A Systematic Review on Cardiometabolic Risks and Perinatal Outcomes among Pregnant Women Living with HIV in the Era of Antiretroviral Therapy
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Eligibility Criteria
2.3. Literature Search
2.4. Study Selection
2.5. Data Extraction and Synthesis
3. Results
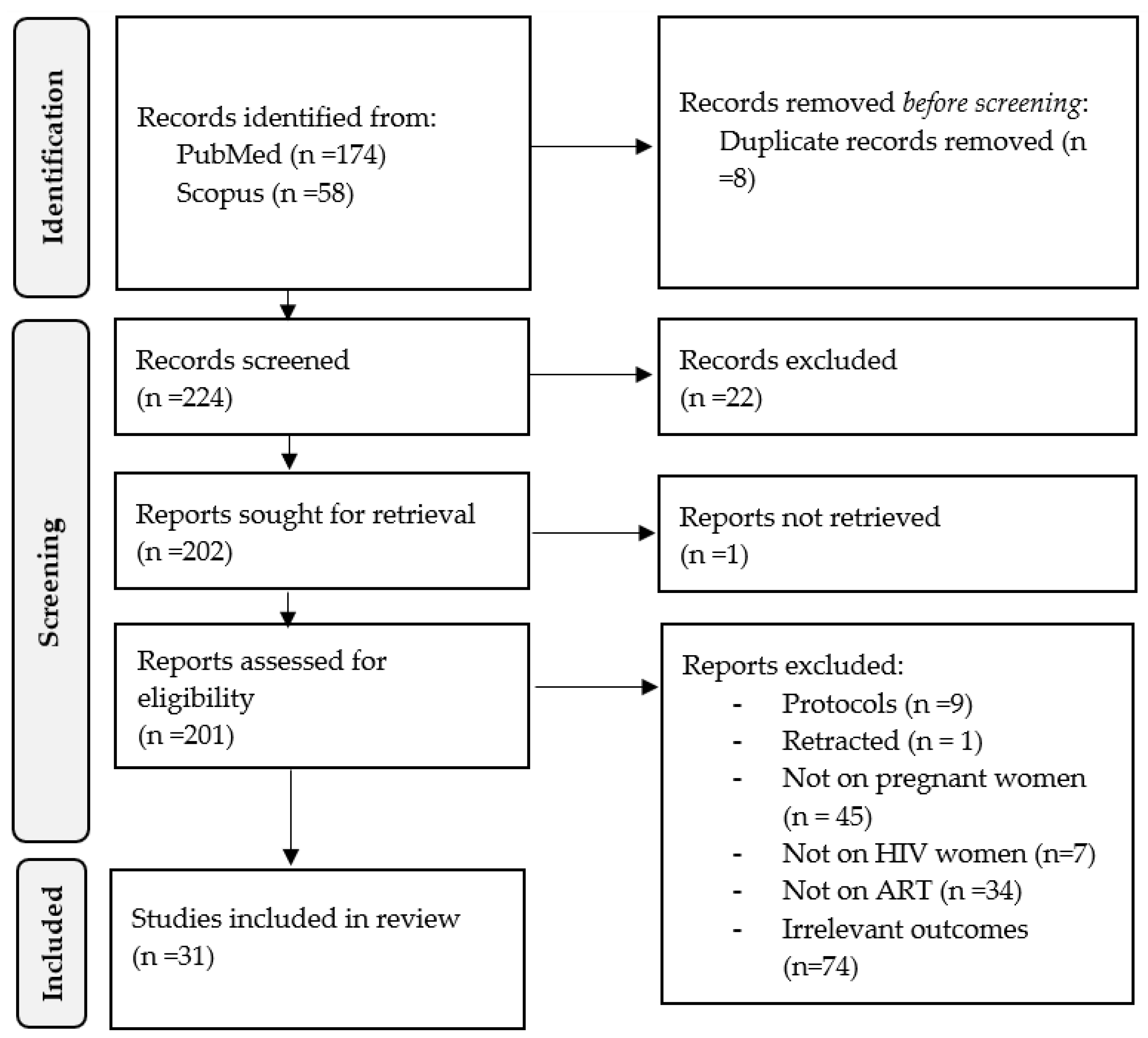
3.1. Search Strategy, Selection Criteria, and Overall Features of Included Studies
3.2. Synthesis of Evidence
3.2.1. Antiretroviral Therapy and Neonatal Outcomes
3.2.2. Antiretroviral Therapy and Cardiometabolic Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Global HIV/AIDS Epidemic. Available online: https://www.kff.org/global-health-policy/fact-sheet/the-global-hivaids-epidemic/# (accessed on 12 April 2023).
- Lule, F. Global Burden of HIV/AIDS. In Handbook of Global Health; Kickbusch, I., Ganten, D., Moeti, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 539–586. ISBN 978-3-030-45009-0. [Google Scholar]
- Pilcher, H. HIV Outpaces Global Response. Nature 2004, 59. [Google Scholar] [CrossRef]
- The Working Group on Mother-To-Child Transmission of HIV. Rates of Mother-to-Child Transmission of HIV-1 in Africa, America, and Europe: Results from 13 Perinatal Studies. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 8, 506–510. [Google Scholar] [CrossRef] [PubMed]
- De Cock, K.M.; Fowler, M.G.; Mercier, E.; De Vincenzi, I.; Saba, J.; Hoff, E.; Alnwick, D.J.; Rogers, M.; Shaffer, N. Prevention of Mother-to-Child HIV Transmission in Resource-Poor Countries. JAMA 2000, 283, 1167–1182. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.; Foroutan, F.; Mirza, R.; Mah Ming, J.; Alexander, P.E.; Agarwal, A.; Lesi, O.; Merglen, A.; Chang, Y.; Zhang, Y.; et al. Antiretroviral Therapy for Pregnant Women Living with HIV or Hepatitis B: A Systematic Review and Meta-Analysis. BMJ Open 2017, 7, e019022. [Google Scholar] [CrossRef] [PubMed]
- Pinnetti, C.; Tintoni, M.; Ammas-sari, A.; Tamburrini, E.; Bernardi, S.; Liuzzi, G.-P.; Scambia, G.; Perno, C.F.; Floridia, M.; Antinori, A.; et al. Successful Prevention of HIV Mother-to-Child Transmission with Dolutegravir-Based Combination Antiretroviral Therapy in a Vertically Infected Pregnant Woman with Multiclass Highly Drug-Resistant HIV-1. AIDS 2015, 29, 2534–2537. [Google Scholar] [CrossRef]
- WHO. Recommends Dolutegravir as Preferred HIV Treatment Option in All Populations. Available online: https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations (accessed on 10 May 2023).
- Zipursky, J.; Loutfy, M. Dolutegravir for Pregnant Women Living with HIV. CMAJ 2020, 192, E217–E218. [Google Scholar] [CrossRef] [PubMed]
- Zash, R.; Holmes, L.B.; Diseko, M.; Jacobson, D.L.; Mayondi, G.K.; Mabuta, J.; Jackson-Gibson, M.; Mmalane, M.; Gaolathe, T.; Lockman, S.; et al. Update on Neural Tube Defects with Antiretroviral Exposure in the Tsepamo Study, Botswana. AIDS 2022, 6–10. [Google Scholar]
- Bollen, P.; Freriksen, J.; Konopnicki, D.; Weizsäcker, K.; Hidalgo Tenorio, C.; Moltó, J.; Taylor, G.; Alba-Alejandre, I.; Van Crevel, R.; Colbers, A.; et al. The Effect of Pregnancy on the Pharmacokinetics of Total and Unbound Dolutegravir and Its Main Metabolite in Women Living with Human Immunodeficiency Virus. Clin. Infect. Dis. 2021, 72, 121–127. [Google Scholar] [CrossRef]
- Snijdewind, I.J.M.; Smit, C.; Godfried, M.H.; Bakker, R.; Nellen, J.F.J.B.; Jaddoe, V.W.V.; Van Leeuwen, E.; Reiss, P.; Steegers, E.A.P.; Van Der Ende, M.E. Preconception Use of CART by HIV-Positive Pregnant Women Increases the Risk of Infants Being Born Small for Gestational Age. PLoS ONE 2018, 13, e0191389. [Google Scholar] [CrossRef]
- Malaba, T.R.; Phillips, T.; Le Roux, S.; Brittain, K.; Zerbe, A.; Petro, G.; Ronan, A.; McIntyre, J.A.; Abrams, E.J.; Myer, L. Antiretroviral Therapy Use during Pregnancy and Adverse Birth Outcomes in South African Women. Int. J. Epidemiol. 2017, 46, 1678–1689. [Google Scholar] [CrossRef]
- Nkeh-Chungag, B.N.; Engwa, G.A.; Businge, C.; Mdondolo, M.; Pajaro Medina, M.; Goswami, N. Assessment of the Impact of HIV Infection and Anti-Retroviral Treatment on the Cardiometabolic Health of Pregnant Mothers and Their Offspring (ARTMOMSBABES). BMC Cardiovasc. Disord. 2021, 21, 322. [Google Scholar] [CrossRef] [PubMed]
- Naicker, T.; Phoswa, W.N.; Onyangunga, O.A.; Gathiram, P.; Moodley, J. Angiogenesis, Lymphangiogenesis, and the Immune Response in South African Preeclamptic Women Receiving HAART. Int. J. Mol. Sci. 2019, 20, 3728. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.T.; Adamson, D. Cardiovascular Disease in Pregnancy. Obstet. Gynaecol. Reprod. Med. 2013, 23, 195–201. [Google Scholar] [CrossRef]
- Luzi, K.; Eckard, A.R.; Lattanzi, A.; Zona, S.; Modena, M.G.; Facchinetti, F.; Guaraldi, G. Effects of Pregnancy on Endothelial Function and Cardiovascular Disease Risk in HIV-Infected Women. Pregnancy Hypertens. 2013, 3, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M.; Newhouse, C.; Chu, B.; Stringer, J.S.A.; Currier, J.S. Non-Communicable Diseases in Pregnant and Postpartum Women Living with HIV: Implications for Health Throughout the Life Course. Curr. HIV/AIDS Rep. 2021, 18, 73–86. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV Infection, Inflammation, Immunosenescence, and Aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; De Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S251–S260. [Google Scholar] [CrossRef]
- Koethe, J.R.; Heimburger, D.C. Nutritional Aspects of HIV-Associated Wasting in Sub-Saharan Africa. Am. J. Clin. Nutr. 2010, 91, 1138S–1142S. [Google Scholar] [CrossRef]
- Kumar, S.; Samaras, K. The Impact of Weight Gain During HIV Treatment on Risk of Pre-Diabetes, Diabetes Mellitus, Cardiovascular Disease, and Mortality. Front. Endocrinol. 2018, 9, 705. [Google Scholar] [CrossRef]
- Rosala-Hallas, A.; Bartlett, J.W.; Filteau, S. Growth of HIV-Exposed Uninfected, Compared with HIV-Unexposed, Zambian Children: A Longitudinal Analysis from Infancy to School Age. BMC Pediatr. 2017, 17, 80. [Google Scholar] [CrossRef]
- Brocklehurst, P. The Association between Maternal HIV Infection and Perinatal Outcome: A Systematic Review of the Literature and Meta-Analysis. Br. J. Obstet. Gynaecol. 1998, 105, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Rollins, N.C.; Coovadia, H.M.; Bland, R.M.; Coutsoudis, A.; Bennish, M.L.; Patel, D.; Newell, M.-L. Pregnancy Outcomes in HIV-Infected and Uninfected Women in Rural and Urban South Africa. J. Acquir. Immune Defic. Syndr. 2007, 44, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, J.; Tan, D.; Huang, G.; Zheng, J.; Xiao, J.; Wang, H.; Huang, Q.; Feng, N.; Zhang, G.; et al. Maternal HIV Infection and Risk of Adverse Pregnancy Outcomes in Hunan Province, China: A Prospective Cohort Study. Medicine 2020, 99, e19213. [Google Scholar] [CrossRef] [PubMed]
- Aizire, J.; Fowler, M.G.; Coovadia, H.M. Operational Issues and Barriers to Implementation of Prevention of Mother-to-Child Transmission of Hiv (PMTCT) Interventions in Sub-Saharan Africa. Curr. HIV Res. 2013, 11, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Moseholm, E.; Helleberg, M.; Sandholdt, H.; Katzenstein, T.L.; Storgaard, M.; Pedersen, G.; Johansen, I.S.; Weis, N. Children Exposed or Unexposed to Human Immunodeficiency Virus: Weight, Height, and Body Mass Index during the First 5 Years of Life-a Danish Nationwide Cohort. Clin. Infect. Dis. 2020, 70, 2168–2177. [Google Scholar] [CrossRef]
- Njom Nlend, A.E.; Motaze, A.C.N.; Sandie, A.; Fokam, J. HIV-1 Transmission and Survival According to Feeding Options in Infants Born to HIV-Infected Women in Yaoundé, Cameroon. BMC Pediatr. 2018, 18, 69. [Google Scholar] [CrossRef]
- Bernstein, H.B.; Wegman, A.D. HIV Infection: Antepartum Treatment and Management. Clin. Obstet. Gynecol. 2017, 61, 122–136. [Google Scholar] [CrossRef]
- Adair, L.S.; Cole, T.J. Rapid Child Growth Raises Blood Pressure in Adolescent Boys Who Were Thin at Birth. Hypertension 2003, 41, 451–456. [Google Scholar] [CrossRef]
- Jasper, E.A.; Cho, H.; Breheny, P.J.; Bao, W.; Dagle, J.M.; Ryckman, K.K. Perinatal Determinants of Growth Trajectories in Children Born Preterm. PLoS ONE 2021, 16, e0245387. [Google Scholar] [CrossRef]
- Nhampossa, T.; González, R.; Nhacolo, A.; Garcia-Otero, L.; Quintó, L.; Mazuze, M.; Mendes, A.; Casellas, A.; Bambo, G.; Couto, A.; et al. Burden, Clinical Presentation and Risk Factors of Advanced HIV Disease in Pregnant Mozambican Women. BMC Pregnancy Childbirth 2022, 22, 756. [Google Scholar] [CrossRef]
- Le Roux, S.M.; Abrams, E.J.; Donald, K.A.; Brittain, K.; Phillips, T.K.; Zerbe, A.; le Roux, D.M.; Kroon, M.; Myer, L. Infectious Morbidity of Breastfed, HIV-Exposed Uninfected Infants under Conditions of Universal Antiretroviral Therapy in South Africa: A Prospective Cohort Study. Lancet Child Adolesc. Health 2020, 4, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.; Langat, A.; Singa, B.; Kinuthia, J.; Itindi, J.; Nyaboe, E.; Ng’Anga, L.W.; Katana, A.; John-Stewart, G.C.; McGrath, C.J. Higher Prevalence of Stunting and Poor Growth Outcomes in HIV-Exposed Uninfected than HIV-Unexposed Infants in Kenya. AIDS 2022, 36, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Ejigu, Y.; Magnus, J.H.; Sundby, J.; Magnus, M.C. Pregnancy Outcome among HIV-Infected Women on Different Antiretroviral Therapies in Ethiopia: A Cohort Study. BMJ Open 2019, 9, e027344. [Google Scholar] [CrossRef] [PubMed]
- Slogrove, A.L.; Johnson, L.F.; Powis, K.M. Population-Level Mortality Associated with HIV Exposure in HIV-Uninfected Infants in Botswana and South Africa: A Model-Based Evaluation. J. Trop. Pediatr. 2019, 65, 373–379. [Google Scholar] [CrossRef]
- Chilyabanyama, O.N.; Chilengi, R.; Laban, N.M.; Chirwa, M.; Simunyandi, M.; Hatyoka, L.M.; Ngaruye, I.; Iqbal, N.T.; Bosomprah, S. Comparing Growth Velocity of HIV Exposed and Non-Exposed Infants: An Observational Study of Infants Enrolled in a Randomized Control Trial in Zambia. PLoS ONE 2021, 16, e0256443. [Google Scholar] [CrossRef]
- Taron-Brocard, C.; Le Chenadec, J.; Faye, A.; Dollfus, C.; Goetghebuer, T.; Gajdos, V.; MarcLabaune, J.; Perilhou, A.; Mandelbrot, L.; Blanche, S.; et al. Increased Risk of Serious Bacterial Infections Due to Maternal Immunosuppression in HIV-Exposed Uninfected Infants in a European Country. Clin. Infect. Dis. 2014, 59, 1332–1345. [Google Scholar] [CrossRef]
- Ejigu, Y.; Magnus, J.H.; Sundby, J.; Magnus, M.C. Differences in Growth of HIV-Exposed Uninfected Infants in Ethiopia According to Timing of in-Utero Antiretroviral Therapy Exposure. Pediatr. Infect. Dis. J. 2020, 39, 730–736. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Osmundo, G.d.S.; da Costa, R.A.; Ruocco, R.M.A.; Francisco, R.P.V. Pregnancy in Women Living with Perinatally Acquired HIV: Perinatal Outcomes and Drug Resistance Profile. Clinics 2023, 78, 100174. [Google Scholar] [CrossRef]
- Tymejczyk, O.; Deschamps, M.M.; Rouzier, V.; McNairy, M.L.; Peck, R.N.; Malha, L.; Macius, Y.; Fitzgerald, D.W.; Pape, J.W.; Nash, D. Estimated Blood Pressure Trajectories and Hypertension Patterns among Pregnant Women Living with HIV, Haiti, 2007–2017. J. Clin. Hypertens. 2022, 24, 237–245. [Google Scholar] [CrossRef]
- Madlala, H.P.; Malaba, T.R.; Newell, M.L.; Myer, L. Elevated Body Mass Index during Pregnancy and Gestational Weight Gain in HIV-Infected and HIV-Uninfected Women in Cape Town, South Africa: Association with Adverse Birth Outcomes. Trop. Med. Int. Health 2020, 25, 702–713. [Google Scholar] [CrossRef]
- Garća-Otero, L.; López, M.; Gómez, O.; Goncé, A.; Bennasar, M.; Martńez, J.M.; Valenzuela-Alcaraz, B.; Rodriguez-López, M.; Sitges, M.; Loncà, M.; et al. Zidovudine Treatment in HIV-Infected Pregnant Women Is Associated with Fetal Cardiac Remodelling. AIDS 2016, 30, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- De la Calle, M.; Rodriguez, R.; Deirós, L.; Bartha, J.L. Fetal Cardiac Biometry and Function in HIV-Infected Pregnant Women Exposed to HAART Therapy. Prenat. Diagn. 2015, 35, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Tuomala, R.E.; Shapiro, D.E.; Mofenson, L.M.; Bryson, Y.; Culnane, M.; Hughes, M.D.; O’Sullivan, M.; Scott, G.; Stek, A.M.; Wara, D.; et al. Antiretroviral Therapy during Pregnancy and the Risk of an Adverse Outcome. N. Engl. J. Med. 2002, 346, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Areechokchai, D.; Bowonwatanuwong, C.; Phonrat, B.; Pitisuttithum, P.; Maek-A-Nantawat, W. Pregnancy Outcomes Among HIV-Infected Women Undergoing Antiretroviral Therapy. Open AIDS J. 2009, 3, 8–13. [Google Scholar] [CrossRef]
- Barral, M.F.M.; de Oliveira, G.R.; Lobato, R.C.; Mendoza-Sassi, R.A.; Martínez, A.M.B.; Gonçalves, C.V. Risk Factors of HIV-1 Vertical Transmission (VT) and the Influence of Antiretroviral Therapy (ART) in Pregnancy Outcome. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 133–138. [Google Scholar] [CrossRef]
- Santini-Oliveira, M.; Friedman, R.K.; Veloso, V.G.; Cunha, C.B.; Pilotto, J.H.; Marins, L.M.S.; João, E.C.; Torres, T.S.; Grinsztejn, B. Incidence of Antiretroviral Adverse Drug Reactions in Pregnant Women in Two Referral Centers for HIV Prevention of Mother-to-Child-Transmission Care and Research in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2014, 18, 372–378. [Google Scholar] [CrossRef]
- Nyemba, D.C.; Kalk, E.; Vinikoor, M.J.; Madlala, H.P.; Mubiana-Mbewe, M.; Mzumara, M.; Moore, C.B.; Slogrove, A.L.; Boulle, A.; Davies, M.-A.; et al. Growth Patterns of Infants with In-Utero HIV and ARV Exposure in Cape Town, South Africa and Lusaka, Zambia. BMC Public Health 2022, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Baltrusaitis, K.; Makanani, B.; Tierney, C.; Fowler, M.G.; Moodley, D.; Theron, G.; Nyakudya, L.H.; Tomu, M.; Fairlie, L.; George, K.; et al. Maternal and Infant Renal Safety Following Tenofovir Disoproxil Fumarate Exposure during Pregnancy in a Randomized Control Trial. BMC Infect. Dis 2022, 22, 634. [Google Scholar] [CrossRef]
- Zash, R.; Jacobson, D.L.; Diseko, M.; Mayondi, G.; Mmalane, M.; Essex, M.; Gaolethe, T.; Petlo, C.; Lockman, S.; Holmes, L.B.; et al. Comparative Safety of Dolutegravir-Based or Efavirenz-Based Antiretroviral Treatment Started during Pregnancy in Botswana: An Observational Study. Lancet Glob. Health 2018, 6, e804–e810. [Google Scholar] [CrossRef] [PubMed]
- Montgomery-Taylor, S.; Hemelaar, J. Management and Outcomes of Pregnancies among Women with HIV in Oxford, UK, in 2008-2012. Int. J. Gynecol. Obstet. 2015, 130, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.S.; Hofer, C.B.; Costa, T.T.; Nogueira, S.A.; Oliveira, R.H.; Abreu, T.F.; Evangelista, L.A.; Farias, I.F.A.; Mercadante, R.T.C.; Garcia, M.F.L.; et al. Pregnancy Outcome in Women Infected with Hiv-1 Receiving Combination Antiretroviral Therapy before versus after Conception. Sex. Transm. Infect. 2009, 85, 82–87. [Google Scholar] [CrossRef]
- Boer, K.; Nellen, J.F.; Patel, D.; Timmermans, S.; Tempelman, C.; Wibaut, M.; Sluman, M.A.; Van Der Ende, M.E.; Godfried, M.H. The AmRo Study: Pregnancy Outcome in HIV-1-Infected Women under Effective Highly Active Antiretroviral Therapy and a Policy of Vaginal Delivery. BJOG 2007, 114, 148–155. [Google Scholar] [CrossRef]
- Aaron, E.; Bonacquisti, A.; Mathew, L.; Alleyne, G.; Bamford, L.P.; Culhane, J.F. Small-for-Gestational-Age Births in Pregnant Women with HIV, Due to Severity of HIV Disease, Not Antiretroviral Therapy. Infect. Dis. Obstet. Gynecol. 2012, 2012, 135030. [Google Scholar] [CrossRef] [PubMed]
- Silverman, N.S.; Watts, D.H.; Hitti, J.; Money, D.M.; Livingston, E.; Axelrod, J.; Ernest, J.M.; Robbins, D.; Divito, M.M.; Silverman, N.S. Initial Multicenter Experience with Double Nucleoside Therapy for Human Immunodeficiency Virus Infection during Pregnancy. Obstet. Gynecol. 1998, 6, 237–243. [Google Scholar]
- McDonald, C.R.; Conroy, A.L.; Gamble, J.L.; Papp, E.; Hawkes, M.; Olwoch, P.; Natureeba, P.; Kamya, M.; Silverman, M.; Cohan, D.; et al. Estradiol Levels Are Altered in Human Immunodeficiency Virus-Infected Pregnant Women Randomized to Efavirenz-Versus Lopinavir/Ritonavir-Based Antiretroviral Therapy. Clin. Infect. Dis. 2018, 66, 428–436. [Google Scholar] [CrossRef]
- González, R.; Rupérez, M.; Sevene, E.; Vala, A.; Maculuve, S.; Bulo, H.; Nhacolo, A.; Mayor, A.; Aponte, J.J.; Macete, E.; et al. Effects of HIV Infection on Maternal and Neonatal Health in Southern Mozambique: A Prospective Cohort Study after a Decade of Antiretroviral Drugs Roll Out. PLoS ONE 2017, 12, e0178134. [Google Scholar] [CrossRef]
- Young, S.; Murray, K.; Mwesigwa, J.; Natureeba, P.; Osterbauer, B.; Achan, J.; Arinaitwe, E.; Clark, T.; Ades, V.; Plenty, A.; et al. Maternal Nutritional Status Predicts Adverse Birth Outcomes among HIV-Infected Rural Ugandan Women Receiving Combination Antiretroviral Therapy. PLoS ONE 2012, 7, e41934. [Google Scholar] [CrossRef]
- Powis, K.M.; Kitch, D.; Ogwu, A.; Hughes, M.D.; Lockman, S.; Leidner, J.; Van Widenfelt, E.; Moffat, C.; Moyo, S.; Makhema, J.; et al. Increased Risk of Preterm Delivery among HIV-Infected Women Randomized to Protease versus Nucleoside Reverse Transcriptase Inhibitor-Based HAART during Pregnancy. J. Infect. Dis. 2011, 204, 506–514. [Google Scholar] [CrossRef]
- Drake, A.L.; Roxby, A.C.; Ongecha-Owuor, F.; Kiarie, J.; John-Stewart, G.; Wald, A.; Richardson, B.A.; Hitti, J.; Overbaugh, J.; Emery, S.; et al. Valacyclovir Suppressive Therapy Reduces Plasma and Breast Milk HIV-1 RNA Levels during Pregnancy and Postpartum: A Randomized Trial. J. Infect. Dis. 2012, 205, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Mofenson, L.M.; Lambert, J.S.; Stiehm, E.R.; Bethel, J.; Meyer, W.A.; Whitehouse, J.; Moye, J., Jr.; Reichelderfer, P.; Harris, D.R.; Fowler, M.G.; et al. Risk Factors for Perinatal Transmission of Human Immunodeficiency Virus Type 1 in Women Treated with Zidovudine a Bstract Background Maternal, Obstetrical, and Infant-Relat. N. Engl. J. Med. 1999, 341, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.L.; Hughes, M.D.; Ogwu, A.; Kitch, D.; Lockman, S.; Moffat, C.; Makhema, J.; Moyo, S.; Thior, I.; McIntosh, K.; et al. Antiretroviral Regimens in Pregnancy and Breast-Feeding in Botswana. N. Engl. J. Med. 2010, 362, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Lallemant, M.; Le Coeur, S.; Sirirungsi, W.; Cressey, T.R.; Ngo-Giang-Huong, N.; Traisathit, P.; Klinbuayaem, V.; Sabsanong, P.; Kanjanavikai, P.; Jourdain, G.; et al. Randomized Noninferiority Trial of Two Maternal Single-Dose Nevirapine-Sparing Regimens to Prevent Perinatal HIV in Thailand. AIDS 2015, 29, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, N.; Best, B.M.; Wang, J.; Capparelli, E.V.; Stek, A.; Barr, E.; Buschur, S.L.; Acosta, E.P.; Smith, E.; Chakhtoura, N.; et al. Dolutegravir Pharmacokinetics in Pregnant and Postpartum Women Living with HIV. AIDS 2018, 32, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Aizire, J.; Brooks, K.M.; Mirochnick, M.; Flynn, P.M.; Butler, K.; Kiser, J.J.; Siberry, G.K.; Fenton, T.; Cababasay, M.; Fowler, M.G. Antenatal Intracellular Concentrations of Tenofovir Diphosphate and Emtricitabine Triphosphate and Associations Between Tenofovir Diphosphate and Severe Adverse Pregnancy Outcomes: IMPAACT-PROMISE (1077BF) Trial. J. Acquir. Immune Defic. Syndr. 2020, 83, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.G.; Venter, W.D.F. Cardiovascular Toxicity of Contemporary Antiretroviral Therapy. Curr. Opin. HIV AIDS 2021, 16, 286–291. [Google Scholar] [CrossRef]
- Wedi, C.O.O.; Kirtley, S.; Hopewell, S.; Corrigan, R.; Kennedy, S.H.; Hemelaar, J. Perinatal Outcomes Associated with Maternal HIV Infection: A Systematic Review and Meta-Analysis. Lancet HIV 2016, 3, e33–e48. [Google Scholar] [CrossRef]
- Shinar, S.; Agrawal, S.; Ryu, M.; Walmsley, S.; Serghides, L.; Yudin, M.H.; Murphy, K.E. Perinatal Outcomes in Women Living with HIV-1 and Receiving Antiretroviral Therapy—A Systematic Review and Meta-Analysis. Acta Obstet. Gynecol. Scand. 2022, 101, 168–182. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Tong, J.; Yin, A.; Wu, L.; Niu, J. Association of Maternal Obesity with Preterm Birth Phenotype and Mediation Effects of Gestational Diabetes Mellitus and Preeclampsia: A Prospective Cohort Study. BMC Pregnancy Childbirth 2022, 22, 459. [Google Scholar] [CrossRef]
- Wei, Y.M.; Yang, H.X.; Zhu, W.W.; Liu, X.Y.; Meng, W.Y.; Wang, Y.Q.; Shang, L.X.; Cai, Z.Y.; Ji, L.P.; Wang, Y.F.; et al. Risk of Adverse Pregnancy Outcomes Stratified for Pre-Pregnancy Body Mass Index. J. Matern.-Fetal Neonatal Med. 2016, 29, 2205–2209. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, Y.; Wang, N.; Lin, W.; Liu, Y.; Wen, D. Maternal Body Mass Index and Risk of Neonatal Adverse Outcomes in China: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2019, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, X.; Yan, Y. Estimated Global Overweight and Obesity Burden in Pregnant Women Based on Panel Data Model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.; Rochow, N.; Kunze, M.; Hesse, V.; Dudenhausen, J.W.; Voigt, M. Obesity in Pregnant Women: A 20-Year Analysis of the German Experience. Eur. J. Clin. Nutr. 2021, 75, 1757–1763. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and Maternal Obesity: Epidemiology and Health Consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Gilleece, Y. Dagny Krankowska ART in Pregnant Women Living with HIV. Lancet 2021, 397, 1240–1241. [Google Scholar] [CrossRef]
- Recommendations for the Use of Antiretroviral Drugs during Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States. Available online: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/recommendations-arv-drugs-pregnancy-overview?view=full (accessed on 4 May 2023).
- Lartey, A.; Marquis, G.S.; Mazur, R.; Perez-Escamilla, R.; Brakohiapa, L.; Ampofo, W.; Sellen, D.; Adu-Afarwuah, S. Maternal HIV Is Associated with Reduced Growth in the First Year of Life among Infants in the Eastern Region of Ghana: The Research to Improve Infant Nutrition and Growth (RIING) Project. Matern. Child Nutr. 2014, 10, 604–616. [Google Scholar] [CrossRef]
- Xiao, P.L.; Zhou, Y.B.; Chen, Y.; Yang, M.X.; Song, X.X.; Shi, Y.; Jiang, Q.W. Association between Maternal HIV Infection and Low Birth Weight and Prematurity: A Meta-Analysis of Cohort Studies. BMC Pregnancy Childbirth 2015, 15, 51. [Google Scholar] [CrossRef]
- Gumbo, F.Z.; Duri, K.; Kandawasvika, G.Q.; Kurewa, N.E.; Mapingure, M.P.; Munjoma, M.W.; Rusakaniko, S.; Chirenje, M.Z.; Stray-Pedersen, B. Risk Factors of HIV Vertical Transmission in a Cohort of Women under a PMTCT Program at Three Peri-Urban Clinics in a Resource-Poor Setting. J. Perinatol. 2010, 30, 717–723. [Google Scholar] [CrossRef]
- Iv, W.A.; Kwiek, J.J. Role of the Placenta in Adverse Perinatal Outcomes among HIV-1 Seropositive Women. J. Nippon Med. Sch. 2013, 80, 90–94. [Google Scholar] [CrossRef]
- Eckard, A.R.; Kirk, S.E.; Hagood, N.L. Contemporary Issues in Pregnancy (and Offspring) in the Current HIV Era. Curr. HIV/AIDS Rep. 2019, 16, 492–500. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year of Publication | Country | Study Design | Population States | Cardiometabolic Markers and Perinatal Outcomes Reported | Summary of Findings |
|---|---|---|---|---|---|
| (Osmundo et al., 2023) [43] | Brazil | Retrospective cohort | 186 pWLWH on ART. | Preterm birth, low birth weight, foetal loss, mother-to-child transmission. | Pregnant women living with HIV (pWLWH) did not increase the risk of adverse perinatal outcomes, and preterm birth [OR = 0.7, 95% CI: (0.3–1.8), p = 0.499]. However, in the third trimester, anaemia was associated with preterm birth (p = 0.039). |
| Tymejczyk et al., 2022 [44] | USA | Retrospective cohort | 1965 pWLWH with about 2306 live births on the PMTCT program. | BMI, body weight, and blood pressure. | Hypertension was increased at about 20 weeks gestation. |
| Nhampossa et al., 2022 [33] | Mozambique | Prospective, retrospective cohort | 260 pWLWH on ART who attended antenatal care. | BMI, preterm birth, low birth weight, neonates deaths. | There were no significant differences in the prevalence of maternal death, preterm delivery [20 (11.9), p = 0.187] compared to [99 (8.4)], low birth weight, and neonatal HIV infection between women with and without advanced HIV diseases. |
| Madlala et al., 2020 [45] | South Africa | Prospective cohort | 249 pWLWH on ART during pregnancy. | Hypertension, preterm, stillbirth, low birth weight. | Maternal obesity was associated with an increased risk of having high birth weight and large size for gestational age infants. In the subset cohort, gestational weight gain was associated with an increased risk of spontaneous preterm delivery [OR = 4.35, 95% CI: (1.55–12.21), p = 0.005] and high birth weight infants. |
| Garća-Otero et al., 2016 [46] | Spain | Prospective cohort | 42 pWLWH on cART. | Preterm, low birth weight, diastolic and heart rate. | Cardiac remodelling and dysfunction were observed in foetuses from HIV-infected mothers on cART. Moreover, HIV infected group had significantly increased preterm birth [(6.0 ± 14.3), p = 0.002] compared to the uninfected-HIV [1.0 ± 1.2] group. |
| De la Calle et al., 2015 [47] | Spain | Longitudinal cohort | 29 pWLWH on HAART. | Systolic and diastolic velocity. | There were no significant differences in foetal cardiac parameters, especially in those born from HIV-infected pregnant women treated with HAART. |
| Tuomala et al., 2002 [48] | USA | Clinical trial | 2123 pWLWH on ART | Stillbirth, low birth weight, premature delivery. | When compared to the group without ART, or monotherapy, the cART group was not associated with an increased risk of premature delivery [OR = 1.80, 95% CI: (0.94–3.43), p > 0.05], low birth weight, or stillbirth in their infants. |
| Areechokchai et al., 2009 [49] | Thailand | Prospective and retrospective cohorts | 246 pWLWH on ART. | Dyslipidaemia, preterm, stillbirth. | Compared to antenatal care clinics, a significant increase in the prevalence of preterm delivery was noted in the groups on cART (19.4%) or initiating PMTCT (19%) during labour without antennal care compared to those on PTMCT during antenatal care (6.9%). Significant dyslipidaemia in ART compared to PMTCT during antenatal care. |
| Barral et al., 2014 [50] | Brazil | Cohorts | 262 pWLWH on ART. | Low-birth weight. | ART showed no effect on the outcome of pregnancy. However, initiation of prenatal care after the first trimester showed an effect on low birth weight and increased risk of prematurity. |
| Santini-Oliveira et al., 2014 [51] | Brazil | Prospective study | 36 pWLWH on ART. | Preterm delivery, low birth weight and birth abnormalities. | Low frequency of preterm delivery in ART-exposed [4 (11.1)] compared to ART-naïve [14 (7.8)]. |
| Nyemba et al., 2022 [52] | South Africa, and Zambia | Prospective cohort | 395 pWLWH on ART. | Low birth weight. | Length for age was lower among infants who were HIV-exposed-uninfected. |
| Baltrusaitis et al., 2022 [53] | USA | Open-label randomised controlled trial | 479 pWLWH treated with ART at week 14 of pregnancy. | Low birth weight. | The TDF-ART regimen showed no observed safety concerns for maternal or infant renal function during pregnancy. |
| Zash et al., 2018 [54] | USA | Observational study | 6322 pWLWH (1729 dolutegravir) and 4593 on EFV. | Preterm, stillbirth, neonatal deaths, and low-birth weight. | There were no significant differences by regimen in the individual outcomes of stillbirth, neonatal death, preterm birth [RR = 0.98, 95% CI: (0.87–1.11)], very preterm birth [RR = 1.09, 95% CI: (0.82–1.45)], and small for gestational age (SGA). |
| Snijdewind et al., 2018 [12] | Netherlands | Retrospective and observational study | 2184 pWLWH receiving cART. | Gestational age, low birth weight, and preterm delivery. | Women starting cART before conception had an increased risk of having SGA infants compared to women starting cART after conception. There was no significant difference in perinatal death or birth weight between women on cART pre- and post-conception. |
| Montgomery, 2015 [55] | UK | Retrospective cohort | 27 pWLWH who started ART during pregnancy. | Preterm births, low birth weight, stillbirth. | One neonate was diagnosed with HIV infection. There were 6 preterm births, 9 cases of low birth weight, 11 small-for-gestational-age neonates, and 1 stillbirth. |
| Machado et al., 2009 [56] | Brazil | Prospective cohort | 696 pWLWH, 130 on ART before pregnancy, and 566 on ART after conception. | Preterm and low-birth weight hypertension. | Patients on HAART pre-conception had an increased risk of low birth weight and preterm delivery [OR = 2.22, 95% CI: (1.08–4.54), p = 0.009]. |
| Ejigu et al., 2019 [36] | Ethiopia | Retrospective cohort study | 1663 pWLWH on ART. | Preterm and low birth weight. | A higher risk of preterm birth among women who initiated HAART before pregnancy [OR = 0.93, 95% CI: (0.78–1.29)] compared with zidovudine monotherapy [OR = 0.35, 95% CI: (0.19–0.64)]. Pregnancies exposed to nevirapine-based HAART also had a greater risk of preterm births [OR = 1.44, 95% CI: (1.06–1.9)] than zidovudine-based HAART [OR = 1.16, 95% CI: (0.83–1.62)] and PI-based HAART [OR = 1.81, 95% CI: (0.78–4.18)]. |
| Boer et al., 2007 [57] | Netherlands | Prospective cohort | 98 pWLWH on HAART. | Mother-to-child transmission, preterm delivery, low birth weight, preeclampsia. | When compared to the control group, there was an increased in preterm delivery in pWLWH [15 (10%)] compared to 6 (3%) in HIV-negative. HAART was associated with increased preterm delivery observed after the first trimester [OR = 2.84, p = 0.04}. |
| Li et al., 2020 [26] | China | Prospective cohort | 414 of 483 pWLWH on ART. | Stillbirth, preterm birth, low birth weight and small for gestational age. | Stillbirth, preterm birth, low birth weight, and SGA were significantly increased by maternal HIV infection but not neonatal asphyxia or birth abnormalities. Compared to untreated HIV infection, mono/dual therapy and HAART protected stillbirth when most HIV-infected pregnant women started ARV therapy during or after the second trimester. |
| Aaron et al., 2012 [58] | USA | Prospective cohort | 183 pWLWH on ART. | Preterm, low birth weight, infant birth weight | Women taking NNRTI had a lower risk of having an SGA infant than women on PIs. |
| Silverman et al., 1998 [59] | USA | Multicenter, prospective observational study | 39 pWLWH on ART. | Birth weight | There were no significant adverse neonatal outcomes except for the three preterm newborns. |
| McDonald et al., 2018 [60] | Uganda, Canada, and the USA | Randomised controlled trial | 326 pWLWH (160 randomised to the EFV arm and 166 women to the LPV-based ART. | Preterm, low birth weight, stillbirth | There was no significant difference on preterm delivery in EFV [24 (15.0), p = 0.46] and LPV/r-based ART [31 (18.7)]. There was no significant difference in both groups on low birth weight and stillbirth. |
| Gonzalez et al., 2017 [61] | Mozambique | Prospective cohort | 561 pWLWH on ART. | Stillbirths, congenital malformations, neonatal deaths, low birth weight, and prematurity | The risk of stillbirths was doubled in HIV-infected women. However, no differences between groups were observed in mean birth weight, prematurity, and maternal and neonatal deaths. |
| Young et al., 2012 [62] | Uganda | Prospective cohort | 166 pWLWH, ART-naïve pregnant women were enrolled between 12- and 28 weeks gestation and treated with a protease inhibitor or non-nucleoside reverse transcriptase inhibitor-based combination regimen. | Preterm, stillbirth, low birth weight | In HIV-infected women initiating cART during pregnancy, inadequate gestational weight gain was observed. Infants whose mothers gained 0.1 kg/week were at increased risk for low birth weight, preterm delivery [OR = 3.46, 95% CI: (1.18–10.15), p = 0.024], and composite adverse birth outcomes. cART did not reduce the burden of adverse birth outcomes among HIV-infected women. |
| Powis et al., 2011 [63] | USA | Retrospective cohort | 560 pWLWH randomised to ART between 26 and 34 weeks of pregnancy. | Preterm infant death | PI-based HAART was associated with increased preterm delivery [24 (25%)] compared to triple NRTI-HAART [42 (16.7%)] without infant hospitalisations or mortality. |
| Drake et al., 2012 [64] | Kenya | Randomised, double-blind trial | 148 pWLWH coinfected with HSV given 500 mg valacyclovir or placebo beginning at 34 weeks gestation. | Preterm | Infants born from HIV on ART had increased weight compared to placebo. |
| Mofenson et al., 1999 [65] | USA | Randomised, controlled trial | 480 pWLWH on zidovudine. | Birth weight | There was no perinatal transmission of HIV-1 among the 84 women who had HIV-1 levels. |
| Shapiro et al., 2010 [66] | Botswana | Randomised controlled trial | 560 pWLWH on abacavir, zidovudine, and lamivudine at 26 to 34 weeks of pregnancy. | Premature, low birth weight, congenital abnormalities, infants deaths | Only 8 children were HIV-infected at 24 months. The NRTI-treated arm had a high preterm delivery [42/283 (15)] compared to the observational [16/156 (10)]. |
| Lallemant et al., 2015 [67] | China | Randomised, partially double-blind and placebo-controlled trial | 405 pWLWH on zidovudine starting at 28 weeks of pregnancy. | Stillbirth, preterm, low birth weight | There was a significant difference in gestation period without difference in birth weight, preterm delivery [21 (14.8%)] in LPV compared to [17 (12.6%)] in NVP, and low birth weight. |
| Mulligan et al., 2018 [68] | USA | Non-randomised, open-label, parallel-group prospective trial | 29 pWLWH and infants on ART. | Preterm, low birth weight | Twenty-nine infants were HIV-negative. Renal abnormalities were noted on ultrasound in two infants associated with the use of dolutegravir. |
| Aizire et al., 2020 [69] | India, Malawi, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe | Retrospective case-control study | 33 pWLWH treated with ART during pregnancy. | Preterm, stillbirth, and infant death. | TFV-DP concentrations in dried blood spots appeared not associated with severe adverse neonatal outcomes, including preterm [OR = 0.96, 95% CI: (0.28, 3.30)], stillbirth and early infant death. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modjadji, P.; Mokgalaboni, K.; Nonterah, E.A.; Lebelo, S.L.; Mchiza, Z.J.-R.; Madiba, S.; Kengne, A.P. A Systematic Review on Cardiometabolic Risks and Perinatal Outcomes among Pregnant Women Living with HIV in the Era of Antiretroviral Therapy. Viruses 2023, 15, 1441. https://doi.org/10.3390/v15071441
Modjadji P, Mokgalaboni K, Nonterah EA, Lebelo SL, Mchiza ZJ-R, Madiba S, Kengne AP. A Systematic Review on Cardiometabolic Risks and Perinatal Outcomes among Pregnant Women Living with HIV in the Era of Antiretroviral Therapy. Viruses. 2023; 15(7):1441. https://doi.org/10.3390/v15071441
Chicago/Turabian StyleModjadji, Perpetua, Kabelo Mokgalaboni, Engelbert A. Nonterah, Sogolo Lucky Lebelo, Zandile June-Rose Mchiza, Sphiwe Madiba, and Andre Pascal Kengne. 2023. "A Systematic Review on Cardiometabolic Risks and Perinatal Outcomes among Pregnant Women Living with HIV in the Era of Antiretroviral Therapy" Viruses 15, no. 7: 1441. https://doi.org/10.3390/v15071441
APA StyleModjadji, P., Mokgalaboni, K., Nonterah, E. A., Lebelo, S. L., Mchiza, Z. J.-R., Madiba, S., & Kengne, A. P. (2023). A Systematic Review on Cardiometabolic Risks and Perinatal Outcomes among Pregnant Women Living with HIV in the Era of Antiretroviral Therapy. Viruses, 15(7), 1441. https://doi.org/10.3390/v15071441








