Recent Developments in Human Papillomavirus (HPV) Vaccinology
Abstract
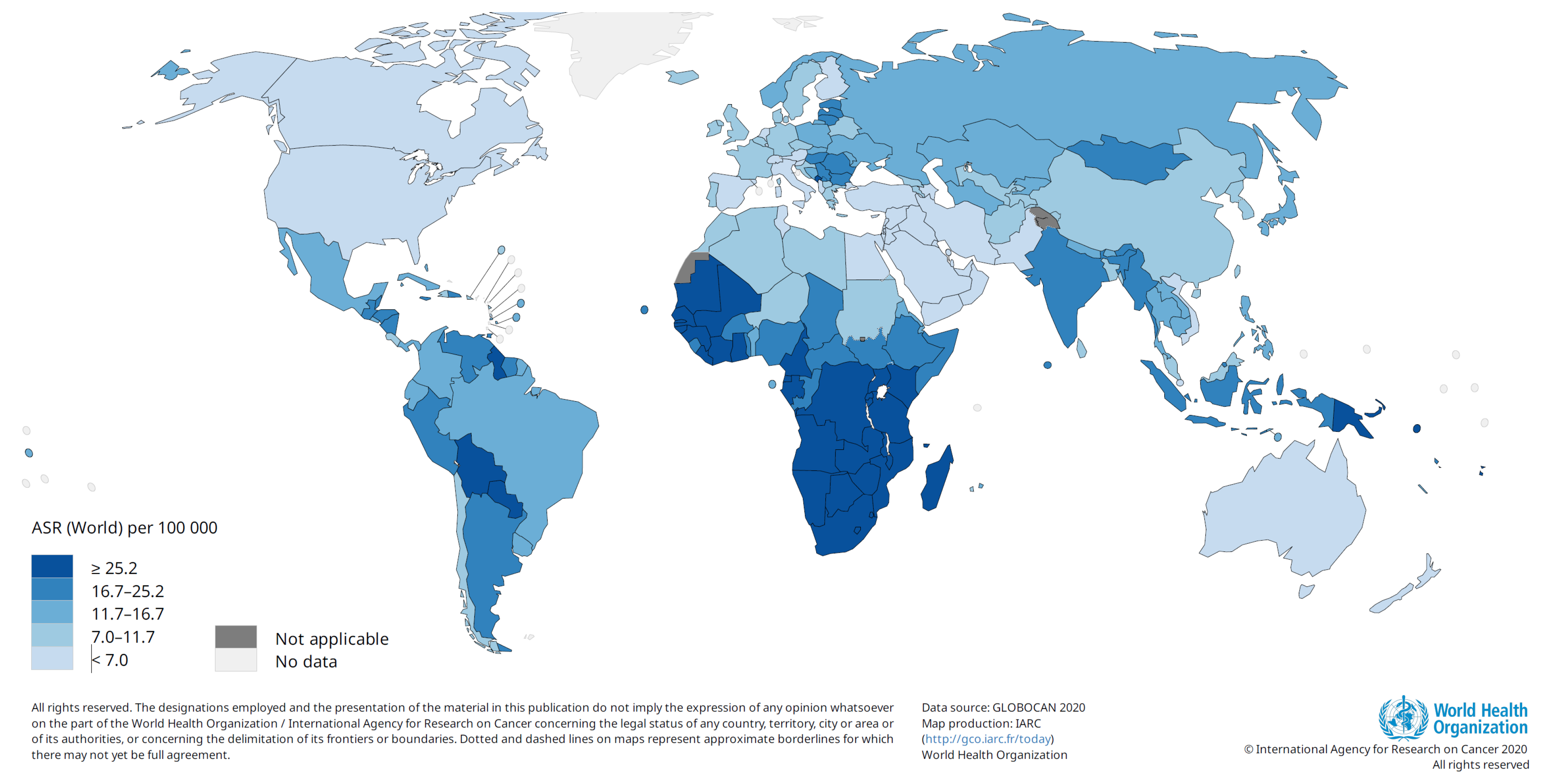
1. Cancers Associated with Human Papillomavirus
2. Structure of Human Papillomavirus
3. HPV Types Associated with Cancer and Genital Warts
4. HPV Vaccines
| Vaccine Name | Valency VLP Types | Manufacturer and Licensure Date or WHO Prequalification Date | Adjuvant | Expression System | Manufacturers’ Schedules |
|---|---|---|---|---|---|
| GARDASIL® * | Quadrivalent, HPV types 6, 11, 16 and 18 | Merck & Co., 2006 | Amorphous aluminium hydroxy phosphate sulphate 225 μg | Yeast, Saccharomyces cerevisiae expressing L1 | GARDASIL® is licensed for girls and boys aged 9–13 years as a 2-dose schedule (6 months apart). From age 14, a 3-dose schedule should be given (at 0, 1–2 and 4–6 months). |
| Cervarix® * | Bivalent, HPV-16, HPV-18 | GlaxoSmithKline, 2007 | 0.5 mg aluminium hydroxide and 50 μg 3-0-desacyl-4′monophosphoryl lipid A | Insect cell line, recombinant baculovirus encoding L1 | Cervarix® is licensed for girls and boys aged 9–14 years as a 2-dose schedule (5–13 months apart). If the recipient’s age at the time of the first dose is ≥15 years, three doses should be given (at 0, 1–2.5 months and 5–12 months) |
| GARDASIL9® * | Nonavalent, HPV types 6, 11, 31, 33, 45, 52 and 58 | Merck & Co., 2014 | Amorphous aluminium hydroxy phosphate sulphate 500 μg | Yeast, Saccharomyces cerevisiae expressing L1 | GARDASIL9® is licensed for girls and boys aged 9–14 years as a 2-dose schedule (5–13 months apart). From age 15, a 3-dose schedule should be followed (at 0, 1–2 and 4–6 months). |
| Cecolin® * | Bivalent, HPV-16, HPV-18 | Xiamen, Innovax Biotechnology, 2020 | Aluminium hydroxide 208 μg | Bacteria, Escherichia coli expressing L1 | Cecolin is licensed for girls aged 9–14 years as a 2-dose schedule (6 months apart). From age 15, a 3-dose schedule is indicated (at 0, 1–2 months and 5–8 months). |
| Walvax recombinant HPV vaccines—WalrinvaxV | Bivalent, HPV-16, HPV-18 | Shanghai Zerun Biotechnology (a subsidiary of Walwax Biotechnology), 2022 | Aluminium phosphate | Yeast, Pichia pastoris expressing L1 | Walrinvax is licensed for girls aged 9–14 years as a 2-dose schedule (6 months apart, with a minimum interval of 5 months). From age 15, a 3-dose schedule is indicated (at 0, 2–3 and 6–7 months). |
| Cervavac® ** | Quadrivalent, HPV 6, 11, 16 and 18 | Serum Institute of India, 2022 | Aluminium based | Cervavac is licensed for girls and boys aged 9–14 years, as a 2-dose schedule (6 months apart). From age 15, a 3-dose schedule should be given (at 0, 2 and 6 months) |
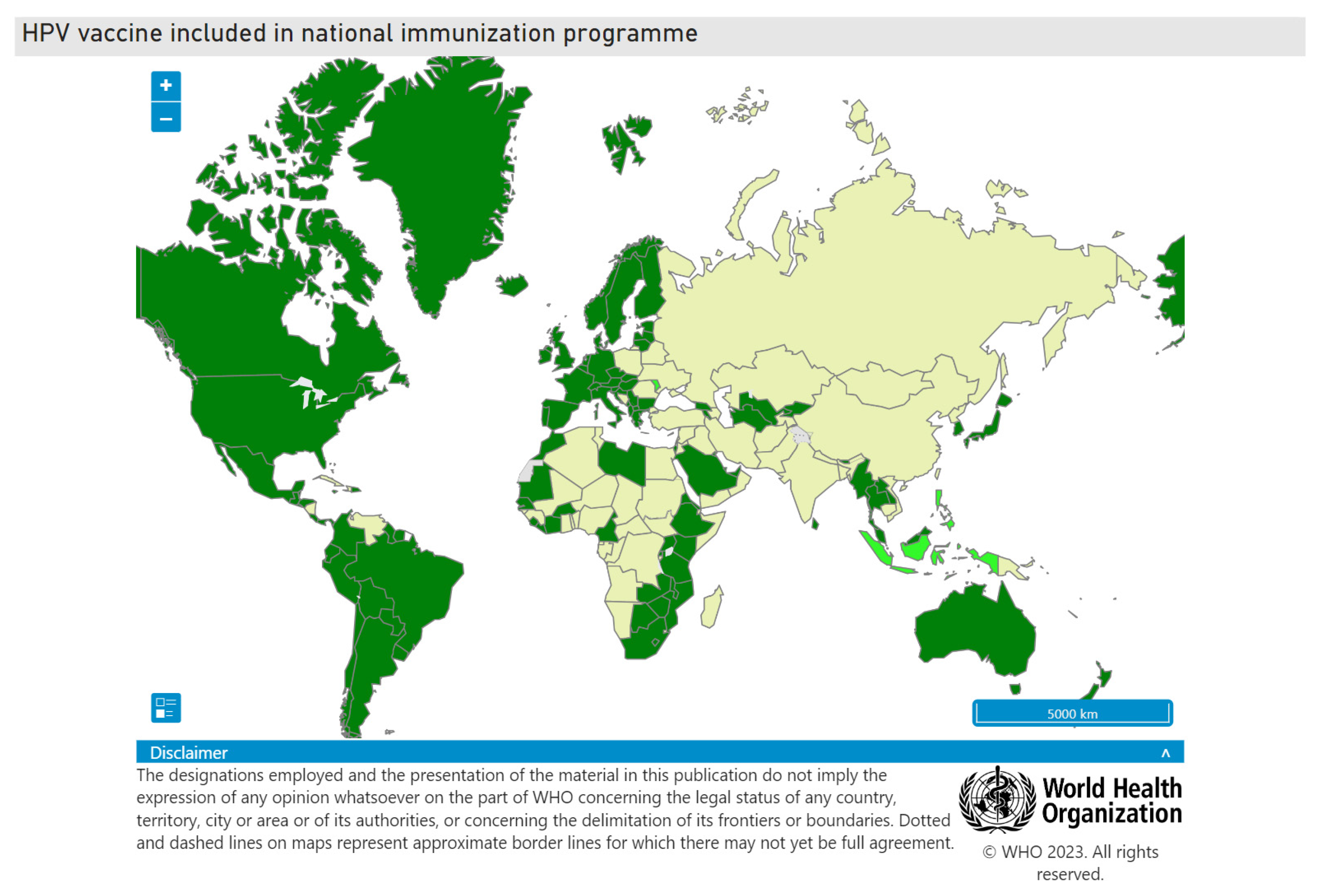
5. HPV Vaccine Implementation Globally
6. Evidence for Reduction of HPV Vaccine Triple Dose to Two- or One-Dose Regimens
7. World Health Organisation Recommendations on HPV Vaccination
- A one- or two-dose schedule for girls aged 9–14 years.
- A one- or two-dose schedule for girls and women aged 15–20 years.
- Two doses with a 6-month interval for women older than 21 years.
8. Catch-Up HPV Vaccination
9. HPV Vaccination of People with HPV-Associated Disease
10. The Case for Vaccinating Boys and Men
11. HPV Vaccination in People Living with HIV
12. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55-78. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, I.M.S. Human papillomavirus-related neoplasia of the ocular adnexa. Acta Ophthalmol. 2022, 100 (Suppl. S272), 3–33. [Google Scholar] [CrossRef]
- Lin, S.; Gao, K.; Gu, S.; You, L.; Qian, S.; Tang, M.; Wang, J.; Chen, K.; Jin, M. Worldwide trends in cervical cancer incidence and mortality, with predictions for the next 15 years. Cancer 2021, 127, 4030–4039. [Google Scholar] [CrossRef]
- Denny, L.; Prendiville, W. Cancer of the cervix: Early detection and cost-effective solutions. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S1), S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Denny, L.; de Sanjose, S.; Mutebi, M.; Anderson, B.O.; Kim, J.; Jeronimo, J.; Herrero, R.; Yeates, K.; Ginsburg, O.; Sankaranarayanan, R. Interventions to close the divide for women with breast and cervical cancer between low-income and middle-income countries and high-income countries. Lancet 2017, 389, 861–870. [Google Scholar] [CrossRef]
- Gooi, Z.; Chan, J.Y.; Fakhry, C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope 2016, 126, 894–900. [Google Scholar] [CrossRef]
- Nogues, J.C.; Fassas, S.; Mulcahy, C.; Zapanta, P.E. Human Papillomavirus-Associated Head and Neck Cancer. J. Am. Board. Fam. Med. 2021, 34, 832–837. [Google Scholar] [CrossRef]
- Rajendra, K.; Sharma, P. Viral Pathogens in Oesophageal and Gastric Cancer. Pathogens 2022, 11, 476. [Google Scholar] [CrossRef]
- Li, S.; Luk, H.Y.; Xia, C.; Chen, Z.; Chan, P.K.S.; Boon, S.S. Oesophageal carcinoma: The prevalence of DNA tumour viruses and therapy. Tumour. Virus. Res. 2022, 13, 200231. [Google Scholar] [CrossRef]
- Sitas, F.; Urban, M.; Stein, L.; Beral, V.; Ruff, P.; Hale, M.; Patel, M.; O’Connell, D.; Qin Yu, X.; Verzijden, A. The relationship between anti-HPV-16 IgG seropositivity and cancer of the cervix, anogenital organs, oral cavity and pharynx, oesophagus and prostate in a black South African population. Infect. Agents Cancer 2007, 2, 1–9. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Damgacioglu, H.; Georges, D.; Sonawane, K.; Ferlay, J.; Bray, F.; Clifford, G.M. Global burden of HPV-attributable squamous cell carcinoma of the anus in 2020, according to sex and HIV status: A worldwide analysis. Int. J. Cancer 2023, 152, 417–428. [Google Scholar] [CrossRef]
- Belnap, D.M.; Olson, N.H.; Cladel, N.M.; Newcomb, W.W.; Brown, J.C.; Kreider, J.W.; Christensen, N.D.; Baker, T.S. Conserved features in papillomavirus and polyomavirus capsids. J. Mol. Biol. 1996, 259, 249–263. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Taub, J.; Greenstone, H.; Roden, R.; Durst, M.; Gissmann, L.; Lowy, D.R.; Schiller, J.T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 1993, 67, 6929–6936. [Google Scholar] [CrossRef]
- Rose, R.C.; Bonnez, W.; Reichman, R.C.; Garcea, R.L. Expression of human papillomavirus type 11 L1 protein in insect cells: In vivo and in vitro assembly of viruslike particles. J. Virol. 1993, 67, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, X.Y.; Louis, K.; Frazer, I.H. Interaction of human papillomavirus (HPV) type 16 capsid proteins with HPV DNA requires an intact L2 N-terminal sequence. J. Virol. 1994, 68, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; DeSalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Muhr, L.S.; Eklund, C.; Dillner, J. Misclassifications in human papillomavirus databases. Virology 2021, 558, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet. Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; de Vuyst, H.; Tenet, V.; Plummer, M.; Tully, S.; Franceschi, S. Effect of HIV Infection on Human Papillomavirus Types Causing Invasive Cervical Cancer in Africa. J. Acquir. Immune. Defic. Syndr. 2016, 73, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Munoz, N.; Franceschi, S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [CrossRef]
- Pimenta, J.M.; Galindo, C.; Jenkins, D.; Taylor, S.M. Estimate of the global burden of cervical adenocarcinoma and potential impact of prophylactic human papillomavirus vaccination. BMC Cancer 2013, 13, 553. [Google Scholar] [CrossRef]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xia, N.; Zhao, Q. Expanded strain coverage for a highly successful public health tool: Prophylactic 9-valent human papillomavirus vaccine. Hum. Vaccin. Immunother. 2017, 13, 2280–2291. [Google Scholar] [CrossRef]
- Denny, L.; Adewole, I.; Anorlu, R.; Dreyer, G.; Moodley, M.; Smith, T.; Snyman, L.; Wiredu, E.; Molijn, A.; Quint, W.; et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int. J. Cancer 2014, 134, 1389–1398. [Google Scholar] [CrossRef]
- van Aardt, M.C.; Dreyer, G.; Pienaar, H.F.; Karlsen, F.; Hovland, S.; Richter, K.L.; Becker, P. Unique human papillomavirus-type distribution in South African women with invasive cervical cancer and the effect of human immunodeficiency virus infection. Int. J. Gynecol. Cancer 2015, 25, 919–925. [Google Scholar] [CrossRef]
- Baandrup, L.; Blomberg, M.; Dehlendorff, C.; Sand, C.; Andersen, K.K.; Kjaer, S.K. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex. Transm. Dis. 2013, 40, 130–135. [Google Scholar] [CrossRef]
- Suzich, J.A.; Ghim, S.J.; Palmer-Hill, F.J.; White, W.I.; Tamura, J.K.; Bell, J.A.; Newsome, J.A.; Jenson, A.B.; Schlegel, R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 1995, 92, 11553–11557. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, R.; Chandrachud, L.M.; O’Neil, B.W.; Wagner, E.R.; Grindlay, G.J.; Armstrong, A.; McGarvie, G.M.; Schiller, J.T.; Lowy, D.R.; Campo, M.S. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 1996, 219, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Breitburd, F.; Kirnbauer, R.; Hubbert, N.L.; Nonnenmacher, B.; Trin-Dinh-Desmarquet, C.; Orth, G.; Schiller, J.T.; Lowy, D.R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 1995, 69, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Ault, K.A.; Giuliano, A.R.; Wheeler, C.M.; Koutsky, L.A.; Malm, C.; Lehtinen, M.; et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005, 6, 271–278. [Google Scholar] [CrossRef]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.M.; Moscicki, A.B.; Romanowski, B.; Roteli-Martins, C.M.; Jenkins, D.; Schuind, A.; Costa Clemens, S.A.; Dubin, G.; et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet 2006, 367, 1247–1255. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; De Carvalho, N.S.; et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmeron, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef]
- Joura, E.A.; Leodolter, S.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Koutsky, L.A.; Garland, S.M.; Harper, D.M.; Tang, G.W.; Ferris, D.G.; et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: A combined analysis of three randomised clinical trials. Lancet 2007, 369, 1693–1702. [Google Scholar] [CrossRef]
- Schiller, J.T.; Castellsague, X.; Villa, L.L.; Hildesheim, A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine 2008, 26 (Suppl. S10), K53–K61. [Google Scholar] [CrossRef]
- Shu, Y.; Yu, Y.; Ji, Y.; Zhang, L.; Li, Y.; Qin, H.; Huang, Z.; Ou, Z.; Huang, M.; Shen, Q.; et al. Immunogenicity and safety of two novel human papillomavirus 4- and 9-valent vaccines in Chinese women aged 20-45 years: A randomized, blinded, controlled with Gardasil (type 6/11/16/18), phase III non-inferiority clinical trial. Vaccine 2022, 40, 6947–6955. [Google Scholar] [CrossRef]
- Diana, G.; Corica, C. Human Papilloma Virus vaccine and prevention of head and neck cancer, what is the current evidence? Oral Oncol. 2021, 115, 105168. [Google Scholar] [CrossRef] [PubMed]
- Aldakak, L.; Huber, V.M.; Ruhli, F.; Bender, N. Sex difference in the immunogenicity of the quadrivalent Human Papilloma Virus vaccine: Systematic review and meta-analysis. Vaccine 2021, 39, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Artemchuk, H.; Eriksson, T.; Poljak, M.; Surcel, H.M.; Dillner, J.; Lehtinen, M.; Faust, H. Long-term Antibody Response to Human Papillomavirus Vaccines: Up to 12 Years of Follow-up in the Finnish Maternity Cohort. J. Infect. Dis. 2019, 219, 582–589. [Google Scholar] [CrossRef]
- Mariz, F.C.; Gray, P.; Bender, N.; Eriksson, T.; Kann, H.; Apter, D.; Paavonen, J.; Pajunen, E.; Prager, K.M.; Sehr, P.; et al. Sustainability of neutralising antibodies induced by bivalent or quadrivalent HPV vaccines and correlation with efficacy: A combined follow-up analysis of data from two randomised, double-blind, multicentre, phase 3 trials. Lancet Infect. Dis. 2021, 21, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Basu, P.; Bender, N.; Herrero, R.; Kemp, T.J.; Kreimer, A.R.; Muller, M.; Panicker, G.; Pawlita, M.; Pinto, L.A.; et al. Evaluation of serological assays to monitor antibody responses to single-dose HPV vaccines. Vaccine 2020, 38, 5997–6006. [Google Scholar] [CrossRef]
- Akhatova, A.; Azizan, A.; Atageldiyeva, K.; Ashimkhanova, A.; Marat, A.; Iztleuov, Y.; Suleimenova, A.; Shamkeeva, S.; Aimagambetova, G. Prophylactic Human Papillomavirus Vaccination: From the Origin to the Current State. Vaccines 2022, 10, 1912. [Google Scholar] [CrossRef]
- Subasinghe, A.K.; Wark, J.D.; Phillips, S.; Cornall, A.; Brotherton, J.M.L.; Garland, S.M. Quadrivalent human papillomavirus vaccination successfully reduces the prevalence of vaccine-targeted genotypes in a young, vaccine-eligible-age sample of Australian females. Sex. Health 2020, 17, 510–516. [Google Scholar] [CrossRef]
- Freire-Salinas, J.; Benito, R.; Azueta, A.; Gil, J.; Mendoza, C.; Nicolas, M.; Garcia-Berbel, P.; Algarate, S.; Gomez-Roman, J. Genotype Distribution Change After Human Papillomavirus Vaccination in Two Autonomous Communities in Spain. Front. Cell. Infect. Microbiol. 2021, 11, 633162. [Google Scholar] [CrossRef]
- Perkins, R.B.; Saslow, D.; Oliver, K. Long-Term Effectiveness of Human Papillomavirus Vaccination: Implications for Future Reduction in Cancer. Ann. Intern. Med. 2022, 175, 1037–1038. [Google Scholar] [CrossRef]
- Hernandez-Aguado, J.J.; Sanchez Torres, D.A.; Martinez Lamela, E.; Aguion Galvez, G.; Sanz Espinosa, E.; Perez Quintanilla, A.; Martinez-Carrillo, D.A.; Ramirez Mena, M.; Coronado Martin, P.J.; Zapardiel, I.; et al. Quadrivalent Human Papillomavirus Vaccine Effectiveness after 12 Years in Madrid (Spain). Vaccines 2022, 10, 387. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Carter, A.; Vickers, T.; Fairley, C.K.; McNulty, A.; Guy, R.J.; Regan, D.G.; Grulich, A.E.; Callander, D.; Khawar, L.; et al. Effect on genital warts in Australian female and heterosexual male individuals after introduction of the national human papillomavirus gender-neutral vaccination programme: An analysis of national sentinel surveillance data from 2004-18. Lancet Infect. Dis. 2021, 21, 1747–1756. [Google Scholar] [CrossRef]
- Meites, E.; Stone, L.; Amiling, R.; Singh, V.; Unger, E.R.; Derkay, C.S.; Markowitz, L.E. Significant Declines in Juvenile-onset Recurrent Respiratory Papillomatosis Following Human Papillomavirus (HPV) Vaccine Introduction in the United States. Clin. Infect. Dis. 2021, 73, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Illah, O.; Olaitan, A. Updates on HPV Vaccination. Diagnostics 2023, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- WHO. Human papillomavirus vaccines: WHO position paper (2022 update). Wkly. Epidemiol. Rec. 2022, 97, 645–672. [Google Scholar]
- Gultekin, M.; Ramirez, P.T.; Broutet, N.; Hutubessy, R. World Health Organization call for action to eliminate cervical cancer globally. Int. J. Gynecol. Cancer 2020, 30, 426–427. [Google Scholar] [CrossRef]
- WHO. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. (Electronic Version). Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 14 June 2023).
- Toh, Z.Q.; Russell, F.M.; Garland, S.M.; Mulholland, E.K.; Patton, G.; Licciardi, P.V. Human Papillomavirus Vaccination After COVID-19. JNCI Cancer Spectr. 2021, 5, pkab011. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Struyf, F.; Del Rosario-Raymundo, M.R.; Hildesheim, A.; Skinner, S.R.; Wacholder, S.; Garland, S.M.; Herrero, R.; David, M.P.; Wheeler, C.M.; et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015, 16, 775–786. [Google Scholar] [CrossRef]
- Safaeian, M.; Porras, C.; Pan, Y.; Kreimer, A.; Schiller, J.T.; Gonzalez, P.; Lowy, D.R.; Wacholder, S.; Schiffman, M.; Rodriguez, A.C.; et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev. Res. 2013, 6, 1242–1250. [Google Scholar] [CrossRef]
- Basu, P.; Malvi, S.G.; Joshi, S.; Bhatla, N.; Muwonge, R.; Lucas, E.; Verma, Y.; Esmy, P.O.; Poli, U.R.R.; Shah, A.; et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre, prospective, cohort study. Lancet Oncol. 2021, 22, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, R.V.; Brown, E.R.; Onono, M.A.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Galloway, D.A.; Pinder, L.F.; Donnell, D.; Wakhungu, I.; et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. 2022, 1, EVIDoa2100056. [Google Scholar] [CrossRef] [PubMed]
- Consortium, S.-D.H.V.E. Review of Current Published Evidence on Single Dose HPV Vaccination. Available online: https://www.path.org/resources/review-current-published-evidence-single-dose-hpv-vaccination (accessed on 10 May 2023).
- Strategic Advisory Group of Experts on Immunization (SAGE). One-Dose Human Papillomavirus (HPV) Vaccine Offers Solid Protection against Cervical Cancer. Available online: https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer (accessed on 15 May 2023).
- Malagon, T.; MacCosham, A.; Burchell, A.N.; El-Zein, M.; Tellier, P.P.; Coutlee, F.; Franco, E.L.; Group, H.S. Proportion of Incident Genital Human Papillomavirus Detections not Attributable to Transmission and Potentially Attributable to Latent Infections: Implications for Cervical Cancer Screening. Clin. Infect. Dis. 2022, 75, 365–371. [Google Scholar] [CrossRef]
- Poynten, I.M.; Jin, F.; Molano, M.; Roberts, J.M.; Hillman, R.J.; Templeton, D.J.; Law, C.; Stanley, M.A.; Waterboer, T.; Farnsworth, A.; et al. Possible Reactivation of Latent Anal Human Papillomavirus Associated with Markers of Immune Dysfunction in Gay and Bisexual Men. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Rebolj, M.; Pesola, F.; Mathews, C.; Mesher, D.; Soldan, K.; Kitchener, H. The impact of catch-up bivalent human papillomavirus vaccination on cervical screening outcomes: An observational study from the English HPV primary screening pilot. Br. J. Cancer 2022, 127, 278–287. [Google Scholar] [CrossRef]
- Silverberg, M.J.; Leyden, W.A.; Lam, J.O.; Chao, C.R.; Gregorich, S.E.; Huchko, M.J.; Kulasingam, S.; Kuppermann, M.; Smith-McCune, K.K.; Sawaya, G.F. Effectiveness of ‘catch-up’ human papillomavirus vaccination to prevent cervical neoplasia in immunosuppressed and non-immunosuppressed women. Vaccine 2020, 38, 4520–4523. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, C.A.; Morettini, M.; Brotherton, J.M.L.; Canfell, K.; Manzoli, L.; Flacco, M.E.; Palmer, M.; Rossi, P.G.; Martellucci, M.; Giacomini, G.; et al. Impact of a Human Papillomavirus Vaccination Program within Organized Cervical Cancer Screening: Cohort Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 588–594. [Google Scholar] [CrossRef]
- Simons, J.J.M.; Westra, T.A.; Postma, M.J. Cost-effectiveness of a male catch-up human papillomavirus vaccination program in the Netherlands. Prev. Med. Rep. 2022, 28, 101872. [Google Scholar] [CrossRef]
- Daniels, V.; Prabhu, V.S.; Palmer, C.; Samant, S.; Kothari, S.; Roberts, C.; Elbasha, E. Public health impact and cost-effectiveness of catch-up 9-valent HPV vaccination of individuals through age 45 years in the United States. Hum. Vaccin. Immunother. 2021, 17, 1943–1951. [Google Scholar] [CrossRef]
- Wang, F.; Jozkowski, K.N.; Zhang, S. Evaluating Risk-Stratified HPV Catch-up Vaccination Strategies: Should We Go beyond Age 26? Med. Decis. Mak. 2022, 42, 524–537. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Bogani, G.; Cavallari, E.N.; Palaia, G.; Perniola, G.; Ralli, M.; Sorrenti, S.; Romeo, U.; Pernazza, A.; et al. HPV Vaccination after Primary Treatment of HPV-Related Disease across Different Organ Sites: A Multidisciplinary Comprehensive Review and Meta-Analysis. Vaccines 2022, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef]
- Eriksen, D.O.; Jensen, P.T.; Schroll, J.B.; Hammer, A. Human papillomavirus vaccination in women undergoing excisional treatment for cervical intraepithelial neoplasia and subsequent risk of recurrence: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2022, 101, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Amiling, R.; Meites, E.; Querec, T.D.; Stone, L.; Singh, V.; Unger, E.R.; Derkay, C.S.; Markowitz, L.E. Juvenile-Onset Recurrent Respiratory Papillomatosis in the United States, Epidemiology and HPV Types-2015-2020. J. Pediatr. Infect. Dis. Soc. 2021, 10, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Ponduri, A.; Azmy, M.C.; Axler, E.; Lin, J.; Schwartz, R.; Chirila, M.; Dikkers, F.G.; Yang, C.J.; Mehta, V.; Gangar, M. The Efficacy of Human Papillomavirus Vaccination as an Adjuvant Therapy in Recurrent Respiratory Papillomatosis. Laryngoscope, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Smahelova, J.; Hamsikova, E.; Ludvikova, V.; Vydrova, J.; Traboulsi, J.; Vencalek, O.; Lukes, P.; Tachezy, R. Outcomes After Human Papillomavirus Vaccination in Patients with Recurrent Respiratory Papillomatosis: A Nonrandomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 654–661. [Google Scholar] [CrossRef]
- Goon, P.; Sauzet, O.; Schuermann, M.; Oppel, F.; Shao, S.; Scholtz, L.U.; Sudhoff, H.; Goerner, M. Recurrent Respiratory Papillomatosis (RRP)-Meta-analyses on the use of the HPV vaccine as adjuvant therapy. NPJ Vaccines 2023, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Bossart, S.; Gabutti, M.P.; Seyed Jafari, S.M.; Hunger, R.E. Nonavalent human papillomavirus vaccination as alternative treatment for genital warts. Dermatol. Ther. 2020, 33, e13771. [Google Scholar] [CrossRef]
- Li, A.J.; Kyesi, F.; Mwengee, W.; Mphuru, A.; Giattas, M.R.; Shayo, B.; Nshunju, R.; Lyimo, D.; Loharikar, A. Impact of the human papillomavirus (HPV) vaccine supply shortage on Tanzania’s national HPV vaccine introduction. Vaccine 2022, 40 (Suppl. S1), A26–A29. [Google Scholar] [CrossRef]
- Garland, S.M.; Stanley, M.A.; Giuliano, A.R.; Moscicki, A.B.; Kaufmann, A.; Bhatla, N.; Woo, Y.L.; Committee, I.P. IPVS statement on “Temporary HPV vaccine shortage: Implications globally to achieve equity”. Papillomavirus Res. 2020, 9, 100195. [Google Scholar] [CrossRef]
- Colzani, E.; Johansen, K.; Johnson, H.; Pastore Celentano, L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma. Euro. Surveill. 2021, 26, 2001659. [Google Scholar] [CrossRef]
- Dykens, J.A.; Peterson, C.E.; Holt, H.K.; Harper, D.M. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Front. Public Health 2023, 11, 1067299. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.; Kann, H.; Pimenoff, V.N.; Eriksson, T.; Luostarinen, T.; Vanska, S.; Surcel, H.M.; Faust, H.; Dillner, J.; Lehtinen, M. Human papillomavirus seroprevalence in pregnant women following gender-neutral and girls-only vaccination programs in Finland: A cross-sectional cohort analysis following a cluster randomized trial. PLoS. Med. 2021, 18, e1003588. [Google Scholar] [CrossRef] [PubMed]
- Machalek, D.A.; Chow, E.P.; Garland, S.M.; Wigan, R.; Cornall, A.M.; Fairley, C.K.; Kaldor, J.M.; Hocking, J.S.; Williams, H.; McNulty, A.; et al. Human Papillomavirus Prevalence in Unvaccinated Heterosexual Men After a National Female Vaccination Program. J. Infect. Dis. 2017, 215, 202–208. [Google Scholar] [CrossRef]
- de Oliveira, T.; Kharsany, A.B.; Graf, T.; Cawood, C.; Khanyile, D.; Grobler, A.; Puren, A.; Madurai, S.; Baxter, C.; Karim, Q.A.; et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: A community-wide phylogenetic study. Lancet HIV 2017, 4, e41–e50. [Google Scholar] [CrossRef]
- Zuma, K.; Simbayi, L.; Zungu, N.; Moyo, S.; Marinda, E.; Jooste, S.; North, A.; Nadol, P.; Aynalem, G.; Igumbor, E.; et al. The HIV Epidemic in South Africa: Key Findings from 2017 National Population-Based Survey. Int. J. Environ. Res. Public Health 2022, 19, 8125. [Google Scholar] [CrossRef] [PubMed]
- Clay, P.A.; Thompson, T.D.; Markowitz, L.E.; Ekwueme, D.U.; Saraiya, M.; Chesson, H.W. Updated estimate of the annual direct medical cost of screening and treatment for human papillomavirus associated disease in the United States. Vaccine 2023, 41, 2376–2381. [Google Scholar] [CrossRef]
- Fabiano, G.; Marcellusi, A.; Mennini, F.S.; Sciattella, P.; Favato, G. Hospital resource utilisation from HPV-related diseases in England: A real-world cost analysis. Eur. J. Health Econ. 2023, 24, 75–80. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Lee, J.Y.; Jay, N.; Goldstone, S.E.; Darragh, T.M.; Dunlevy, H.A.; Rosa-Cunha, I.; Arons, A.; Pugliese, J.C.; Vena, D.; et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N. Engl. J. Med. 2022, 386, 2273–2282. [Google Scholar] [CrossRef]
- Kristiansen, S.; Bjartling, C.; Torbrand, C.; Grelaud, D.; Lindstrom, M.; Svensson, A.; Forslund, O. Increased prevalence of human papillomavirus in fresh tissue from penile cancers compared to non-malignant penile samples: A case-control study. BMC Cancer 2022, 22, 1227. [Google Scholar] [CrossRef]
- Barroso, L.F.; Stier, E.A.; Hillman, R.; Palefsky, J. Anal Cancer Screening and Prevention: Summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infection Guidelines. Clin. Infect. Dis. 2022, 74, S179–S192. [Google Scholar] [CrossRef]
- Graham, D.M.; Isaranuwatchai, W.; Habbous, S.; de Oliveira, C.; Liu, G.; Siu, L.L.; Hoch, J.S. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer 2015, 121, 1785–1792. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.; Coetzee, D.; Marais, D.J.; Kamupira, M.; Zwane, E.; Allan, B.; Constant, D.; Moodley, J.R.; Hoffman, M.; Williamson, A.-L. Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. J. Infect. Dis. 2009, 199, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, M.K.; Gray, R.H.; Serwadda, D.; Kigozi, G.; Gravitt, P.E.; Nalugoda, F.; Reynolds, S.J.; Wawer, M.J.; Watya, S.; Quinn, T.C.; et al. High-risk human papillomavirus viral load and persistence among heterosexual HIV-negative and HIV-positive men. Sex. Transm. Infect. 2014, 90, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Mbulawa, Z.Z.; Coetzee, D.; Williamson, A.L. Human papillomavirus prevalence in South African women and men according to age and human immunodeficiency virus status. BMC Infect. Dis. 2015, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Lissouba, P.; Van de Perre, P.; Auvert, B. Association of genital human papillomavirus infection with HIV acquisition: A systematic review and meta-analysis. Sex. Transm. Infect. 2013, 89, 350–356. [Google Scholar] [CrossRef]
- Auvert, B.; Marais, D.; Lissouba, P.; Zarca, K.; Ramjee, G.; Williamson, A.-L. High-risk human papillomavirus is associated with HIV acquisition among South African female sex workers. Infect. Dis. Obstet. Gynecol. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Marais, D.J.; Passmore, J.A.; Denny, L.; Sampson, C.; Allan, B.R.; Williamson, A.L. Cervical and oral human papillomavirus types in HIV-1 positive and negative women with cervical disease in South Africa. J. Med. Virol. 2008, 80, 953–959. [Google Scholar] [CrossRef]
- Castilho, J.L.; Levi, J.E.; Luz, P.M.; Cambou, M.C.; Vanni, T.; de Andrade, A.; Derrico, M.; Veloso, V.G.; Grinsztejn, B.; Friedman, R.K. A cross-sectional study of high-risk human papillomavirus clustering and cervical outcomes in HIV-infected women in Rio de Janeiro, Brazil. BMC Cancer 2015, 15, 478. [Google Scholar] [CrossRef]
- Asangbeh-Kerman, S.L.; Davidovic, M.; Taghavi, K.; Kachingwe, J.; Rammipi, K.M.; Muzingwani, L.; Pascoe, M.; Jousse, M.; Mulongo, M.; Mwanahamuntu, M.; et al. Cervical cancer prevention in countries with the highest HIV prevalence: A review of policies. BMC Public Health 2022, 22, 1530. [Google Scholar] [CrossRef]
- Ibrahim Khalil, A.; Mpunga, T.; Wei, F.; Baussano, I.; de Martel, C.; Bray, F.; Stelzle, D.; Dryden-Peterson, S.; Jaquet, A.; Horner, M.J.; et al. Age-specific burden of cervical cancer associated with HIV: A global analysis with a focus on sub-Saharan Africa. Int. J. Cancer 2022, 150, 761–772. [Google Scholar] [CrossRef]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef] [PubMed]
- Debeaudrap, P.; Sobngwi, J.; Tebeu, P.M.; Clifford, G.M. Residual or Recurrent Precancerous Lesions After Treatment of Cervical Lesions in Human Immunodeficiency Virus-infected Women: A Systematic Review and Meta-analysis of Treatment Failure. Clin. Infect. Dis. 2019, 69, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.A.; Damgacioglu, H.; Georges, D.; Sonawane, K.; Clifford, G.M. Human papillomavirus-associated anal cancer incidence and burden among US men, according to sexual orientation, HIV status, and age. Clin. Infect. Dis. 2023, ciad205. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williamson, A.-L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440. https://doi.org/10.3390/v15071440
Williamson A-L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses. 2023; 15(7):1440. https://doi.org/10.3390/v15071440
Chicago/Turabian StyleWilliamson, Anna-Lise. 2023. "Recent Developments in Human Papillomavirus (HPV) Vaccinology" Viruses 15, no. 7: 1440. https://doi.org/10.3390/v15071440
APA StyleWilliamson, A.-L. (2023). Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses, 15(7), 1440. https://doi.org/10.3390/v15071440






