Exploring the Expression and Function of cTyro3, a Candidate Zika Virus Receptor, in the Embryonic Chicken Brain and Inner Ear
Abstract
1. Introduction
2. Materials and Methods
2.1. ZIKV Production and Titration
2.2. Tissue Processing and Cryosectioning
2.3. RNAscope In Situ Hybridization
2.4. Plasmid Construct Design and Cloning
2.5. Plasmid Transfection and RCAS(A) Infection of DF-1 Cells
2.6. Western Blot
2.7. Animal Handling and Injections
2.8. Immunohistochemistry
2.9. Imaging
3. Results
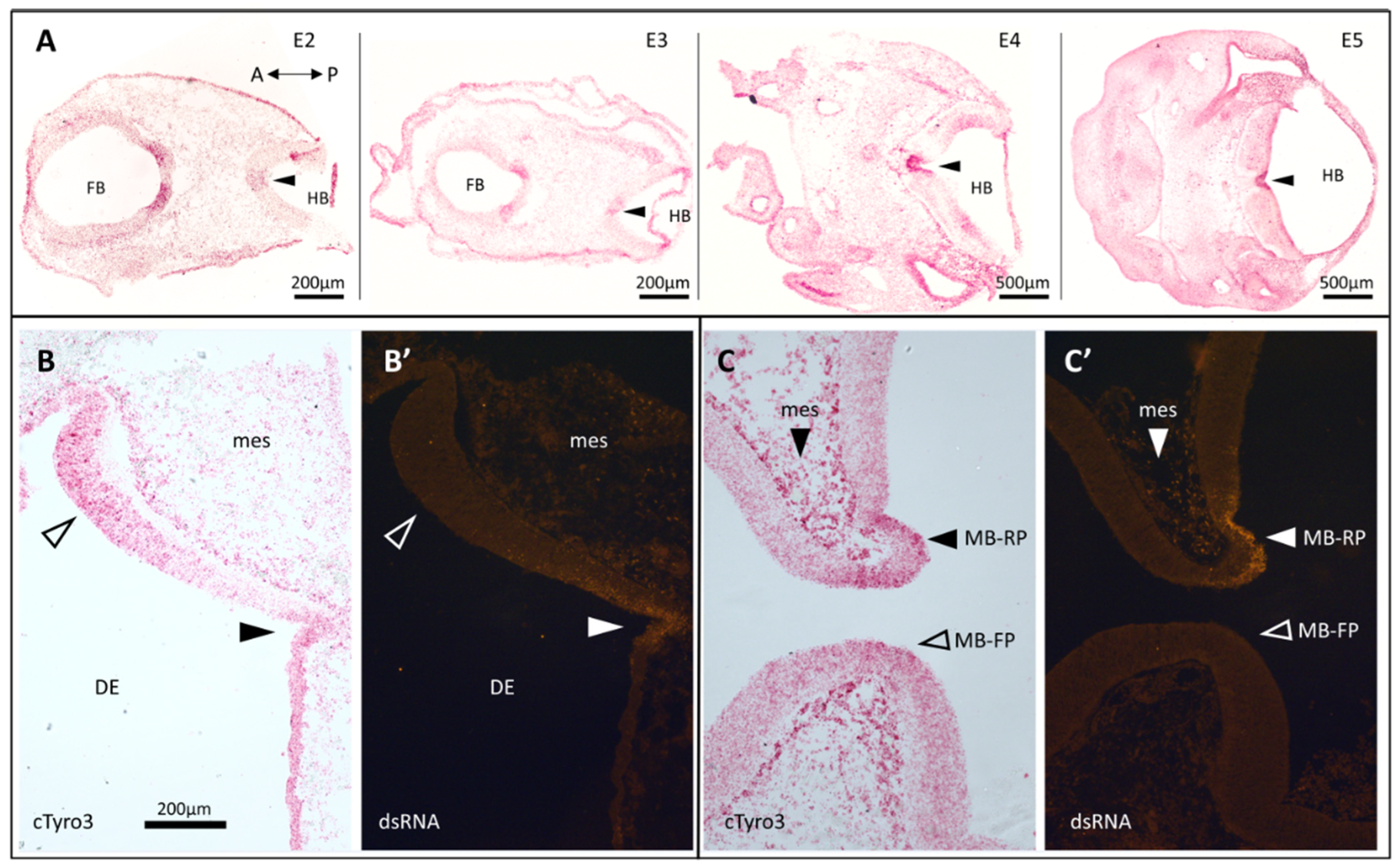
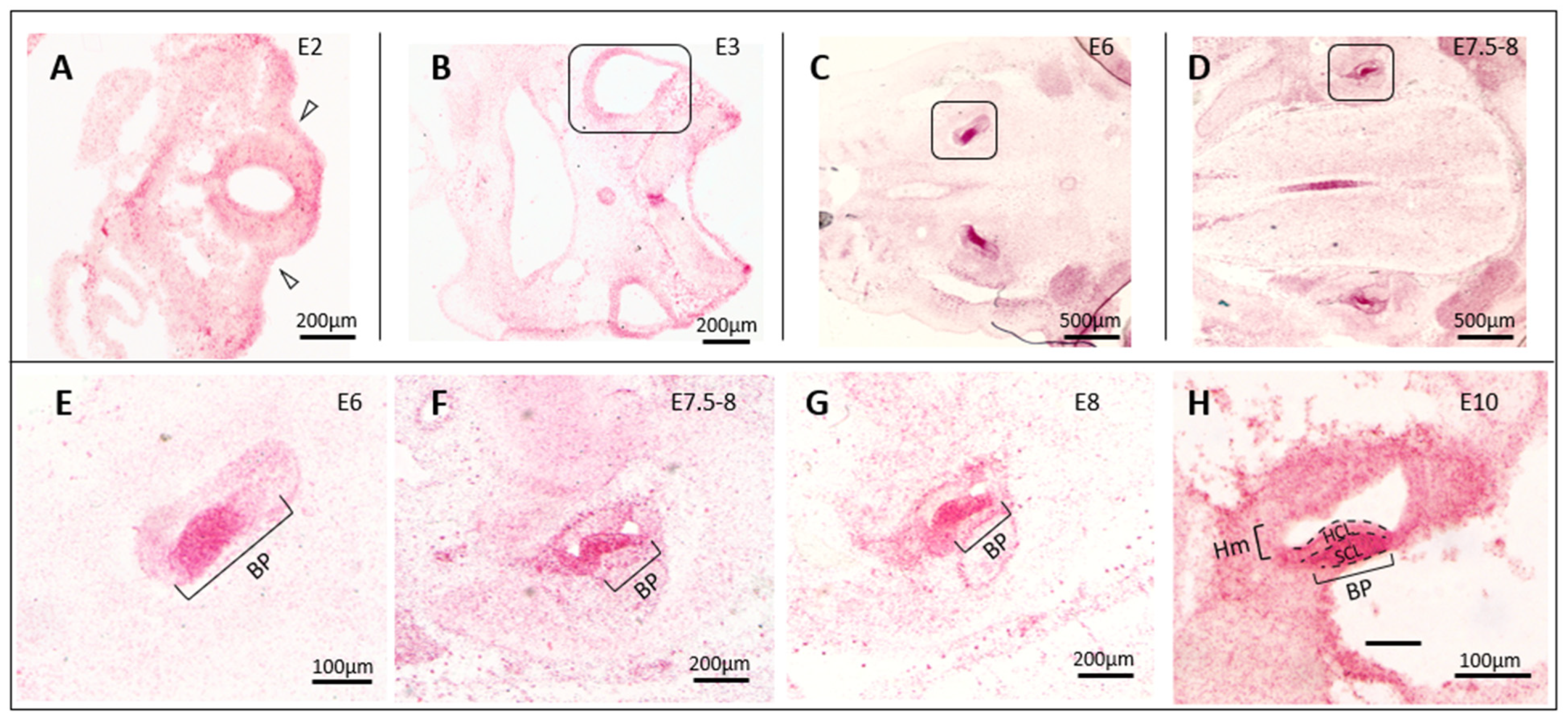
3.1. In Situ Hybridization Indicates a Correlation between cTyro3 Expression and ZIKV Infection Hotspots
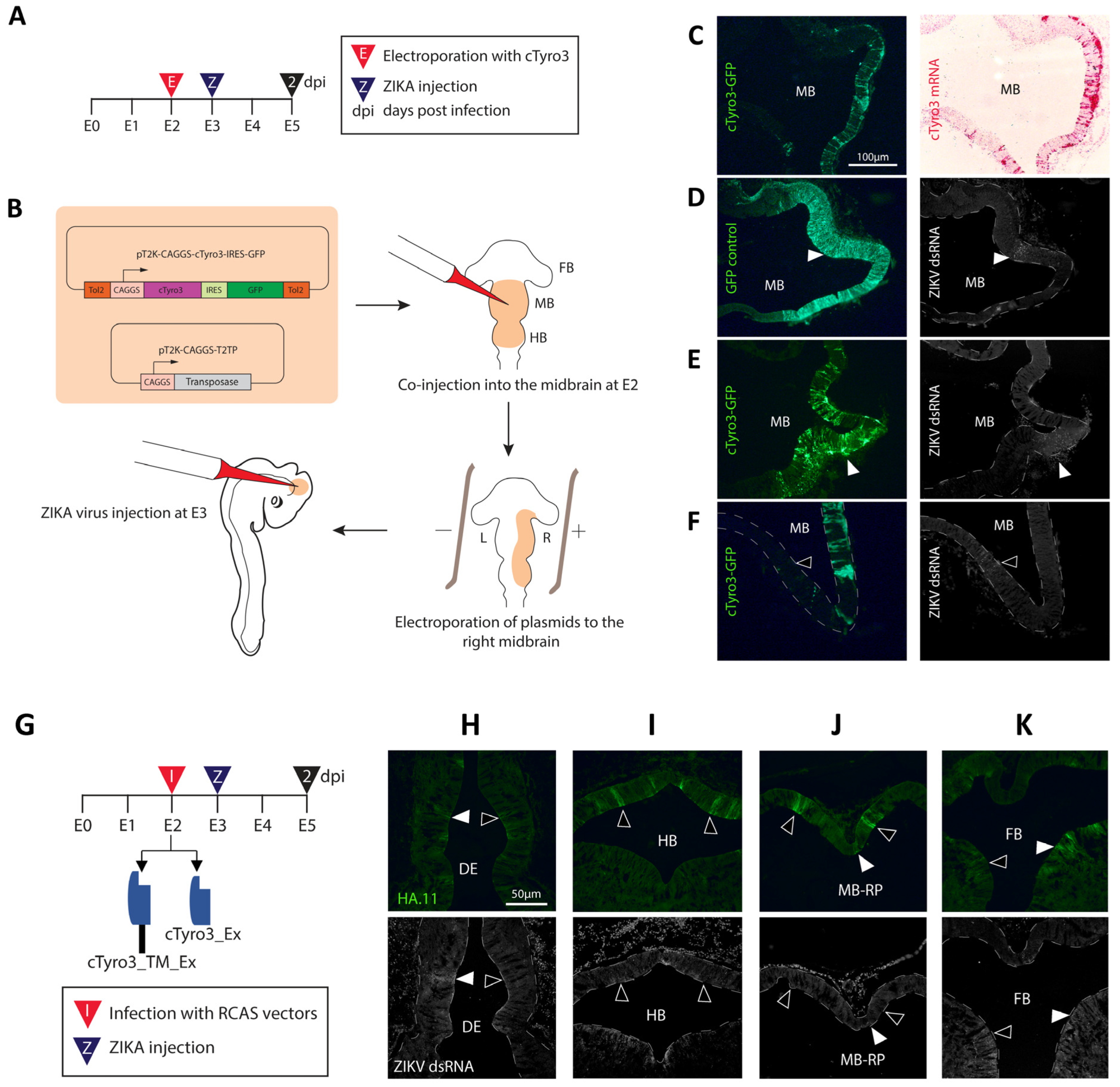
3.2. Ectopic cTyro3 Overexpression Does Not Increase ZIKV Infection beyond the Usual Hotspots of the Embryonic Brain
3.3. Expression of Truncated Versions of cTyro3 Does Not Reduce ZIKV Infection in the Embryonic Brain
3.4. Infection of the Inner Ear with cTyro3 Dominant-Negative Viruses Does Not Affect Cell Death or Hair Cell Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.L.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.R.; Neri, J.I.; Neto, J.M.D.P.; Wanderley, H.Y.; et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Nogueira, R.M.R.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.-A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro-Preliminary report. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Martines, R.B.; Bhatnagar, J.; Keating, M.K.; Silva-Flannery, L.; Muehlenbachs, A.; Gary, J.; Goldsmith, C.; Hale, G.; Ritter, J.; Rollin, D.; et al. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses—Brazil, 2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 159–160. [Google Scholar] [CrossRef]
- Zanluca, C.; De Melo, V.C.A.; Mosimann, A.L.P.; Dos Santos, G.I.V.; Dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. 2015, 110, 569–572. Memórias Do Inst. Oswaldo Cruz 2015, 110, 569–570. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; DuBray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Simpson, D.I. Zika virus infection in man. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 335–338. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- World Health Organization. Zika Situation Report: Neurological Syndrome And congenital Anomalies; Technical Documents; World Health Organization: Geneve, Switzerland, 2016.
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef]
- Leal, M.C.; Muniz, L.F.; Ferreira, T.S.; Santos, C.M.; Almeida, L.C.; Van Der Linden, V.; Ramos, R.C.; Rodrigues, L.C.; Neto, S.S.C. Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection—Brazil, November 2015–May 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Heang, V.; Yasuda, C.Y.; Sovann, L.; Haddow, A.; Da Rosa, A.P.T.; Tesh, R.B.; Kasper, M.R. Zika Virus Infection, Cambodia, 2010. Emerg. Infect. Dis. 2012, 18, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Tappe, D.; Nachtigall, S.; Kapaun, A.; Schnitzler, P.; Günther, S.; Schmidt-Chanasit, J. Acute Zika Virus Infection after Travel to Malaysian Borneo, September 2014. Emerg. Infect. Dis. 2015, 21, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Furtado, J.M.; Espósito, D.L.; Klein, T.M.; Teixeira-Pinto, T.; da Fonseca, B.A. Uveitis Associated with Zika Virus Infection. N. Engl. J. Med. 2016, 375, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Sene, A.; Richner, J.; Smith, A.M.; Santeford, A.; Ban, N.; Weger-Lucarelli, J.; Manzella, F.; Rückert, C.; Govero, J.; et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016, 16, 3208–3218. [Google Scholar] [CrossRef]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Adams, L.E.; et al. Persistence of Zika Virus in Body Fluids—Final Report. N. Engl. J. Med. 2018, 379, 1234–1243. [Google Scholar] [CrossRef]
- Foy, B.D.; Kobylinski, K.; Foy, J.L.C.; Blitvich, B.J.; Da Rosa, A.T.; Haddow, A.; Lanciotti, R.S.; Tesh, R.B. Probable Non–Vector-borne Transmission of Zika Virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef]
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Wettersten, H.I.; Beck, S.; Clark, A.E.; Wu, Q.; et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin αvβ5 Axis. Cell Stem Cell 2020, 26, 187–204.e10. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Tiwari, S.K.; Lichinchi, G.; Yau, E.H.; Hui, H.; Li, W.; Furnari, F.; Rana, T.M. Integrin αvβ5 Internalizes Zika Virus during Neural Stem Cells Infection and Provides a Promising Target for Antiviral Therapy. Cell Rep. 2020, 30, 969–983.e4. [Google Scholar] [CrossRef]
- Srivastava, M.; Zhang, Y.; Chen, J.; Sirohi, D.; Miller, A.; Chen, Z.; Lu, H.; Xu, J.; Kuhn, R.J.; Tao, W.A. Chemical proteomics tracks virus entry and uncovers NCAM1 as Zika virus receptor. Nat. Commun. 2020, 11, 3896. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, D.; Kuhn, R.J. Zika Virus Structure, Maturation, and Receptors. J. Infect. Dis. 2017, 216, S935–S944. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sanchez, E.; Altmeyer, R.; Amara, A.; Schwartz, O.; Fieschi, F.; Virelizier, J.; Arenzana-Seisdedos, F.; Desprès, P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003, 4, 723–728. [Google Scholar] [CrossRef]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef]
- Richard, A.S.; Zhang, A.; Park, S.-J.; Farzan, M.; Zong, M.; Choe, H. Virion-associated phosphatidylethanolamine promotes TIM1-mediated infection by Ebola, dengue, and West Nile viruses. Proc. Natl. Acad. Sci. USA 2015, 112, 14682–14687. [Google Scholar] [CrossRef]
- Paixão, E.S.; Teixeira, M.G.; Costa, M.D.C.N.; Barreto, M.L.; Rodrigues, L.C. Symptomatic Dengue during Pregnancy and Congenital Neurologic Malformations. Emerg. Infect. Dis. 2018, 24, 1748–1750. [Google Scholar] [CrossRef]
- Wiley, C.A.; Chimelli, L. Human Zika and West Nile virus neurological infections: What is the difference? Neuropathology 2017, 37, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.S.; Shim, B.-S.; Kwon, Y.-C.; Zhang, R.; Otsuka, Y.; Schmitt, K.; Berri, F.; Diamond, M.S.; Choe, H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; DeLalio, L.; Isakson, B.E.; Wang, T.T. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ. Res. 2016, 119, 1183–1189. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.-M.; et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.F.; Salick, M.R.; Wiskow, O.; Ho, D.J.; Worringer, K.A.; Ihry, R.J.; Kommineni, S.; Bilican, B.; Klim, J.R.; Hill, E.J.; et al. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell 2016, 19, 703–708. [Google Scholar] [CrossRef]
- Hastings, A.K.; Yockey, L.J.; Jagger, B.W.; Hwang, J.; Uraki, R.; Gaitsch, H.F.; Parnell, L.A.; Cao, B.; Mysorekar, I.U.; Rothlin, C.V.; et al. TAM Receptors Are not Required for Zika Virus Infection in Mice. Cell Rep. 2017, 19, 558–568. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Thawani, A.; Sirohi, D.; Kuhn, R.J.; Fekete, D.M. Zika Virus Can Strongly Infect and Disrupt Secondary Organizers in the Ventricular Zone of the Embryonic Chicken Brain. Cell Rep. 2018, 23, 692–700. [Google Scholar] [CrossRef]
- Thawani, A.; Sammudin, N.H.; Reygaerts, H.S.; Wozniak, A.N.; Munnamalai, V.; Kuhn, R.J.; Fekete, D.M. Zika virus can directly infect and damage the auditory and vestibular components of the embryonic chicken inner ear. Dev. Dyn. 2020, 249, 867–883. [Google Scholar] [CrossRef]
- Goodfellow, F.T.; Tesla, B.; Simchick, G.; Zhao, Q.; Hodge, T.; Brindley, M.A.; Stice, S.L. Zika Virus Induced Mortality and Microcephaly in Chicken Embryos. Stem Cells Dev. 2016, 25, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Wachholz, G.E.; Varela, A.P.M.; Teixeira, T.F.; Matos, S.M.S.; Soster, P.; Vianna, F.S.L.; Souza, D.O.G.; Roehe, P.M.; Schuler-Faccini, L.; Fraga, L.R. Zika virus-induced brain malformations in chicken embryos. Birth Defects Res. 2020, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Willard, K.A.; Demakovsky, L.; Tesla, B.; Goodfellow, F.T.; Stice, S.L.; Murdock, C.C.; Brindley, M.A. Zika Virus Exhibits Lineage-Specific Phenotypes in Cell Culture, in Aedes aegypti Mosquitoes, and in an Embryo Model. Viruses 2017, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lemke, G. Homeostatic Regulation of the Immune System by Receptor Tyrosine Kinases of the Tyro 3 Family. Science 2001, 293, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Carrera-Silva, E.A.; Bosurgi, L.; Ghosh, S. TAM Receptor Signaling in Immune Homeostasis. Annu. Rev. Immunol. 2015, 33, 355–391. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM Receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Seitz, H.M.; Camenisch, T.D.; Lemke, G.; Earp, H.S.; Matsushima, G.K. Macrophages and Dendritic Cells Use Different Axl/Mertk/Tyro3 Receptors in Clearance of Apoptotic Cells. J. Immunol. 2007, 178, 5635–5642. [Google Scholar] [CrossRef]
- Hunt, C.L.; Kolokoltsov, A.A.; Davey, R.A.; Maury, W. The Tyro3 Receptor Kinase Axl Enhances Macropinocytosis of Zaire Ebolavirus. J. Virol. 2011, 85, 334–347. [Google Scholar] [CrossRef]
- Fibriansah, G.; Ibarra, K.D.; Ng, T.-S.; Smith, S.A.; Tan, J.L.; Lim, X.-N.; Ooi, J.S.G.; Kostyuchenko, V.A.; Wang, J.; de Silva, A.M.; et al. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 2015, 349, 88–91. [Google Scholar] [CrossRef]
- Cherrier, M.V.; Kaufmann, B.; Nybakken, G.E.; Lok, S.-M.; Warren, J.; Chen, B.R.; Nelson, C.A.; Kostyuchenko, V.; Holdaway, H.A.; Chipman, P.R.; et al. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J. 2009, 28, 3269–3276. [Google Scholar] [CrossRef]
- Jemielity, S.; Wang, J.J.; Chan, Y.K.; Ahmed, A.A.; Li, W.; Monahan, S.; Bu, X.; Farzan, M.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-family Proteins Promote Infection of Multiple Enveloped Viruses through Virion-associated Phosphatidylserine. PLoS Pathog. 2013, 9, e1003232. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Vaccinia Virus Uses Macropinocytosis and Apoptotic Mimicry to Enter Host Cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Apoptotic mimicry: Phosphatidylserine-mediated macropinocytosis of vaccinia virus. Ann. N. Y. Acad. Sci. 2010, 1209, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Strange, D.P.; Jiyarom, B.; Pourhabibi Zarandi, N.; Xie, X.; Baker, C.; Sadri-Ardekani, H.; Shi, P.-Y.; Verma, S. Axl Promotes Zika Virus Entry and Modulates the Antiviral State of Human Sertoli Cells. mBio 2019, 10, e01372-19. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Zagórska, A.; Lew, E.D.; Shrestha, B.; Rothlin, C.V.; Naughton, J.; Diamond, M.S.; Lemke, G.; Young, J.A. Enveloped Viruses Disable Innate Immune Responses in Dendritic Cells by Direct Activation of TAM Receptors. Cell Host Microbe 2013, 14, 136–147. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.-F.; Yang, Y.; Zou, P.; Chen, J.; He, Y.; Shui, S.-L.; Cui, Y.-R.; Bai, R.; Liang, Y.-J.; et al. AXL promotes Zika virus infection in astrocytes by antagonizing type I interferon signalling. Nat. Microbiol. 2018, 3, 302–309. [Google Scholar] [CrossRef]
- Nazerai, L.; Christensen, J.P.; Thomsen, A.R. A ‘Furry-Tale’ of Zika Virus Infection: What Have We Learned from Animal Models? Viruses 2019, 11, 29. [Google Scholar] [CrossRef]
- Benazzato, C.; Russo, F.B.; Beltrão-Braga, P.C.B. An update on preclinical pregnancy models of Zika virus infection for drug and vaccine discovery. Expert Opin. Drug Discov. 2022, 17, 19–25. [Google Scholar] [CrossRef]
- Wachholz, G.E.; Rengel, B.D.; Vargesson, N.; Fraga, L.R. From the Farm to the Lab: How Chicken Embryos Contribute to the Field of Teratology. Front. Genet. 2021, 12, 666726. Available online: https://www.frontiersin.org/articles/10.3389/fgene.2021.666726 (accessed on 6 August 2022). [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Stoller, M.L.; Fekete, D.M. Tol2-Mediated Delivery of miRNAs to the Chicken Otocyst Using Plasmid Electroporation. In Auditory and Vestibular Research: Methods and Protocols; Sokolowski, B., Ed.; Springer: New York, NY, USA, 2016; Volume 1427, pp. 27–42. [Google Scholar] [CrossRef]
- Potts, W.M.; Olsen, M.; Boettiger, D.; Vogt, V.M. Epitope Mapping of Monoclonal Antibodies to gag Protein p19 of Avian Sarcoma and Leukaemia Viruses. J. Gen. Virol. 1987, 68, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Chicken galGal6 chr5:25149919-25189179 UCSC Genome Browser v437. Available online: http://genome.ucsc.edu/cgi-bin/hgTracks?db=galGal6&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosition=&position=chr5%3A25149919%2D25189179&hgsid=1469643211_OhxXqvUrI9C1iBRzZxhnsz7efyHo (accessed on 9 October 2022).
- NCBI RefSeq Genes, Curated Subset (NM_*, NR_*, and YP_*)—NM_204627.2. Available online: http://genome.ucsc.edu/cgi-bin/hgc?hgsid=1305564669_4PaYptdE8IEvoEVXvfZR7UY7tQ43&db=galGal6&c=chr5&l=25149918&r=25189179&o=25149918&t=25189179&g=ncbiRefSeqCurated&i=NM_204627.2 (accessed on 9 October 2022).
- Tyrosine-Protein Kinase Receptor TYRO3 Precursor [Gallus Gallus]—Protein—NCBI. Available online: https://www.ncbi.nlm.nih.gov/protein/NP_989958 (accessed on 9 October 2022).
- Geisha. Available online: http://geisha.arizona.edu/geisha/search.jsp?search=gene+name+or+description&text=Tyro3 (accessed on 11 August 2022).
- Bell, G.W.; Yatskievych, T.A.; Antin, P.B. GEISHA, a whole-mount in situ hybridization gene expression screen in chicken embryos. Dev. Dyn. 2004, 229, 677–687. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, J.P.; Frye, R.A.; Cogswell, P.C.; Neubauer, A.; Kitch, B.; Prokop, C.; Espinosa, R.; Le Beau, M.M.; Earp, H.S.; Liu, E.T. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 1991, 11, 5016–5031. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Tsou, W.-I.; Nguyen, K.-Q.N.; Calarese, D.A.; Garforth, S.J.; Antes, A.L.; Smirnov, S.V.; Almo, S.C.; Birge, R.B.; Kotenko, S.V. Receptor Tyrosine Kinases, TYRO3, AXL, and MER, Demonstrate Distinct Patterns and Complex Regulation of Ligand-induced Activation. J. Biol. Chem. 2014, 289, 25750–25763. [Google Scholar] [CrossRef]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.-C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.-T.; Ma, X.-J.; Luo, Y. RNAscope: A Novel in Situ RNA Analysis Platform for Formalin-Fixed, Paraffin-Embedded Tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Evans, D. Viral Receptors. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 319–324. [Google Scholar] [CrossRef]
- Brindley, M.A.; Hunt, C.L.; Kondratowicz, A.S.; Bowman, J.; Sinn, P.L.; McCray, P.B., Jr.; Quinn, K.; Weller, M.L.; Chiorini, J.A.; Maury, W. Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology 2011, 415, 83–94. [Google Scholar] [CrossRef]
- Zwernik, S.D.; Adams, B.H.; Raymond, D.A.; Warner, C.M.; Kassam, A.B.; Rovin, R.A.; Akhtar, P. AXL receptor is required for Zika virus strain MR-766 infection in human glioblastoma cell lines. Mol. Ther. Oncolytics 2021, 23, 447–457. [Google Scholar] [CrossRef]
- Biscardi, J.S.; Denhez, F.; Buehler, G.F.; Chesnutt, D.A.; Baragona, S.C.; O’Bryan, J.P.; Der, C.J.; Fiordalisi, J.J.; Fults, D.W.; Maness, P.F. rek, a Gene Expressed in Retina and Brain, Encodes a Receptor Tyrosine Kinase of the Axl/Tyro3 Family. J. Biol. Chem. 1996, 271, 29049–29059. [Google Scholar] [CrossRef]
- Butler, H.; Juurlink, B.H.J. An Atlas for Staging Mammalian and Chick Embryos; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Bass, D.M.; Greenberg, H.B. Strategies for the identification of icosahedral virus receptors. J. Clin. Investig. 1992, 89, 3–9. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Fourgeaud, L.; Través, P.G.; Tufail, Y.; Leal-Bailey, H.; Lew, E.D.; Burrola, P.G.; Callaway, P.; Zagórska, A.; Rothlin, C.V.; Nimmerjahn, A.; et al. TAM receptors regulate multiple features of microglial physiology. Nature 2016, 532, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G.; Burstyn-Cohen, T. TAM receptors and the clearance of apoptotic cells. Ann. N. Y. Acad. Sci. 2010, 1209, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Manipulating Gene Expression with Replication-Competent Retroviruses—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8722477/ (accessed on 10 October 2022).
- Fekete, D.M.; Homburger, S.; Waring, M.; Riedl, A.; Garcia, L. Involvement of programmed cell death in morphogenesis of the vertebrate inner ear. Development 1997, 124, 2451–2461. [Google Scholar] [CrossRef]
- Lang, H.; Bever, M.M.; Fekete, D.M. Cell proliferation and cell death in the developing chick inner ear: Spatial and temporal patterns. J. Comp. Neurol. 2000, 417, 205–220. [Google Scholar] [CrossRef]
- Katayama, A.; Corwin, J.T. Cell production in the chicken cochlea. J. Comp. Neurol. 1989, 281, 129–135. [Google Scholar] [CrossRef]
- De Araújo, T.V.B.; Rodrigues, L.C.; de Alencar Ximenes, R.A.; de Barros Miranda-Filho, D.; Montarroyos, U.R.; de Melo, A.P.L.; Valongueiro, S.; de Albuquerque, M.D.F.P.M.; Souza, W.V.; Braga, C.; et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 2016, 16, 1356–1363. [Google Scholar] [CrossRef]
- Mercer, J. Viral Apoptotic Mimicry Party: P.S. Bring Your Own Gas6. Cell Host Microbe 2011, 9, 255–257. [Google Scholar] [CrossRef]
- Zhang, L.; Richard, A.S.; Jackson, C.B.; Ojha, A.; Choe, H. Phosphatidylethanolamine and Phosphatidylserine Synergize To Enhance GAS6/AXL-Mediated Virus Infection and Efferocytosis. J. Virol. 2020, 95, e02079-20. [Google Scholar] [CrossRef]
- Silva-Filho, J.L.; de Oliveira, L.G.; Monteiro, L.; Parise, P.L.; Zanluqui, N.G.; Polonio, C.M.; de Freitas, C.L.; Toledo-Teixeira, D.A.; de Souza, W.M.; Bittencourt, N.; et al. Gas6 drives Zika virus-induced neurological complications in humans and congenital syndrome in immunocompetent mice. Brain Behav. Immun. 2021, 97, 260–274. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell 2016, 18, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Wang, Z.; Zhen, Z.-D.; Feng, K.-H.; Guo, J.; Gao, N.; Fan, D.-Y.; Han, D.-S.; Wang, P.-G.; An, J. Axl is not an indispensable factor for Zika virus infection in mice. J. Gen. Virol. 2017, 98, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Gornalusse, G.G.; Zhang, M.; Wang, R.; Rwigamba, E.; Kirby, A.C.; Fialkow, M.; Nance, E.; Hladik, F.; Vojtech, L. HSV-2 Infection Enhances Zika Virus Infection of Primary Genital Epithelial Cells Independently of the Known Zika Virus Receptor AXL. Front. Microbiol. 2022, 12, 825049. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2021.825049 (accessed on 4 August 2022). [CrossRef]
- Garcia, P.; Wang, Y.; Viallet, J.; Jilkova, Z.M. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Bódi, I.; Oláh, I. Avian dendritic cells: Phenotype and ontogeny in lymphoid organs. Dev. Comp. Immunol. 2016, 58, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Kaiser, P. Antigen presenting cells in a non-mammalian model system, the chicken. Immunobiology 2011, 216, 1177–1183. [Google Scholar] [CrossRef]
- Taylor, P.; Martinez-Pomares, L.; Stacey, M.; Lin, H.-H.; Brown, G.; Gordon, S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005, 23, 901–944. [Google Scholar] [CrossRef]
- Smith, A.L.; Göbel, T.W. Chapter 5—Avian T Cells: Antigen Recognition and Lineages. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 91–102. [Google Scholar] [CrossRef]
- Bueno, M.G.; Martinez, N.; Abdalla, L.; dos Santos, C.N.D.; Chame, M. Animals in the Zika Virus Life Cycle: What to Expect from Megadiverse Latin American Countries. PLoS Neglected Trop. Dis. 2016, 10, e0005073. [Google Scholar] [CrossRef]
- Prieto, A.L.; Weber, J.L.; Tracy, S.; Heeb, M.J.; Lai, C. Gas6, a ligand for the receptor protein-tyrosine kinase Tyro-3, is widely expressed in the central nervous system. Brain Res. 1999, 816, 646–661. [Google Scholar] [CrossRef]
- Zagórska, A.; Través, P.G.; Lew, E.D.; Dransfield, I.; Lemke, G. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol. 2014, 15, 920–928. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xie, L.; Liu, C.; Zhang, Q.; Sun, S. PTEN/PI3k/AKT Regulates Macrophage Polarization in Emphysematous mice. Scand. J. Immunol. 2017, 85, 395–405. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, V.; Kuhn, R.J.; Fekete, D.M. Exploring the Expression and Function of cTyro3, a Candidate Zika Virus Receptor, in the Embryonic Chicken Brain and Inner Ear. Viruses 2023, 15, 247. https://doi.org/10.3390/v15010247
Negi V, Kuhn RJ, Fekete DM. Exploring the Expression and Function of cTyro3, a Candidate Zika Virus Receptor, in the Embryonic Chicken Brain and Inner Ear. Viruses. 2023; 15(1):247. https://doi.org/10.3390/v15010247
Chicago/Turabian StyleNegi, Vashi, Richard J. Kuhn, and Donna M. Fekete. 2023. "Exploring the Expression and Function of cTyro3, a Candidate Zika Virus Receptor, in the Embryonic Chicken Brain and Inner Ear" Viruses 15, no. 1: 247. https://doi.org/10.3390/v15010247
APA StyleNegi, V., Kuhn, R. J., & Fekete, D. M. (2023). Exploring the Expression and Function of cTyro3, a Candidate Zika Virus Receptor, in the Embryonic Chicken Brain and Inner Ear. Viruses, 15(1), 247. https://doi.org/10.3390/v15010247






