Progress in PRRSV Infection and Adaptive Immune Response Mechanisms
Abstract
1. Introduction
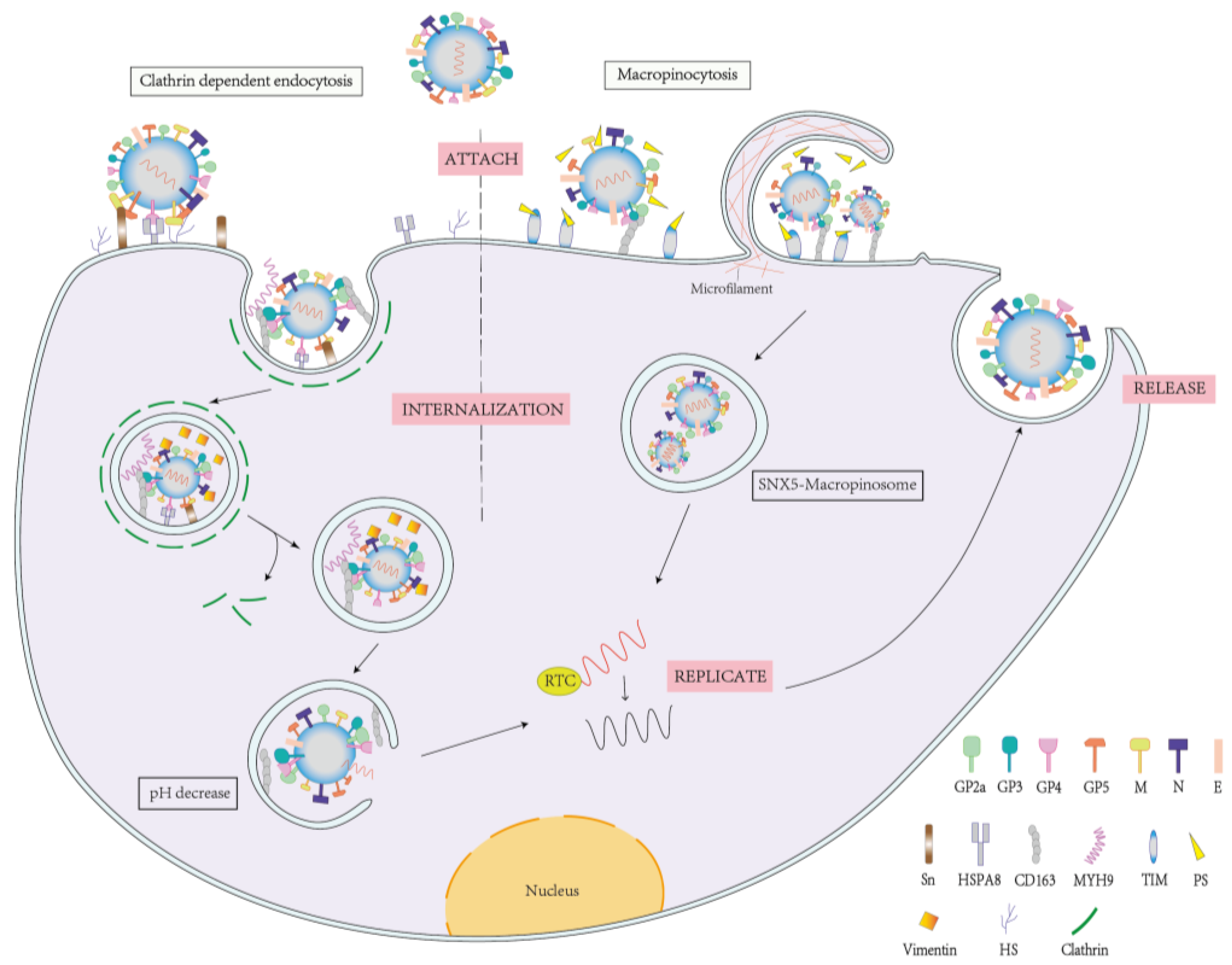
2. Mechanism of PRRSV Infection
2.1. PRRSV Infection and Receptor Proteins
2.2. Persistent Infection
3. PRRSV and the Adaptive Immune Response
3.1. Cellular Immunity
3.1.1. Cellular Immune Response of the Host
3.1.2. Mechanism of PRRSV in Anti-Cellular Immunity
3.1.3. Cellular Immunity and Vaccine Development
3.2. Humoral Immunity
3.2.1. Delayed Production of Neutralizing Antibodies
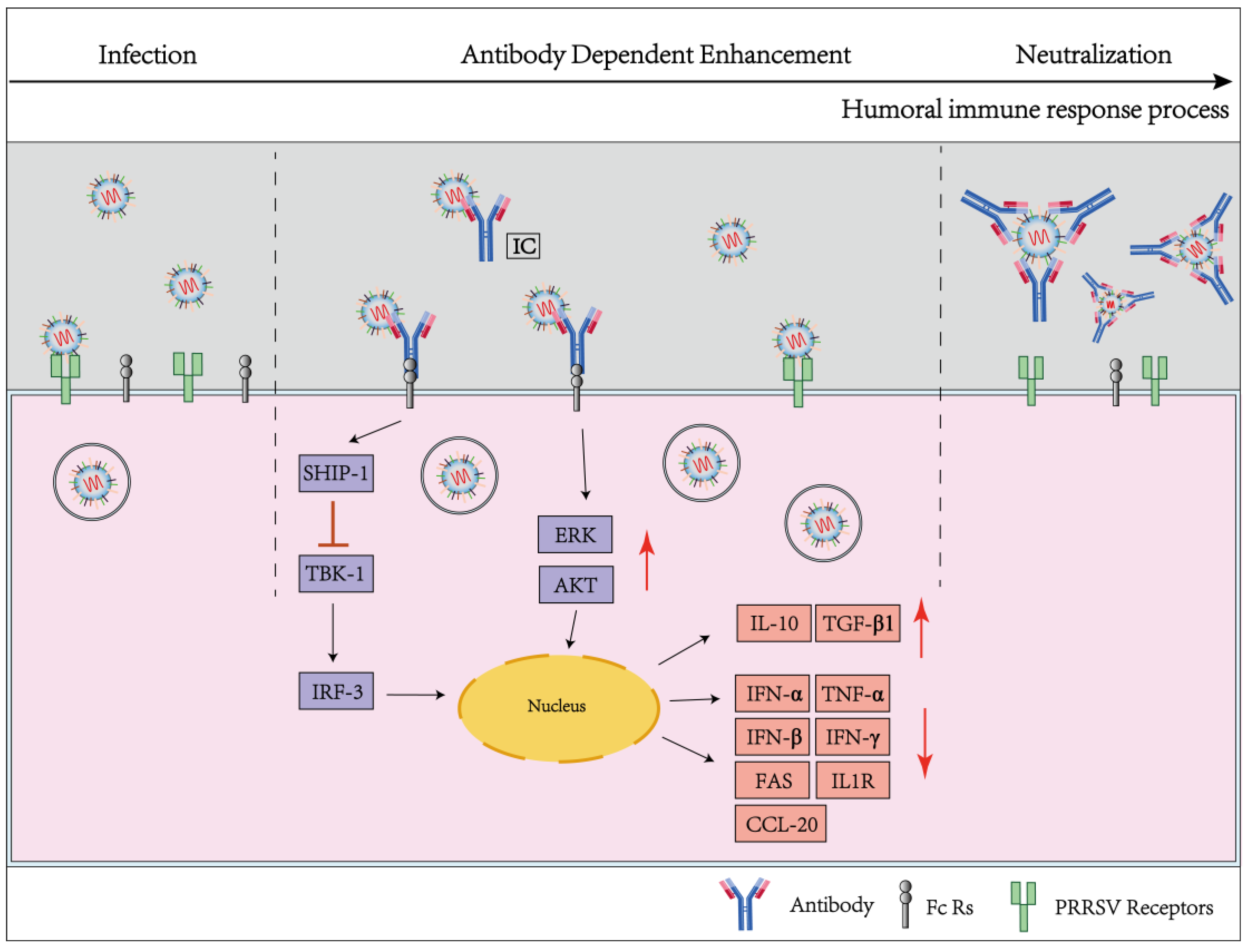
3.2.2. Non-Neutralizing Antibodies and ADE
3.2.3. The Virucidal Effect of Non-Neutralizing Antibodies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GP | Glycoprotein |
| GP2a | Glycoprotein 2a |
| GP3 | Glycoprotein 3 |
| GP4 | Glycoprotein 4 |
| GP5 | Glycoprotein 5 |
| M | Membrane protein |
| N | Nucleocapsid |
| GP5/M heterodimer | Glycoprotein 5/Membrane protein heterodimer |
| NSP | Nonstructural protein |
| NSP2 | Nonstructural protein 2 |
| NSP5 | Nonstructural protein 5 |
| NSP9 | Nonstructural protein 9 |
| PAMs | Pulmonary alveolar macrophages |
| CD163 | Cysteine-rich scavenger receptor |
| PAM-CD163 | PAM cell-derived CD163 |
| Sn or CD169 | Sialoadhesin |
| CD163+ Sn+ cells | exist CD163 and Sn cells |
| DCs | Dendritic cells |
| NK | Natural killer |
| HS | Heparin sulfate |
| CD151 | Cluster of differentiation 151 |
| MYH9 | Non-muscle myosin heavy chain 9 |
| HSPA8 | Heat shock protein member 8 |
| CME | Clathrin-mediated endocytosis |
| TIM | T-cell immunoglobulin mucin structural domain |
| PS | Phosphatidylserine |
| SRCR | Scavenger receptor cysteine-rich |
| DC-SIGN or CD209 | Dendritic cell-specific intercellular adhesion mole-Cule-3-grabbing non-integrin |
| Marc145 | Epithelial cells generated from monkey kidney |
| Nabs | Neutralizing antibodies |
| Tfh | Follicular helper T cells |
| ADE | Antibody-dependent enhancement |
| Fc Rs | Immune cell surface receptors, mainly Fc receptors |
| CRs | Complement receptors |
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| CDC | Antibody-dependent complement-mediated cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
References
- Wensvoort, G.; Terpstra, C.; Pol, J.M.; ter Laak, E.A.; Bloemraad, M.; de Kluyver, E.P.; Kragten, C.; van Buiten, L.; den Besten, A.; Wagenaar, F.; et al. Mystery swine disease in The Netherlands: The isolation of Lelystad virus. Vet. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef]
- Collins, J.E.; Benfield, D.A.; Christianson, W.T.; Harris, L.; Hennings, J.C.; Shaw, D.P.; Goyal, S.M.; McCullough, S.; Morrison, R.B.; Joo, H.S.; et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Investig. 1992, 4, 117–126. [Google Scholar] [CrossRef]
- Ruedas-Torres, I.; Rodríguez-Gómez, I.M.; Sánchez-Carvajal, J.M.; Larenas-Muñoz, F.; Pallarés, F.J.; Carrasco, L.; Gómez-Laguna, J. The jigsaw of PRRSV virulence. Vet. Microbiol. 2021, 260, 109168. [Google Scholar] [CrossRef] [PubMed]
- Dokland, T. The structural biology of PRRSV. Virus Res. 2010, 154, 86–97. [Google Scholar] [CrossRef]
- Nelson, E.A.; Christopher-Hennings, J.; Drew, T.; Wensvoort, G.; Collins, J.E.; Benfield, D.A. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J. Clin. Microbiol. 1993, 31, 3184–3189. [Google Scholar] [CrossRef]
- Yu, F.; Yan, Y.; Shi, M.; Liu, H.Z.; Zhang, H.L.; Yang, Y.B.; Huang, X.Y.; Gauger, P.C.; Zhang, J.; Zhang, Y.H.; et al. Phylogenetics, Genomic Recombination, and NSP2 Polymorphic Patterns of Porcine Reproductive and Respiratory Syndrome Virus in China and the United States in 2014–2018. J. Virol. 2020, 94, e01813-19. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, H.; Zhao, J.; Gong, B.; Sun, Q.; Xiang, L.; Li, W.; Guo, Z.; Li, J.; Tang, Y.D.; et al. Epidemiological investigation and genetic evolutionary analysis of PRRSV-1 on a pig farm in China. Front. Microbiol. 2022, 13, 1067173. [Google Scholar] [CrossRef]
- Yu, F.; Liu, L.; Tian, X.; Chen, L.; Huang, X.; Sun, Y.; Yan, Y.; Tian, Z.; Cai, X.; Liu, D.; et al. Genomic Analysis of Porcine Reproductive and Respiratory Syndrome Virus 1 Revealed Extensive Recombination and Potential Introduction Events in China. Vet. Sci. 2022, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, Z.; Xu, T.; Zhou, Y.; Li, J.; Deng, H.; Li, F.; Xu, L.; Sun, X.; Zhu, L. Molecular Characterization of the Nsp2 and ORF5s of PRRSV Strains in Sichuan China during 2012–2020. Animals 2022, 12, 3309. [Google Scholar] [CrossRef]
- Hume, D.A. Macrophages as APC and the dendritic cell myth. J. Immunol. 2008, 181, 5829–5835. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Genzow, M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS). Vaccine 2011, 29, 8192–8204. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E.M. Antiviral Strategies against PRRSV Infection. Trends Microbiol. 2017, 25, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Rowland, R.R.; Lawson, S.; Rossow, K.; Benfield, D.A. Lymphoid tissue tropism of porcine reproductive and respiratory syndrome virus replication during persistent infection of pigs originally exposed to virus in utero. Vet. Microbiol. 2003, 96, 219–235. [Google Scholar] [CrossRef]
- Ye, N.; Wang, B.; Feng, W.; Tang, D.; Zeng, Z. PRRS virus receptors and an alternative pathway for viral invasion. Virus. Res. 2022, 320, 198885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sha, H.; Qin, L.; Wang, N.; Kong, W.; Huang, L.; Zhao, M. Research Progress in Porcine Reproductive and Respiratory Syndrome Virus-Host Protein Interactions. Animals 2022, 12, 1381. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Chen, X.X.; Zhi, Y.; Deng, R.; Zhou, E.M.; Qiao, S.; Zhang, G. Nonmuscle Myosin Heavy Chain IIA Recognizes Sialic Acids on Sialylated RNA Viruses to Suppress Proinflammatory Responses via the DAP12-Syk Pathway. mBio 2019, 10, e00574-19. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Geng, R.; Zhang, L.; Chen, X.X.; Qiao, S.; Zhang, G. Heat Shock Protein Member 8 (HSPA8) Is Involved in Porcine Reproductive and Respiratory Syndrome Virus Attachment and Internalization. Microbiol. Spectr. 2022, 10, e0186021. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Hu, Q.; Wang, T.; Zhu, G.; Zhao, Q.; Zhou, E.M. Identification of MYH9 Key Domain Involved in the Entry of PRRSV Into Permissive Cells. Front. Microbiol. 2022, 13, 865343. [Google Scholar] [CrossRef]
- Hou, G.; Xue, B.; Li, L.; Nan, Y.; Zhang, L.; Li, K.; Zhao, Q.; Hiscox, J.A.; Stewart, J.P.; Wu, C.; et al. Direct Interaction Between CD163 N-Terminal Domain and MYH9 C-Terminal Domain Contributes to Porcine Reproductive and Respiratory Syndrome Virus Internalization by Permissive Cells. Front. Microbiol. 2019, 10, 1815. [Google Scholar] [CrossRef]
- Van Breedam, W.; Van Gorp, H.; Zhang, J.Q.; Crocker, P.R.; Delputte, P.L.; Nauwynck, H.J. The M/GP(5) glycoprotein complex of porcine reproductive and respiratory syndrome virus binds the sialoadhesin receptor in a sialic acid-dependent manner. PLoS Pathog. 2010, 6, e1000730. [Google Scholar] [CrossRef]
- Das, P.B.; Dinh, P.X.; Ansari, I.H.; de Lima, M.; Osorio, F.A.; Pattnaik, A.K. The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163. J. Virol. 2010, 84, 1731–1740. [Google Scholar] [CrossRef]
- Kim, J.K.; Fahad, A.M.; Shanmukhappa, K.; Kapil, S. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J. Virol. 2006, 80, 689–696. [Google Scholar] [CrossRef]
- Zheng, X.X.; Li, R.; Qiao, S.; Chen, X.X.; Zhang, L.; Lu, Q.; Xing, G.; Zhou, E.M.; Zhang, G. Vimentin rearrangement by phosphorylation is beneficial for porcine reproductive and respiratory syndrome virus replication in vitro. Vet. Microbiol. 2021, 259, 109133. [Google Scholar] [CrossRef]
- Wei, X.; Li, R.; Qiao, S.; Chen, X.X.; Xing, G.; Zhang, G. Porcine Reproductive and Respiratory Syndrome Virus Utilizes Viral Apoptotic Mimicry as an Alternative Pathway to Infect Host Cells. J. Virol. 2020, 94, e00709-20. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wei, R.; Dong, W.; Zhu, Z.; Zhang, X.; Chen, Y.; Liu, X.; Guo, C. CD163(ΔSRCR5) MARC-145 Cells Resist PRRSV-2 Infection via Inhibiting Virus Uncoating, Which Requires the Interaction of CD163 with Calpain 1. Front. Microbiol. 2019, 10, 3115. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.D.; Bardot, R.; Whitworth, K.M.; Trible, B.R.; Fang, Y.; Mileham, A.; Kerrigan, M.A.; Samuel, M.S.; Prather, R.S.; Rowland, R.R.R. Replacement of Porcine CD163 Scavenger Receptor Cysteine-Rich Domain 5 with a CD163-Like Homolog Confers Resistance of Pigs to Genotype 1 but Not Genotype 2 Porcine Reproductive and Respiratory Syndrome Virus. J. Virol. 2017, 91, e01521-16. [Google Scholar] [CrossRef] [PubMed]
- Burkard, C.; Opriessnig, T.; Mileham, A.J.; Stadejek, T.; Ait-Ali, T.; Lillico, S.G.; Whitelaw, C.B.A.; Archibald, A.L. Pigs Lacking the Scavenger Receptor Cysteine-Rich Domain 5 of CD163 Are Resistant to Porcine Reproductive and Respiratory Syndrome Virus 1 Infection. J. Virol. 2018, 92, JVI.00415-18. [Google Scholar] [CrossRef]
- Stoian, A.M.M.; Rowland, R.R.R.; Brandariz-Nuñez, A. Mutations within scavenger receptor cysteine-rich (SRCR) protein domain 5 of porcine CD163 involved in infection with porcine reproductive and respiratory syndrome virus (PRRS). J. Gen. Virol. 2022, 103, 001740. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, C.K.; Nam, E.; Kim, S.H.; Lee, O.S.; Lee, D.S.; Lee, C. Generation of a porcine alveolar macrophage cell line for the growth of porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2010, 163, 410–415. [Google Scholar] [CrossRef]
- Frydas, I.S.; Nauwynck, H.J. Replication characteristics of eight virulent and two attenuated genotype 1 and 2 porcine reproductive and respiratory syndrome virus (PRRSV) strains in nasal mucosa explants. Vet. Microbiol. 2016, 182, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, A.; Liu, K.; Feng, C.; Hou, Y.; Chen, J.; Ma, S.; Huang, L.; Dai, X. Nano-LYTACs for Degradation of Membrane Proteins and Inhibition of CD24/Siglec-10 Signaling Pathway. Adv. Sci. 2023, 10, e2300288. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.W.; Zimmerman, J.J.; Yoon, K.J.; Swenson, S.L.; McGinley, M.J.; Hill, H.T.; Platt, K.B.; Christopher-Hennings, J.; Nelson, E.A. Porcine reproductive and respiratory syndrome virus: A persistent infection. Vet. Microbiol. 1997, 55, 231–240. [Google Scholar] [CrossRef]
- Rowland, R.R.; Morrison, R.B. Challenges and opportunities for the control and elimination of porcine reproductive and respiratory syndrome virus. Transbound Emerg. Dis. 2012, 59 (Suppl. 1), 55–59. [Google Scholar] [CrossRef] [PubMed]
- Pertich, A.; Barna, Z.; Makai, O.; Farkas, J.; Molnár, T.; Bálint, Á.; Szabó, I.; Albert, M. Elimination of porcine reproductive and respiratory syndrome virus infection using an inactivated vaccine in combination with a roll-over method in a Hungarian large-scale pig herd. Acta Vet. Scand. 2022, 64, 12. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Munoz, N.; El Najjar, F.; Dutch, R.E. Viral cell-to-cell spread: Conventional and non-conventional ways. Adv. Virus Res. 2020, 108, 85–125. [Google Scholar] [CrossRef]
- Guo, R.; Davis, D.; Fang, Y. Intercellular transfer of mitochondria rescues virus-induced cell death but facilitates cell-to-cell spreading of porcine reproductive and respiratory syndrome virus. Virology 2018, 517, 122–134. [Google Scholar] [CrossRef]
- Guo, R.; Katz, B.B.; Tomich, J.M.; Gallagher, T.; Fang, Y. Porcine Reproductive and Respiratory Syndrome Virus Utilizes Nanotubes for Intercellular Spread. J. Virol. 2016, 90, 5163–5175. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhou, X.; Guo, T.; Qiao, S.; Guo, Z.; Li, R.; Jin, Q.; Hu, X.; Xing, G.; Deng, R.; et al. Efficacy of a live attenuated highly pathogenic PRRSV vaccine against a NADC30-like strain challenge: Implications for ADE of PRRSV. BMC Vet. Res. 2021, 17, 260. [Google Scholar] [CrossRef]
- Madapong, A.; Saeng-Chuto, K.; Chaikhumwang, P.; Tantituvanont, A.; Saardrak, K.; Pedrazuela Sanz, R.; Miranda Alvarez, J.; Nilubol, D. Immune response and protective efficacy of intramuscular and intradermal vaccination with porcine reproductive and respiratory syndrome virus 1 (PRRSV-1) modified live vaccine against highly pathogenic PRRSV-2 (HP-PRRSV-2) challenge, either alone or in combination with of PRRSV-1. Vet. Microbiol. 2020, 244, 108655. [Google Scholar] [CrossRef]
- Qiu, M.; Li, S.; Ye, M.; Li, J.; Sun, Z.; Li, X.; Xu, Y.; Xiao, Y.; Li, C.; Feng, B.; et al. Systemic Homologous Neutralizing Antibodies Are Inadequate for the Evaluation of Vaccine Protective Efficacy against Coinfection by High Virulent PEDV and PRRSV. Microbiol. Spectr. 2022, 10, e0257421. [Google Scholar] [CrossRef]
- Nam, B.; Mekuria, Z.; Carossino, M.; Li, G.; Zheng, Y.; Zhang, J.; Cook, R.F.; Shuck, K.M.; Campos, J.R.; Squires, E.L.; et al. Intrahost Selection Pressure Drives Equine Arteritis Virus Evolution during Persistent Infection in the Stallion Reproductive Tract. J. Virol. 2019, 93, e00045-19. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Cai, X.; Zhou, E.M.; Zimmerman, J.J. Effects of PRRSV Infection on the Porcine Thymus. Trends Microbiol. 2020, 28, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Rahe, M.C.; Murtaugh, M.P. Mechanisms of Adaptive Immunity to Porcine Reproductive and Respiratory Syndrome Virus. Viruses 2017, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Han, Y.; Wu, X.; Wang, Y.; Su, Q.; Shen, Y.; Guan, K.; Michal, J.J.; Jiang, Z.; Liu, B.; et al. Integrated time-series transcriptomic and metabolomic analyses reveal different inflammatory and adaptive immune responses contributing to host resistance to PRRSV. Front. Immunol. 2022, 13, 960709. [Google Scholar] [CrossRef] [PubMed]
- Loving, C.L.; Osorio, F.A.; Murtaugh, M.P.; Zuckermann, F.A. Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Immunol. Immunopathol. 2015, 167, 1–14. [Google Scholar] [CrossRef]
- Tian, Y.; Hao, Y.; Dong, M.; Li, S.; Wang, D.; Jiang, F.; Wang, Q.; Hao, X.; Yang, Y.; Chen, N.; et al. Development of a Monoclonal Antibody to Pig CD69 Reveals Early Activation of T Cells in Pig after PRRSV and ASFV Infection. Viruses 2022, 14, 1343. [Google Scholar] [CrossRef]
- Ruedas-Torres, I.; Gómez-Laguna, J.; Sánchez-Carvajal, J.M.; Larenas-Muñoz, F.; Barranco, I.; Pallarés, F.J.; Carrasco, L.; Rodríguez-Gómez, I.M. Activation of T-bet, FOXP3, and EOMES in Target Organs from Piglets Infected with the Virulent PRRSV-1 Lena Strain. Front. Immunol. 2021, 12, 773146. [Google Scholar] [CrossRef]
- Kick, A.R.; Amaral, A.F.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Käser, T. The T-Cell Response to Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Viruses 2019, 11, 796. [Google Scholar] [CrossRef]
- Li, Y.; Díaz, I.; Martín-Valls, G.; Beyersdorf, N.; Mateu, E. Systemic CD4 cytotoxic T cells improve protection against PRRSV-1 transplacental infection. Front. Immunol. 2022, 13, 1020227. [Google Scholar] [CrossRef]
- Cao, J.; Grauwet, K.; Vermeulen, B.; Devriendt, B.; Jiang, P.; Favoreel, H.; Nauwynck, H. Suppression of NK cell-mediated cytotoxicity against PRRSV-infected porcine alveolar macrophages in vitro. Vet. Microbiol. 2013, 164, 261–269. [Google Scholar] [CrossRef]
- Crisci, E.; Fraile, L.; Montoya, M. Cellular Innate Immunity against PRRSV and Swine Influenza Viruses. Vet. Sci. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Batista, L.; Dee, S.; Halbur, P.; Murtaugh, M.P. The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J. Virol. 2004, 78, 5923–5933. [Google Scholar] [CrossRef] [PubMed]
- Nedumpun, T.; Sirisereewan, C.; Thanmuan, C.; Techapongtada, P.; Puntarotairung, R.; Naraprasertkul, S.; Thanawongnuwech, R.; Suradhat, S. Induction of porcine reproductive and respiratory syndrome virus (PRRSV)-specific regulatory T lymphocytes (Treg) in the lungs and tracheobronchial lymph nodes of PRRSV-infected pigs. Vet. Microbiol. 2018, 216, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nedumpun, T.; Techakriengkrai, N.; Thanawongnuwech, R.; Suradhat, S. Negative Immunomodulatory Effects of Type 2 Porcine Reproductive and Respiratory Syndrome Virus-Induced Interleukin-1 Receptor Antagonist on Porcine Innate and Adaptive Immune Functions. Front. Immunol. 2019, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, J.; Liew, C.S.; Riethoven, J.M.; Sillman, S.; Vu, H.L.X. Porcine Reproductive and Respiratory Syndrome Virus Infection Upregulates Negative Immune Regulators and T-Cell Exhaustion Markers. J. Virol. 2021, 95, e0105221. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Torres, I.; Rodríguez-Gómez, I.M.; Sánchez-Carvajal, J.M.; Guil-Luna, S.; Larenas-Muñoz, F.; Pallarés, F.J.; Carrasco, L.; Gómez-Laguna, J. Up-Regulation of Immune Checkpoints in the Thymus of PRRSV-1-Infected Piglets in a Virulence-Dependent Fashion. Front. Immunol. 2021, 12, 671743. [Google Scholar] [CrossRef]
- Ruedas-Torres, I.; Sánchez-Carvajal, J.M.; Carrasco, L.; Pallarés, F.J.; Larenas-Muñoz, F.; Rodríguez-Gómez, I.M.; Gómez-Laguna, J. PRRSV-1 induced lung lesion is associated with an imbalance between costimulatory and coinhibitory immune checkpoints. Front. Microbiol. 2022, 13, 1007523. [Google Scholar] [CrossRef]
- Bautista, E.M.; Suárez, P.; Molitor, T.W. T cell responses to the structural polypeptides of porcine reproductive and respiratory syndrome virus. Arch. Virol. 1999, 144, 117–134. [Google Scholar] [CrossRef]
- Chung, C.J.; Cha, S.H.; Grimm, A.L.; Ajithdoss, D.; Rzepka, J.; Chung, G.; Yu, J.; Davis, W.C.; Ho, C.S. Pigs that recover from porcine reproduction and respiratory syndrome virus infection develop cytotoxic CD4+CD8+ and CD4+CD8- T-cells that kill virus infected cells. PLoS ONE 2018, 13, e0203482. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, N.; Wei, X.; Jiang, Y.; Chen, R.; Li, Q.; Liang, R.; Zhang, L.; Ma, L.; Xia, C. Illumination of PRRSV Cytotoxic T Lymphocyte Epitopes by the Three-Dimensional Structure and Peptidome of Swine Lymphocyte Antigen Class I (SLA-I). Front. Immunol. 2019, 10, 2995. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Subramaniam, S.; Heffron, C.L.; Mahsoub, H.M.; Sooryanarain, H.; Wang, B.; Cao, Q.M.; Hassebroek, A.; LeRoith, T.; Foss, D.L.; et al. Construction and efficacy evaluation of novel swine leukocyte antigen (SLA) class I and class II allele-specific poly-T cell epitope vaccines against porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2020, 101, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xia, Q.; Zhou, J.; Liu, H.; Chen, Y.; Liu, Y.; Ding, P.; Qi, Y.; Wang, A. Identification of potential SLA-I-restricted CTL epitopes within the M protein of porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2021, 259, 109131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.M.; Tian, D.; Heffron, C.L.; Subramaniam, S.; Opriessnig, T.; Foss, D.L.; Calvert, J.G.; Meng, X.J. Cytotoxic T lymphocyte epitopes identified from a contemporary strain of porcine reproductive and respiratory syndrome virus enhance CD4+CD8+ T, CD8+ T, and γδ T cell responses. Virology 2019, 538, 35–44. [Google Scholar] [CrossRef]
- Mötz, M.; Stas, M.R.; Hammer, S.E.; Duckova, T.; Fontaine, F.; Kiesler, A.; Seitz, K.; Ladinig, A.; Müller, A.C.; Riedel, C.; et al. Identification of MHC-I-Presented Porcine Respiratory and Reproductive Syndrome Virus (PRRSV) Peptides Reveals Immunogenic Epitopes within Several Non-Structural Proteins Recognized by CD8(+) T Cells. Viruses 2022, 14, 1891. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, H.; Wang, M.; Bai, X.; Cao, J.; Zhang, Z.; Wang, Q. Mannosylated gelatin nanoparticles enhanced inactivated PRRSV targeting dendritic cells and increased T cell immunity. Vet. Immunol. Immunopathol. 2021, 235, 110237. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.M.; Kim, J.H.; Cho, C.W.; Jeon, J.W.; Park, J.K.; Lee, S.H.; Jung, B.G.; Lee, B.J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 2018, 200, 40–51. [Google Scholar] [CrossRef]
- Welner, S.; Ruggli, N.; Liniger, M.; Summerfield, A.; Larsen, L.E.; Jungersen, G. Reduced Virus Load in Lungs of Pigs Challenged with Porcine Reproductive and Respiratory Syndrome Virus after Vaccination with Virus Replicon Particles Encoding Conserved PRRSV Cytotoxic T-Cell Epitopes. Vaccines 2021, 9, 208. [Google Scholar] [CrossRef]
- Tang, T.; Wang, C.; Pu, Q.; Peng, J.; Liu, S.; Ren, C.; Jiang, M.; Tian, Z. Vaccination of Mice with Listeria ivanovii Expressing the Truncated M Protein of Porcine Reproductive and Respiratory Syndrome Virus Induces both Antigen-Specific CD4+ and CD8+ T Cell-Mediated Immunity. J. Mol. Microbiol. Biotechnol. 2019, 29, 74–82. [Google Scholar] [CrossRef]
- Bernelin-Cottet, C.; Urien, C.; Stubsrud, E.; Jakob, V.; Bouguyon, E.; Bordet, E.; Barc, C.; Boulesteix, O.; Contreras, V.; Barnier-Quer, C.; et al. A DNA-Modified Live Vaccine Prime-Boost Strategy Broadens the T-Cell Response and Enhances the Antibody Response against the Porcine Reproductive and Respiratory Syndrome Virus. Viruses 2019, 11, 551. [Google Scholar] [CrossRef]
- Cao, Q.M.; Ni, Y.Y.; Cao, D.; Tian, D.; Yugo, D.M.; Heffron, C.L.; Overend, C.; Subramaniam, S.; Rogers, A.J.; Catanzaro, N.; et al. Recombinant Porcine Reproductive and Respiratory Syndrome Virus Expressing Membrane-Bound Interleukin-15 as an Immunomodulatory Adjuvant Enhances NK and γδ T Cell Responses and Confers Heterologous Protection. J. Virol. 2018, 92, e00007-18. [Google Scholar] [CrossRef] [PubMed]
- Montaner-Tarbes, S.; Del Portillo, H.A.; Montoya, M.; Fraile, L. Key Gaps in the Knowledge of the Porcine Respiratory Reproductive Syndrome Virus (PRRSV). Front. Vet. Sci. 2019, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Y.; Feng, W. Porcine Reproductive and Respiratory Syndrome Virus: Immune Escape and Application of Reverse Genetics in Attenuated Live Vaccine Development. Vaccines 2021, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.A.; Galeota, J.A.; Nelson, E.; Brodersen, B.; Doster, A.; Wills, R.; Zuckermann, F.; Laegreid, W.W. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 2002, 302, 9–20. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Lopez, O.J.; Osorio, F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 2004, 102, 155–163. [Google Scholar] [CrossRef]
- Luo, Q.; Zheng, Y.; Zhang, H.; Yang, Z.; Sha, H.; Kong, W.; Zhao, M.; Wang, N. Research Progress on Glycoprotein 5 of Porcine Reproductive and Respiratory Syndrome Virus. Animals 2023, 13, 813. [Google Scholar] [CrossRef]
- Vu, H.L.; Kwon, B.; Yoon, K.J.; Laegreid, W.W.; Pattnaik, A.K.; Osorio, F.A. Immune evasion of porcine reproductive and respiratory syndrome virus through glycan shielding involves both glycoprotein 5 as well as glycoprotein 3. J. Virol. 2011, 85, 5555–5564. [Google Scholar] [CrossRef]
- Ansari, I.H.; Kwon, B.; Osorio, F.A.; Pattnaik, A.K. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J. Virol. 2006, 80, 3994–4004. [Google Scholar] [CrossRef]
- Paploski, I.A.D.; Makau, D.N.; Pamornchainavakul, N.; Baker, J.P.; Schroeder, D.; Rovira, A.; VanderWaal, K. Potential Novel N-Glycosylation Patterns Associated with the Emergence of New Genetic Variants of PRRSV-2 in the U.S. Vaccines 2022, 10, 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xiao, S.; Yang, X.; Chen, X.; Wu, P.; Song, J.; Ma, Z.; Cai, Z.; Jiang, M.; et al. High-frequency mutation and recombination are responsible for the emergence of novel porcine reproductive and respiratory syndrome virus in northwest China. Arch. Virol. 2019, 164, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, X.; Noor, A.U.; Zhang, X.; Song, C.; Sun, H. Enhancing half-life and cytotoxicity of porcine respiratory and reproductive syndrome virus soluble receptors by taming their Fc domains. Vet. Microbiol. 2022, 273, 109526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Li, W.; Feng, X.; Han, F.; Zhang, Y.; Chen, J.; Liu, D.; Xia, P. Activating Fc Gamma Receptors and Viral Receptors Are Required for Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection. Vet. Sci. 2022, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhang, L.; Wang, J.; Lu, D.; Li, Y.; Ren, J.; Shen, M.; Zhang, L.; Huang, J. Porcine FcεRI Mediates Porcine Reproductive and Respiratory Syndrome Virus Multiplication and Regulates the Inflammatory Reaction. Virol. Sin. 2018, 33, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.; Sun, Y.; Kong, L.; Xu, P.; Xia, P.; Zhang, G. Antibody-Mediated Porcine Reproductive and Respiratory Syndrome Virus Infection Downregulates the Production of Interferon-α and Tumor Necrosis Factor-α in Porcine Alveolar Macrophages via Fc Gamma Receptor I and III. Viruses 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Chen, X.; Li, Y.; Pang, M.; Chen, H.; Nie, X.; Pan, Y.; Qiao, S.; Bao, D. Porcine FcγRIIb mediated PRRSV ADE infection through inhibiting IFN-β by cytoplasmic inhibitory signal transduction. Int. J. Biol. Macromol. 2019, 138, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, W.; Zhao, S.; Cui, Z.; Chen, Y.; Zhang, Y.N.; Chen, J.; Xia, P. Proteomic Characterization of PAMs with PRRSV-ADE Infection. Viruses 2022, 15, 36. [Google Scholar] [CrossRef]
- Bao, D.; Wang, R.; Qiao, S.; Wan, B.; Wang, Y.; Liu, M.; Shi, X.; Guo, J.; Zhang, G. Antibody-dependent enhancement of PRRSV infection down-modulates TNF-α and IFN-β transcription in macrophages. Vet. Immunol. Immunopathol. 2013, 156, 128–134. [Google Scholar] [CrossRef]
- Gu, W.; Guo, L.; Yu, H.; Niu, J.; Huang, M.; Luo, X.; Li, R.; Tian, Z.; Feng, L.; Wang, Y. Involvement of CD16 in antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection. J. Gen. Virol. 2015, 96, 1712–1722. [Google Scholar] [CrossRef]
- Sautter, C.A.; Trus, I.; Nauwynck, H.; Summerfield, A. No Evidence for a Role for Antibodies during Vaccination-Induced Enhancement of Porcine Reproductive and Respiratory Syndrome. Viruses 2019, 11, 829. [Google Scholar] [CrossRef]
- Rahe, M.C.; Murtaugh, M.P. Effector mechanisms of humoral immunity to porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2017, 186, 15–18. [Google Scholar] [CrossRef]
- Paudyal, B.; Mwangi, W.; Rijal, P.; Schwartz, J.C.; Noble, A.; Shaw, A.; Sealy, J.E.; Bonnet-Di Placido, M.; Graham, S.P.; Townsend, A.; et al. Fc-Mediated Functions of Porcine IgG Subclasses. Front. Immunol. 2022, 13, 903755. [Google Scholar] [CrossRef]
- Gunn, B.M.; Yu, W.H.; Karim, M.M.; Brannan, J.M.; Herbert, A.S.; Wec, A.Z.; Halfmann, P.J.; Fusco, M.L.; Schendel, S.L.; Gangavarapu, K.; et al. A Role for Fc Function in Therapeutic Monoclonal Antibody-Mediated Protection against Ebola Virus. Cell Host Microbe 2018, 24, 221–233.e225. [Google Scholar] [CrossRef]
- Flórez-Álvarez, L.; Hernandez, J.C.; Zapata, W. NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies. Front. Immunol. 2018, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Astorga-Gamaza, A.; Grau-Expósito, J.; Burgos, J.; Navarro, J.; Curran, A.; Planas, B.; Suanzes, P.; Falcó, V.; Genescà, M.; Buzon, M.J. Identification of HIV-reservoir cells with reduced susceptibility to antibody-dependent immune response. Elife 2022, 11, e78294. [Google Scholar] [CrossRef]
- Vietzen, H.; Danklmaier, V.; Zoufaly, A.; Puchhammer-Stöckl, E. High-affinity FcγRIIIa genetic variants and potent NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) responses contributing to severe COVID-19. Genet. Med. 2022, 24, 1449–1458. [Google Scholar] [CrossRef]
- Goldberg, B.S.; Ackerman, M.E. Antibody-mediated complement activation in pathology and protection. Immunol. Cell. Biol. 2020, 98, 305–317. [Google Scholar] [CrossRef]
- Costers, S.; Delputte, P.L.; Nauwynck, H.J. Porcine reproductive and respiratory syndrome virus-infected alveolar macrophages contain no detectable levels of viral proteins in their plasma membrane and are protected against antibody-dependent, complement-mediated cell lysis. J. Gen. Virol. 2006, 87, 2341–2351. [Google Scholar] [CrossRef]
- Nelson, C.S.; Huffman, T.; Jenks, J.A.; Cisneros de la Rosa, E.; Xie, G.; Vandergrift, N.; Pass, R.F.; Pollara, J.; Permar, S.R. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc. Natl. Acad. Sci. USA 2018, 115, 6267–6272. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, I.; Kropff, B.; Ambrose, L.; McIntosh, M.; McLean, G.R.; Pichon, S.; Atkinson, C.; Milne, R.S.B.; Mach, M.; Griffiths, P.D.; et al. Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
| Design System | Ingredients | Test Results | Reference |
|---|---|---|---|
| MnGNP | Mannose-modified gelatin nanoparticles (MnGNP) as a carrier to encapsulate inactivated PRRSV virus | Improves T-cell activation, proliferation, and immunity | [66] |
| CH/AL-BV | Chitosan/sodium alginate (CH/AL) nanoparticle-encapsulated bee venom (BV) | Effectively induces Th1-related immune responses, stimulates T cells to secrete IFN-γ, and reduces immunosuppressive effects | [67] |
| VRPs | Expression of PRRSV cytotoxic T cell epitopes using viral replicon particles (VRPs) of swine fever virus | Significantly reduces viral load in the lung tail and improves cell-mediated immune response | [68] |
| LI-M’ | Integration of the hydrophilic structural domain of the PRRSV M protein into Listeria monocytogenes | Significantly enhances CD8+ T cell-mediated immunity | [69] |
| DNA-MLV | DNA vaccines encoding conserved B and T cell epitopes among European subtype 1 strains and known strains | An expanded T-cell response and enhanced antibody response | [70] |
| IL-15-MLV | Interleukin-15 (IL-15) and MLV fused with glycosylphosphatidylinositol (GPI) | Enhances NK and T cell immune responses and provides some allogenic protection | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Zhang, H.; Cheng, H.; Liu, M.; Wen, S.; Ren, J. Progress in PRRSV Infection and Adaptive Immune Response Mechanisms. Viruses 2023, 15, 1442. https://doi.org/10.3390/v15071442
Cai H, Zhang H, Cheng H, Liu M, Wen S, Ren J. Progress in PRRSV Infection and Adaptive Immune Response Mechanisms. Viruses. 2023; 15(7):1442. https://doi.org/10.3390/v15071442
Chicago/Turabian StyleCai, Huanchang, Hewei Zhang, Huai Cheng, Min Liu, Shubo Wen, and Jingqiang Ren. 2023. "Progress in PRRSV Infection and Adaptive Immune Response Mechanisms" Viruses 15, no. 7: 1442. https://doi.org/10.3390/v15071442
APA StyleCai, H., Zhang, H., Cheng, H., Liu, M., Wen, S., & Ren, J. (2023). Progress in PRRSV Infection and Adaptive Immune Response Mechanisms. Viruses, 15(7), 1442. https://doi.org/10.3390/v15071442








