilar protein motions in the Mpro systems, as mutually confirmed by the RMSD, Rg, and RMSF data. Interestingly, the KLQSGA system, which also showed structural instability in its RMSD and Rg values, was classified in Group 3 and not alongside the KLQSVQ, KLQAEQ, and KLQAND systems. Of note, the dendrogram showed the KLQSGA system displaying the highest dissimilarity with the rest of the systems within Group 3, showing a weak correlation in terms of protein motions and conformational changes with its “similar” systems.
Systems in Groups 2 to 4 all showed varying degrees of similarity within and across themselves. Systems in Group 3 displayed the least amount of divergence in the hierarchical dendrogram when compared to Groups 2 and 4 (
Figure 7). Notably, Group 3 was occupied by systems that attained high medians and/or multimodal distribution in their RMSD and Rg values. Systems that demonstrated stability were split across the three groups.
The PCA results highly aligned with the RMSD, Rg, and RMSF values in terms of overall trends. Most of the M
pro systems registered PCA coordinates within the range of 5 and −5 for PC1 and PC2, just as they attained RMSD and Rg equilibration around 0.2 nm and 2.60 nm, respectively. Importantly, there were only two unbinding events registered to the 131 KLQ***-bound M
pro systems—an indication of strong intermolecular interaction in the binding pocket. The retention of hexapeptides by the protein could be attributed to the substrate recognition and specificity postulated in the docking studies. Despite binding substrates with minor chemical differences, the protein achieved and retained similar flexibility, compaction, and prominent protein motions, which altogether caused similar behaviors and patterns of stability in the M
pro systems. Consequently, these results suggested that SARS-CoV-2 M
pro has an intrinsic mechanism that enables the binding of different peptide substrates without rendering drastic instability to the entire structure. Even in the PCA, the majority of the systems seemingly occupied the same positions on the PCA plot throughout the course of the simulation. The exceptions to these trends consistently included the KLQAEQ, KLQAND, KLQSGA, and KLQSVQ systems, as clearly demonstrated by their high dissimilarity to the rest of the systems. While the dissimilarities in protein motions depicted in the dendrogram (
Figure 7) could be easily explained, the similarities also required verification. Henceforth, at least two relevant systems from each hierarchical classification (or group) were selected for trajectory visualization using VMD.
The visualization of the trajectories for the KLQAEQ and KLQSVQ systems in Group 1 confirmed the weak correlation in protein motions reported in the dendrogram. The protein backbone for both systems showed no overlap at any point in the simulations (
Supplementary Figure S9). Dissimilarity in these systems was expected as the KLQAEQ system showed substrate unbinding while the KLQSVQ system showed dimer dissociation. Therefore, these systems only fell within the same classification due to their drastic changes in conformation and location, as shown by their RMSD and Rg values. While the ejection of non-covalently bound ligands from the binding pocket normally does not register such extreme changes in RMSD and Rg values for the protein backbone, the steep hikes displayed in the KLQAEQ system were attributed to the inclusion of substrates as third chains of M
pro by the GROMACS software prior to the MD simulations. The protein motions of the hexapeptide when it was ejected and later bound again affected the values for RMSD, Rg, and PCA. The KLQAND system seemingly displayed similarity to the KLQAEQ system, even though the former demonstrated dimer dissociation. The visualization of these systems refuted this similarity as the protein backbones did not exhibit overlap during the simulations (
Supplementary Figure S9). However, the relationship between their PCA results could be attributed to the substrate unbinding (KLQAEQ) and dissociation (KLQAND) that were later restored for both systems. Therefore, the similarity between the PCA results was owing to both systems having registered steep hikes in RMSD and Rg values rather than steep gradients in RMSD and Rg values.
The visualization of the systems (
apo-M
pro and M
pro-KLQSEG) in Group 2 showed a strong similarity in protein motions as the structures overlapped throughout all frames of the trajectories (
Supplementary Figure S10). Particularly, Domains I and II (the chymotrypsin-like structure) consistently overlapped throughout the simulation, whereas the helices of Domain III displayed the most structural deviation in both structures. The mapping of high-RMSF-value residues indicated that residues in this domain were highly flexible. The KLQAVV and KLQSGA systems in Group 3 displayed little similarity in protein motions at any point of the simulations (
Supplementary Figure S11). This was supported by the placement of these systems in the dendrogram; wherein the KLQSGA system exhibited high dissimilarity with the rest of the systems in Group 3 owing to the unbinding of the hexapeptide towards the end of the simulation. However, the similarity between the KLQSGA system and other Group 3 systems could have been the result of overlapping M
pro motions prior to unbinding of the hexapeptide. The KLQAVV system demonstrated structural stability as well as ligand stability throughout the simulation and also showed conformational similarity with the closely related KLQSAN system (
Supplementary Figure S11).
Similarly, the systems (M
pro-KLQAAA and M
pro-KLQSKG) in Group 4 also showed little similarity in protein motions (
Supplementary Figure S12). While overlapping of the structures was visible in the β-sheets of Domain I, a strong similarity was highly unlikely considering their placement in the dendrogram (
Figure 7). It could be, in general, that the protein motions were within a broad range, but it was visually impossible to assess this detail. Lastly, the visualization of the systems from all four groups revealed little to no similarity in protein motions between groups (
Supplementary Figure S13). Structural overlaps rarely occurred during the simulations. The dissimilarity in protein motions was highly expected given the fact that these classifications diverged from the highest point of dissimilarity, as illustrated in
Figure 7. Thus, this observation validated the hierarchical clustering since these systems exhibited no similarity in their prominent protein motions throughout the 20 ns MD simulation.
The visualization of the four clades of PCA progression allowed the inspection and confirmation of the similarities in protein motions of the systems. The outcomes of the visualization indicated that this type of PCA progression analysis is able, certainly in some cases, to identify both similar and dissimilar protein motions in comparable systems. With refinement, it could provide information for general use. In this case, it certainly enabled us to focus on particular systems given the number of simulations to assess.