The Host Cytoskeleton Functions as a Pleiotropic Scaffold: Orchestrating Regulation of the Viral Life Cycle and Mediating Host Antiviral Innate Immune Responses
Abstract
1. Introduction
| Cytoskeleton Types | Main Members | Polymer Formation | Functions | References |
|---|---|---|---|---|
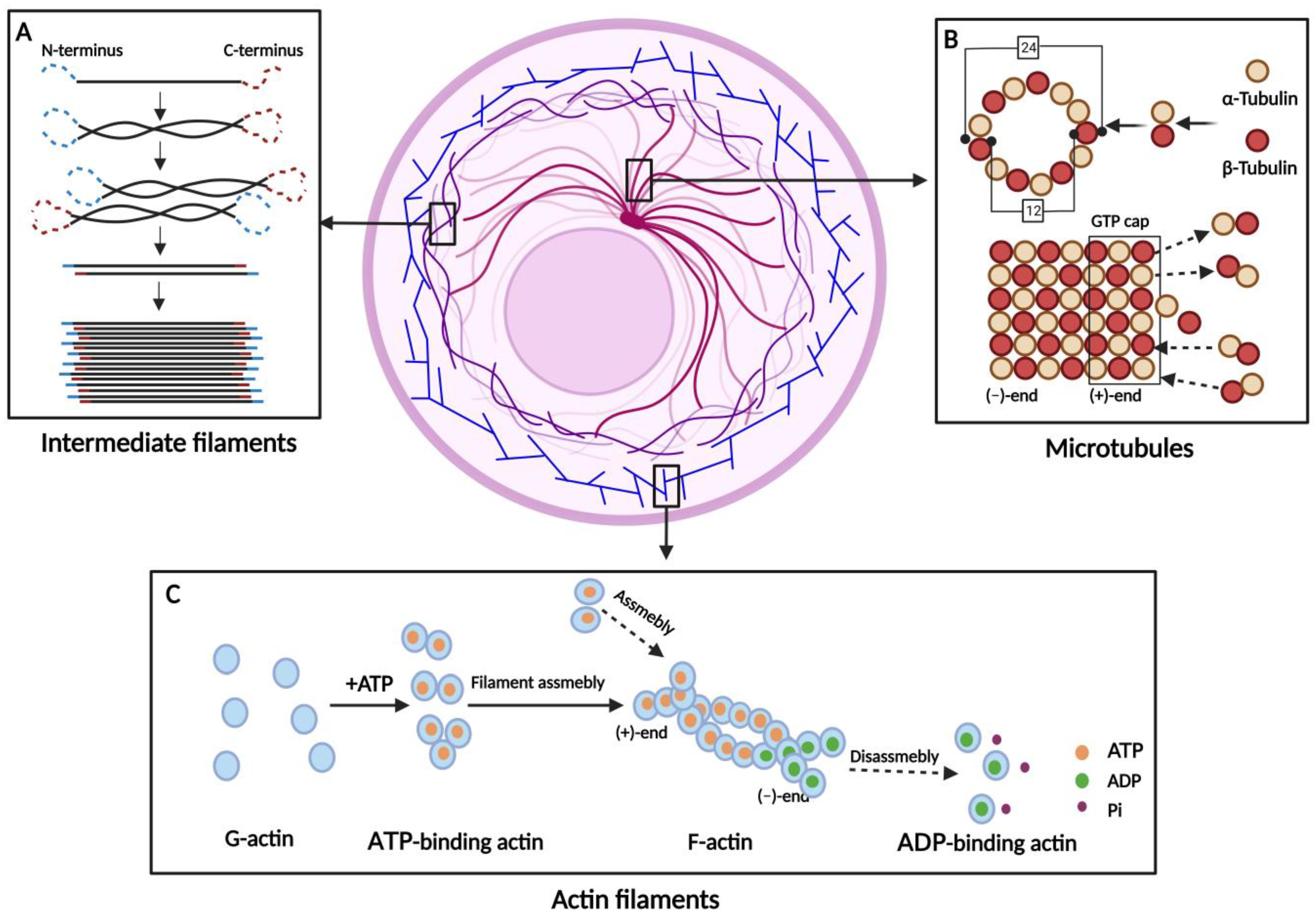
| Actin filaments (AFs) | β-Actin γ-Actin | G-actin forms an unstable dimer or trimer, and then the filaments are elongated by the addition of monomers. | Muscle contraction/ Maintenance of cell surface shape/ Deformable movement/ Cytokinesis | [1,16,17,18] |
| Microtubules (MTs) | α-Tubulin β-Tubulin | α- and β-Tubulin form a heterodimer, which is continuously extended. Thirteen extended tubulin protofilaments form a hollow tube. | Maintaining cell shape/ Transport of substances/ Assistant in mitosis | [1,19,20,21,22,23,24] |
| Intermediate filaments (IFs) | Acidic Keratins Basic Keratins Vimentin Lamins | IFs arise from the monomers spiraling around each other to form dimers. Two dimers aggregate to a tetramer and eight tetramers to a unit-length filament. | Maintaining cell morphology/ Signal transduction/ Involved in cellular stress | [1,25,26,27,28,29] |
2. Physiological Functions of the Cytoskeleton on Normal Conditions
3. Pathological Roles of the Cytoskeleton on Abnormal Conditions
3.1. Neoplasm and Cancer
3.2. Passive Infection with Bacteria, Viruses, or Parasites
3.3. Pathological Process
4. Multiple Engagements of the Cytoskeleton in Viral Life Cycle by Targeting Various Stages
4.1. Entry and Internalization
4.2. Transport
4.3. Replication, Transcription, and Translation
4.4. Assembly and Egress
5. The Cytoskeleton Mediates Virus Transmission and Spread from Cell to Cell
5.1. Direct Transmission
5.2. Indirect Transmission
6. The Cytoskeleton Is Involved in the Immune Responses to Viral Infections
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hohmann, T.; Dehghani, F. The cytoskeleton—A complex interacting meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Abouelezz, A.; Almeida-Souza, L. The mammalian endocytic cytoskeleton. Eur. J. Cell Biol. 2022, 101, 151222. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Gammon, D.B. Manipulation of the host cytoskeleton by viruses: Insights and mechanisms. Viruses 2022, 14, 1586. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J. Mechanical tumor microenvironment and transduction: Cytoskeleton mediates cancer cell invasion and metastasis. Int. J. Biol. Sci. 2020, 16, 2014–2028. [Google Scholar] [CrossRef]
- Molinie, N.; Rubtsova, S.N.; Fokin, A.; Visweshwaran, S.P.; Rocques, N.; Polesskaya, A.; Schnitzler, A.; Vacher, S.; Denisov, E.V.; Tashireva, L.A.; et al. Cortical branched actin determines cell cycle progression. Cell Res. 2019, 29, 432–445. [Google Scholar] [CrossRef]
- Wang, I.H.; Burckhardt, C.J.; Yakimovich, A.; Greber, U.F. Imaging, tracking and computational analyses of virus entry and egress with the cytoskeleton. Viruses 2018, 10, 166. [Google Scholar] [CrossRef]
- Buchwalter, R.A.; Ogden, S.C.; York, S.B.; Sun, L.; Zheng, C.; Hammack, C.; Cheng, Y.; Chen, J.V.; Cone, A.S.; Meckes, D.G., Jr.; et al. Coordination of Zika virus infection and viroplasm organization by microtubules and microtubule-organizing centers. Cells 2021, 10, 3335. [Google Scholar] [CrossRef]
- Dutour-Provenzano, G.; Etienne-Manneville, S. Intermediate filaments. Curr. Biol. 2021, 31, R522–R529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, S.; Li, Y.; Feng, F.; Li, M.; Xue, Y.; Cui, J.; Xu, T.; Jin, X.; Jiu, Y. Host cytoskeletal vimentin serves as a structural organizer and an RNA-binding protein regulator to facilitate Zika viral replication. Proc. Natl. Acad. Sci. USA 2022, 119, e2113909119. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Shao, Q.; Yu, H.; Liu, J.; Li, Y.; Wang, B.; Sang, H.; Li, D.; Bing, A.; Hou, Y.; et al. Tight junctions, the key factor in virus-related disease. Pathogens 2022, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Reis, R.; Volcic, M.; Liu, G.; Wang, M.K.; Chia, B.S.; Nchioua, R.; Gross, R.; Munch, J.; Kirchhoff, F.; et al. Actin cytoskeleton remodeling primes RIG-I-like receptor activation. Cell 2022, 185, 3588–3602. [Google Scholar] [CrossRef] [PubMed]
- de Rivero Vaccari, J.P.; Minkiewicz, J.; Wang, X.; de Rivero Vaccari, J.C.; German, R.; Marcillo, A.E.; Dietrich, W.D.; Keane, R.W. Astrogliosis involves activation of retinoic acid-inducible gene-like signaling in the innate immune response after spinal cord injury. Glia 2012, 60, 14–21. [Google Scholar] [CrossRef]
- Liu, H.; Ye, G.; Liu, X.; Xue, M.; Zhou, Q.; Zhang, L.; Zhang, K.; Huang, L.; Weng, C. Vimentin inhibits type I interferon production by disrupting the TBK1-IKKepsilon-IRF3 axis. Cell Rep. 2022, 41, 111469. [Google Scholar] [CrossRef] [PubMed]
- Bovellan, M.; Romeo, Y.; Biro, M.; Boden, A.; Chugh, P.; Yonis, A.; Vaghela, M.; Fritzsche, M.; Moulding, D.; Thorogate, R.; et al. Cellular control of cortical actin nucleation. Curr. Biol. 2014, 24, 1628–1635. [Google Scholar] [CrossRef]
- Kloc, M.; Chanana, P.; Vaughn, N.; Uosef, A.; Kubiak, J.Z.; Ghobrial, R.M. New insights into cellular functions of nuclear actin. Biology 2021, 10, 304. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef]
- Nogales, E.; Wang, H.W. Structural intermediates in microtubule assembly and disassembly: How and why? Curr. Opin. Cell Biol. 2006, 18, 179–184. [Google Scholar] [CrossRef]
- Gudimchuk, N.B.; McIntosh, J.R. Regulation of microtubule dynamics, mechanics and function through the growing tip. Nat. Rev. Mol. Cell Biol. 2021, 22, 777–795. [Google Scholar] [CrossRef]
- Akıl, C.; Ali, S.; Tran, L.T.; Gaillard, J.; Li, W.; Hayashida, K.; Hirose, M.; Kato, T.; Oshima, A.; Fujishima, K.; et al. Structure and dynamics of Odinarchaeota tubulin and the implications for eukaryotic microtubule evolution. Sci. Adv. 2022, 8, eabm2225. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Saravanan, S.; Sharma, D.; Narasimha, M. The microtubule end-binding proteins EB1 and patronin modulate the spatiotemporal dynamics of myosin and pattern pulsed apical constriction. Development 2022, 149, dev199759. [Google Scholar] [CrossRef]
- Martin, M.; Akhmanova, A. Coming into focus: Mechanisms of microtubule minus-end organization. Trends Cell Biol. 2018, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Ferro, L.S.; Can, S.; Turner, M.A.; ElShenawy, M.M.; Yildiz, A. Kinesin and dynein use distinct mechanisms to bypass obstacles. eLife 2019, 8, e48629. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, J.; Pallari, H.M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Bott, C.J.; Winckler, B. Intermediate filaments in developing neurons: Beyond structure. Cytoskeleton 2020, 77, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, F1000 Faculty Rev-1796. [Google Scholar] [CrossRef]
- Redmond, C.J.; Coulombe, P.A. Intermediate filaments as effectors of differentiation. Curr. Opin. Cell Biol. 2021, 68, 155–162. [Google Scholar] [CrossRef]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Dugina, V.B.; Shagieva, G.S.; Kopnin, P.B. Biological role of actin isoforms in mammalian cells. Biochemistry 2019, 84, 583–592. [Google Scholar] [CrossRef]
- Rubenstein, P.A. The functional importance of multiple actin isoforms. Bioessays 1990, 12, 309–315. [Google Scholar] [CrossRef]
- Carlier, M.F.; Shekhar, S. Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat. Rev. Mol. Cell Biol. 2017, 18, 389–401. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielinska, W.; Halas-Wisniewska, M.; Grzanka, A. Involvement of actin and actin-binding proteins in carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef]
- Rotty, J.D.; Wu, C.; Bear, J.E. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kage, F.; Winterhoff, M.; Dimchev, V.; Mueller, J.; Thalheim, T.; Freise, A.; Brühmann, S.; Kollasser, J.; Block, J.; Dimchev, G.; et al. FMNL formins boost lamellipodial force generation. Nat. Commun. 2017, 8, 14832. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M.; Cadot, B. Actin on and around the nucleus. Trends Cell Biol. 2021, 31, 211–223. [Google Scholar] [CrossRef]
- Tann, J.Y.; Moore, A.W. MTOC organization and competition during neuron differentiation. Results Probl. Cell Differ. 2019, 67, 337–357. [Google Scholar] [PubMed]
- Zehner, Z.E.; Paterson, B.M. Characterization of the chicken vimentin gene: Single copy gene producing multiple mRNAs. Proc. Natl. Acad. Sci. USA 1983, 80, 911–915. [Google Scholar] [CrossRef]
- Lundin, V.F.; Leroux, M.R.; Stirling, P.C. Quality control of cytoskeletal proteins and human disease. Trends Biochem. Sci. 2010, 35, 288–297. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Sakata-Yanagimoto, M.; Enami, T.; Yoshida, K.; Shiraishi, Y.; Ishii, R.; Miyake, Y.; Muto, H.; Tsuyama, N.; Sato-Otsubo, A.; Okuno, Y.; et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat. Genet. 2014, 46, 171–175. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, U.; In’t Veld, P.; Gilles, C.; Sermon, K.; De, R.M.; Van, V.H.; Van, S.A.; Liebaers, I. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol. Hum. Reprod. 2007, 13, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.G.; Kojima, S.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Hartland, E.L.; Ghosal, D.; Giogha, C. Manipulation of epithelial cell architecture by the bacterial pathogens Listeria and Shigella. Curr. Opin. Cell Biol. 2022, 79, 102131. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Schwan, C.; Jank, T. Clostridium difficile toxin biology. Annu. Rev. Microbiol. 2017, 71, 281–307. [Google Scholar] [CrossRef]
- Garzon, M.; Sosik, P.; Drastík, J.; Skalli, O. A self-controlled and self-healing model of bacterial cells. Membranes 2022, 12, 678. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Listeria monocytogenes: Cell biology of invasion and intracellular growth. Microbiol. Spectr. 2018, 6, 6. [Google Scholar] [CrossRef]
- Agaisse, H. Molecular and cellular mechanisms of Shigella flexneri dissemination. Front. Cell. Infect. Microbiol. 2016, 11, 29. [Google Scholar] [CrossRef]
- Miao, C.; Zhao, S.; Etienne-Manneville, S.; Jiu, Y. The diverse actions of cytoskeletal vimentin in bacterial infection and host defense. J. Cell Sci. 2023, 136, jcs260509. [Google Scholar] [CrossRef]
- Guo, Y.; Duan, M.; Wang, X.; Gao, J.; Guan, Z.; Zhang, M. Early events in rabies virus infection-Attachment, entry, and intracellular trafficking. Virus Res. 2019, 263, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, L.; Ricketts, S.N.; Rust, M.J.; Das, M.; Robertson-Anderson, R.M.; Ross, J.L. Actin and microtubule crosslinkers tune mobility and control co-localization in a composite cytoskeletal network. Soft Matter. 2020, 16, 7191–7201. [Google Scholar] [CrossRef]
- Cyrklaff, M.; Sanchez, C.P.; Kilian, N.; Bisseye, C.; Simpore, J.; Frischknecht, F.; Lanzer, M. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science 2011, 334, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Kang, J.K.; Kim, S.K.; Ahn, K.J.; Seok, H.; Park, S.J.; Chang, J.S.; Pothoulakis, C.; Lamont, J.T.; Kim, H. Clostridium difficile toxin A decreases acetylation of tubulin, leading to microtubule depolymerization through activation of histone deacetylase 6, and this mediates acute inflammation. J. Biol. Chem. 2010, 285, 32888–32896. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.; Han, M.; Woottum, M.; Bracq, L.; Bouchet, J.; Xie, M.; Benichou, S. Virus-mediated cell-cell fusion. Int. J. Mol. Sci. 2020, 21, 9644. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Niu, Z.; Zhang, B.; Wang, C.; Yao, X.; Peng, H.; Franca, D.N.; Wang, Y.; Zhu, Y.; et al. SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination. Cell Death Differ. 2021, 28, 2765–2777. [Google Scholar] [CrossRef]
- Kloc, M.; Uosef, A.; Wosik, J.; Kubiak, J.Z.; Ghobrial, R.M. Virus interactions with the actin cytoskeleton--what we know and do not know about SARS-CoV-2. Arch. Virol. 2022, 167, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of tau as a microtubule-associated protein: Structural and functional aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Alhogbani, T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann. Saudi Med. 2016, 36, 78–80. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Lee, J.Y.; Cerikan, B.; Neufeldt, C.J.; Oorschot, V.M.J.; Köhrer, S.; Hennies, J.; Schieber, N.L.; Ronchi, P.; Mizzon, G.; et al. Integrative imaging reveals SARS-CoV-2-induced reshaping of subcellular morphologies. Cell Host Microbe 2020, 28, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Saksena, M.; Denes, C.E.; Diefenbach, R.J.; Cunningham, A.L. Infection and transport of herpes simplex virus type 1 in neurons: Role of the cytoskeleton. Viruses 2018, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Paluck, A.; Osan, J.; Hollingsworth, L.; Talukdar, S.N.; Saegh, A.A.; Mehedi, M. Role of ARP2/3 complex-driven actin polymerization in RSV infection. Pathogens 2021, 11, 26. [Google Scholar] [CrossRef]
- Liu, X.; Nawaz, Z.; Guo, C.; Ali, S.; Naeem, M.A.; Jamil, T.; Ahmad, W.; Siddiq, M.U.; Ahmed, S.; Asif Idrees, M.; et al. Rabies virus exploits cytoskeleton network to cause early disease progression and cellular dysfunction. Front. Vet. Sci. 2022, 9, 889873. [Google Scholar] [CrossRef]
- Piccinotti, S.; Kirchhausen, T.; Whelan, S.P. Uptake of rabies virus into epithelial cells by clathrin-mediated endocytosis depends upon actin. J. Virol. 2013, 87, 11637–11647. [Google Scholar] [CrossRef]
- Lehmann, M.J.; Sherer, N.M.; Marks, C.B.; Pypaert, M.; Mothes, W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 2005, 170, 317–325. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Turney, S.; Qian, H.; Elson, E.L. Nanometre-level analysis demonstrates that lipid flow does not drive membrane glycoprotein movements. Nature 1989, 340, 284–288. [Google Scholar] [CrossRef]
- Svitkina, T.M.; Verkhovsky, A.B.; McQuade, K.M.; Borisy, G.G. Analysis of the actin-myosin II system in fish epidermal keratocytes: Mechanism of cell body translocation. J. Cell Biol. 1997, 139, 397–415. [Google Scholar] [CrossRef]
- Cheng, Y.; Lou, J.X.; Liu, C.C.; Liu, Y.Y.; Chen, X.N.; Liang, X.D.; Zhang, J.; Yang, Q.; Go, Y.Y.; Zhou, B. Microfilaments and microtubules alternately coordinate the multi-step endosomal trafficking of classical swine fever virus. J. Virol. 2021, 95, e02436-20. [Google Scholar] [CrossRef]
- Mylvaganam, S.; Freeman, S.A.; Grinstein, S. The cytoskeleton in phagocytosis and macropinocytosis. Curr. Biol. 2021, 31, R619–R632. [Google Scholar] [CrossRef] [PubMed]
- Pastey, M.K.; Gower, T.L.; Spearman, P.W.; Crowe, J.E., Jr.; Graham, B.S. A RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3. Nat. Med. 2000, 6, 35–40. [Google Scholar] [CrossRef]
- Hoppe, S.; Schelhaas, M.; Jaeger, V.; Liebig, T.; Petermann, P.; Knebel-Mörsdorf, D. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J. Gen. Virol. 2006, 87, 3483–3494. [Google Scholar] [CrossRef]
- Cuartas-López, A.M.; Hernández-Cuellar, C.E.; Gallego-Gómez, J.C. Disentangling the role of PI3K/Akt, Rho GTPase and the actin cytoskeleton on dengue virus infection. Virus Res. 2018, 256, 153–165. [Google Scholar] [CrossRef]
- Miller, M.S.; Hertel, L. Onset of human cytomegalovirus replication in fibroblasts requires the presence of an intact vimentin cytoskeleton. J. Virol. 2009, 83, 7015–7028. [Google Scholar] [CrossRef] [PubMed]
- Sodeik, B. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 2000, 8, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.H. HIV-1 capsid exploitation of the host microtubule cytoskeleton during early infection. Retrovirology 2021, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Schmidt, E.E.; Halford, W.P. ICP0 dismantles microtubule networks in herpes simplex virus-infected cells. PLoS ONE 2010, 5, e10975. [Google Scholar] [CrossRef]
- Wang, J.; Fang, S.; Xiao, H.; Chen, B.; Tam, J.P.; Liu, D.X. Interaction of the coronavirus infectious bronchitis virus membrane protein with beta-actin and its implication in virion assembly and budding. PLoS ONE 2009, 4, e4908. [Google Scholar]
- Richards, A.; Berth, S.H.; Brady, S.; Morfini, G. Engagement of neurotropic viruses in fast axonal transport: Mechanisms, potential role of host kinases and implications for neuronal dysfunction. Front. Cell Neurosci. 2021, 15, 684762. [Google Scholar] [CrossRef]
- Shanda, S.K.; Wilson, D.W. UL36p is required for efficient transport of membrane-associated Herpes simplex virus type 1 along microtubules. J. Virol. 2008, 82, 7388–7394. [Google Scholar] [CrossRef]
- Zaichick, S.V.; Bohannon, K.P.; Hughes, A.; Sollars, P.J.; Pickard, G.E.; Smith, G.A. The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion. Cell Host Microbe 2013, 13, 193–203. [Google Scholar] [CrossRef]
- Dodding, M.P.; Way, M. Coupling viruses to dynein and kinesin-1. EMBO J. 2011, 30, 3527–3539. [Google Scholar] [CrossRef]
- Naghavi, M.H.; Walsh, D. Microtubule regulation and function during virus infection. J. Virol. 2017, 91, e00538-17. [Google Scholar] [CrossRef]
- Pawlica, P.; Berthoux, L. Cytoplasmic dynein promotes HIV-1 uncoating. Viruses 2014, 6, 4195–4211. [Google Scholar] [CrossRef]
- Yoder, A.; Guo, J.; Yu, D.; Cui, Z.; Zhang, X.E.; Wu, Y. Effects of microtubule modulators on HIV-1 infection of transformed and resting CD4 T cells. J. Virol. 2011, 85, 3020–3024. [Google Scholar] [CrossRef]
- Neufeldt, C.J.; Cortese, M. Membrane architects: How positive-strand RNA viruses restructure the cell. J. Gen. Virol. 2022, 103, 10. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Lin, Z.; Shi, K.; Jiu, Y. Cytoskeleton--a crucial key in host cell for coronavirus infection. J. Mol. Cell Biol. 2020, 12, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Mallardo, M.; Schleich, S.; Krijnse Locker, J. Microtubule-dependent organization of vaccinia virus core-derived early mRNAs into distinct cytoplasmic structures. Mol. Biol. Cell 2001, 12, 3875–3891. [Google Scholar] [CrossRef] [PubMed]
- Schepis, A.; Schramm, B.; de Haan, C.A.; Locker, J.K. Vaccinia virus-induced microtubule-dependent cellular rearrangements. Traffic 2006, 7, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Cibulka, J.; Fraiberk, M.; Forstova, J. Nuclear actin and lamins in viral infections. Viruses 2012, 4, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Forest, T.; Barnard, S.; Baines, J.D. Active intranuclear movement of herpesvirus capsids. Nat. Cell Biol. 2005, 7, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Monaghan, P.; Way, M.; Wileman, T. Transport of African swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin. J. Virol. 2004, 78, 7990–8001. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.L.; Gillespie, L.K.; Mackenzie, J.M. Mouse norovirus 1 utilizes the cytoskeleton network to establish localization of the replication complex proximal to the microtubule organizing center. J. Virol. 2012, 86, 4110–4122. [Google Scholar] [CrossRef]
- Appenzeller-Herzog, C.; Hauri, H.P. The ER-Golgi intermediate compartment (ERGIC): In search of its identity and function. J. Cell Sci. 2006, 119, 2173–2183. [Google Scholar] [CrossRef]
- Yu, S.F.; Eastman, S.W.; Linial, M.L. Foamy virus capsid assembly occurs at a pericentriolar region through a cytoplasmic targeting/retention signal in Gag. Traffic 2006, 7, 966–977. [Google Scholar] [CrossRef]
- Ng, M.L.; Lee, J.W.; Leong, M.L.; Ling, A.E.; Tan, H.C.; Ooi, E.E. Topographic changes in SARS coronavirus-infected cells at late stages of infection. Emerg. Infect. Dis. 2004, 10, 1907–1914. [Google Scholar] [CrossRef]
- Bohn, W.; Rutter, G.; Hohenberg, H.; Mannweiler, K.; Nobis, P. Involvement of actin filaments in budding of measles virus: Studies on cytoskeletons of infected cells. Virology 1986, 149, 91–106. [Google Scholar] [CrossRef]
- Milbradt, J.; Sonntag, E.; Wagner, S.; Strojan, H.; Wangen, C.; Lenac Rovis, T.; Lisnic, B.; Jonjic, S.; Sticht, H.; Britt, W.J.; et al. Human cytomegalovirus nuclear capsids associate with the core nuclear egress complex and the viral protein kinase pUL97. Viruses 2018, 10, 35. [Google Scholar] [CrossRef]
- Iwami, S.; Takeuchi, J.S.; Nakaoka, S.; Mammano, F.; Clavel, F.; Inaba, H.; Kobayashi, T.; Misawa, N.; Aihara, K.; Koyanagi, Y.; et al. Cell-to-cell infection by HIV contributes over half of virus infection. eLife 2015, 4, e08150. [Google Scholar] [CrossRef]
- Reh, L.; Magnus, C.; Schanz, M.; Weber, J.; Uhr, T.; Rusert, P.; Trkola, A. Capacity of broadly neutralizing antibodies to inhibit HIV-1 cell-cell transmission is strain- and epitope-dependent. PLoS Pathog. 2015, 11, e1004966. [Google Scholar] [CrossRef]
- Zhong, P.; Agosto, L.M.; Ilinskaya, A.; Dorjbal, B.; Truong, R.; Derse, D.; Uchil, P.D.; Heidecker, G.; Mothes, W. Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV. PLoS ONE 2013, 8, e53138. [Google Scholar] [CrossRef]
- Kim, J.T.; Chang, E.; Sigal, A.; Baltimore, D. Dendritic cells efficiently transmit HIV to T cells in a tenofovir and raltegravir insensitive manner. PLoS ONE 2018, 13, e0189945. [Google Scholar] [CrossRef] [PubMed]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Abounit, S.; Zurzolo, C. Wiring through tunneling nanotubes—From electrical signals to organelle transfer. J. Cell Sci. 2012, 125, 1089–1098. [Google Scholar] [CrossRef]
- Zurzolo, C. Tunneling nanotubes: Reshaping connectivity. Curr. Opin. Cell Biol. 2021, 71, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kazanietz, M.G.; Cooke, M. Rho GTPases and the emerging role of tunneling nanotubes in physiology and disease. Am. J. Physiol. Cell Physiol. 2020, 319, C877–C884. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Koganti, R.; Russell, G.; Sharma, A.; Shukla, D. Role of tunneling nanotubes in viral infection, neurodegenerative disease, and cancer. Front. Immunol. 2021, 12, 680891. [Google Scholar] [CrossRef]
- Sewald, X.; Gonzalez, D.G.; Haberman, A.M.; Mothes, W. In vivo imaging of virological synapses. Nat. Commun. 2012, 3, 1320. [Google Scholar] [CrossRef]
- Piguet, V.; Sattentau, Q. Dangerous liaisons at the virological synapse. J. Clin. Investig. 2004, 114, 605–610. [Google Scholar] [CrossRef]
- Igakura, T.; Stinchcombe, J.C.; Goon, P.K.; Taylor, G.P.; Weber, J.N.; Griffiths, G.M.; Tanaka, Y.; Osame, M.; Bangham, C.R. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 2003, 299, 1713–1716. [Google Scholar] [CrossRef]
- Yashavantha Rao, H.C.; Jayabaskaran, C. The emergence of a novel coronavirus (SARS-CoV-2) disease and their neuroinvasive propensity may affect in COVID-19 patients. J. Med. Virol. 2020, 92, 786–790. [Google Scholar] [CrossRef]
- Martin, N.; Welsch, S.; Jolly, C.; Briggs, J.A.; Vaux, D.; Sattentau, Q.J. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J. Virol. 2010, 84, 3516–3527. [Google Scholar] [CrossRef]
- Vasiliver-Shamis, G.; Cho, M.W.; Hioe, C.E.; Dustin, M.L. Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse. J. Virol. 2009, 83, 11341–11355. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-cell junctions organize structural and signaling networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef]
- Heuser, S.; Hufbauer, M.; Marx, B.; Tok, A.; Majewski, S.; Pfister, H.; Akgül, B. The levels of epithelial anchor proteins β-catenin and zona occludens-1 are altered by E7 of human papillomaviruses 5 and 8. J. Gen. Virol. 2016, 97, 463–472. [Google Scholar] [CrossRef]
- Labudová, M. Cell-to-cell transport in viral families: Faster than usual. Acta Virol. 2020, 64, 154–166. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T.; Akira, S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol. Rev. 2011, 243, 61–73. [Google Scholar] [CrossRef] [PubMed]
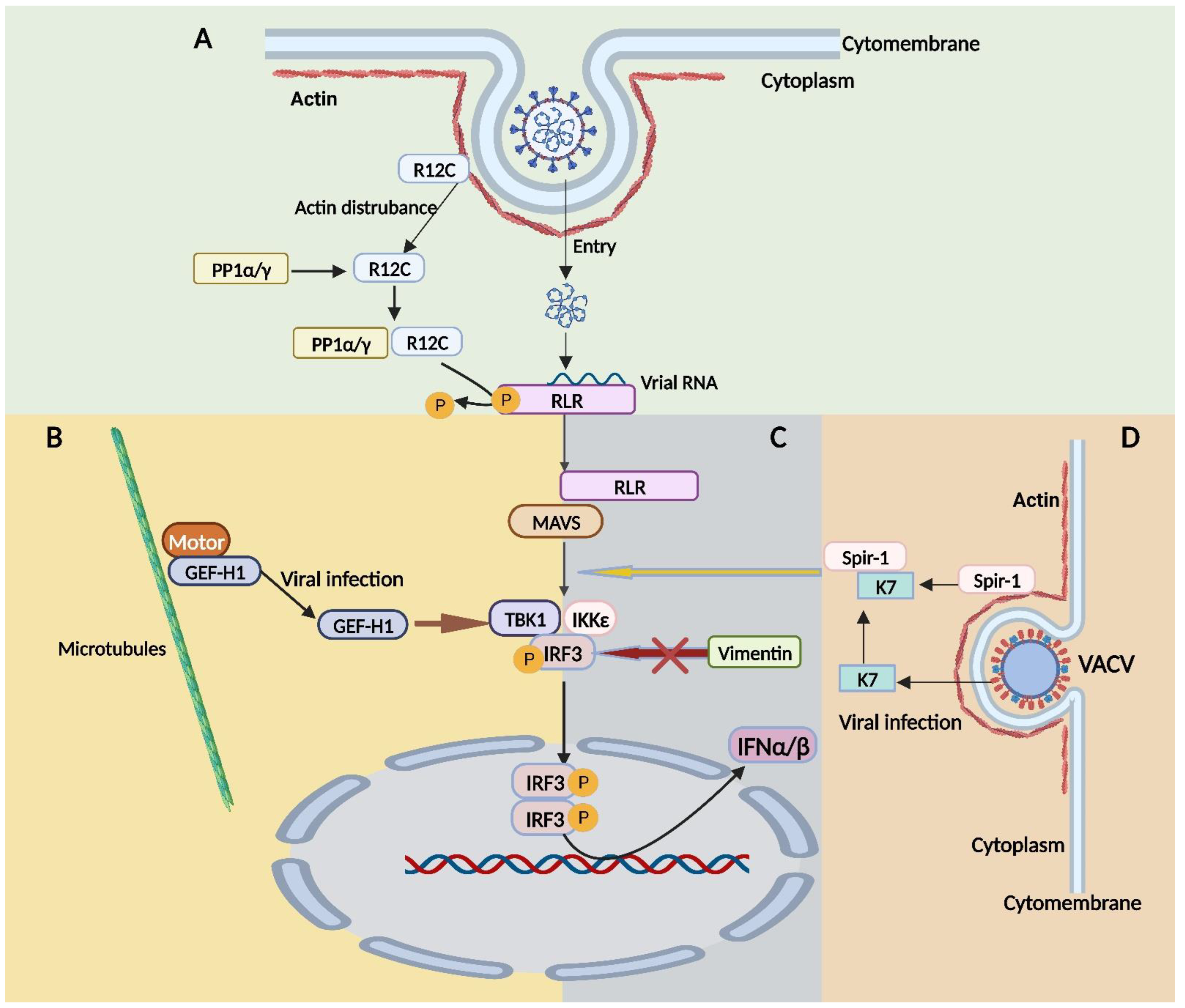
- Gack, M.U.; Nistal-Villán, E.; Inn, K.S.; García-Sastre, A.; Jung, J.U. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 2010, 84, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Wies, E.; Wang, M.K.; Maharaj, N.P.; Chen, K.; Zhou, S.; Finberg, R.W.; Gack, M.U. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity 2013, 38, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Wellington, A.; Emmons, S.; James, B.; Calley, J.; Grover, M.; Tolias, P.; Manseau, L. Spire contains actin binding domains and is related to ascidian posterior end mark-5. Development 1999, 126, 5267–5274. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.A.; Macilwee, S.L.; Rashid, A.; Cox, S.E.; Albarnaz, J.D.; Bonjardim, C.A.; Smith, G.L. The actin nucleator Spir-1 is a virus restriction factor that promotes innate immune signalling. PLoS Pathog. 2022, 18, e1010277. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.; Dimitriou, I.D.; Rottapel, R. Go with the flow: GEF-H1 mediated shear stress mechanotransduction in neutrophils. Small GTPases 2020, 11, 23–31. [Google Scholar] [CrossRef]
- Chiang, H.S.; Zhao, Y.; Song, J.H.; Liu, S.; Wang, N.; Terhorst, C.; Sharpe, A.H.; Basavappa, M.; Jeffrey, K.L.; Reinecker, H.C. GEF-H1 controls microtubule-dependent sensing of nucleic acids for antiviral host defenses. Nat. Immunol. 2014, 15, 63–71. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.M.; Maniatis, T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef]
- Stevens, C.; Henderson, P.; Nimmo, E.R.; Soares, D.C.; Dogan, B.; Simpson, K.W.; Barrett, J.C.; Wilson, D.C.; Satsangi, J. The intermediate filament protein, vimentin, is a regulator of NOD2 activity. Gut 2013, 62, 695–707. [Google Scholar] [CrossRef]
- Hu, K.; Onintsoa Diarimalala, R.; Yao, C.; Li, H.; Wei, Y. EV-A71 mechanism of entry: Receptors/co-receptors, related pathways and inhibitors. Viruses 2023, 15, 785. [Google Scholar] [CrossRef]
- Schäfer, G.; Graham, L.M.; Lang, D.M.; Blumenthal, M.J.; Bergant Marušič, M.; Katz, A.A. Vimentin modulates infectious internalization of human papillomavirus 16 pseudovirions. J. Virol. 2017, 91, e00307-17. [Google Scholar] [CrossRef]


| Types of Pathogeneses | Changes in the Cytoskeleton | The Effects of the Changes | Pathological Roles | References |
|---|---|---|---|---|
| Cancers | Depolymerization and polymerization of actin | Contributing to cell migration | Devoting to cancer cells spread and replicate quickly | [41,42,43,44,45] |
| Depolymerization, polymerization and modification of microtubules | Participating in cell movement through signal transduction and as a transport structure | |||
| Interaction of vimentin with actin and microtubules. | Contributing to cell–matrix adhesion and migration | |||
| Activation of vimentin expression, and interaction of vimentin with motor proteins | Aims to enhance cell motility, which is conducive to the process of epithelial–mesenchymal transition (EMT) | |||
| Intracellular bacteria infected | Actin is recruited and interacts with actin regulatory factors Arp2/3 | Leading to bacterial engulfment and internalization in a membrane-bound vacuole | Promoting the infection of intracellular bacteria | [46,47,48,49,50,51,55] |
| Microtubule depolymerization and the activity of the Rho family of enzymes that control microtubules are affected and interfered with by bacterial production of Clostridium difficile toxin A (TcdA) | Participating in bacterial transportation and the consequential immune-inflammatory responses | |||
| Vimentin is expressed on the cell surface, secreted and located extracellularly | Contributing to stress reaction; vimentin can be both pro- and anti-bacterial, favoring bacterial invasion in some contexts, but also involved in bacterial-induced inflammation regulation | |||
| Viruses infected | Actin depolymerizes and polymerizes, and kinetoproteins are recruited. | Contributing to entry and internalization | Assisting the virus to complete its life cycle | [15,52,53] |
| Microtubule and motor proteins interact with viral proteins, microtubule depolymerization and polymerization, motor proteins are changed | Transporting viral components, formation of replicative organelles | |||
| The vimentin expression is changed | Contributing to viral replication and signaling | |||
| Parasites infected | Plasmodium can promote actin polymerization in vitro | Inhibiting the movement of cargo vesicles to the erythrocyte plasma membrane | Promoting severe Plasmodium falciparum malaria infection | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Peng, D.; Cao, H.; Yang, X.; Li, S.; Qiu, H.-J.; Li, L.-F. The Host Cytoskeleton Functions as a Pleiotropic Scaffold: Orchestrating Regulation of the Viral Life Cycle and Mediating Host Antiviral Innate Immune Responses. Viruses 2023, 15, 1354. https://doi.org/10.3390/v15061354
Li M, Peng D, Cao H, Yang X, Li S, Qiu H-J, Li L-F. The Host Cytoskeleton Functions as a Pleiotropic Scaffold: Orchestrating Regulation of the Viral Life Cycle and Mediating Host Antiviral Innate Immune Responses. Viruses. 2023; 15(6):1354. https://doi.org/10.3390/v15061354
Chicago/Turabian StyleLi, Meilin, Dingkun Peng, Hongwei Cao, Xiaoke Yang, Su Li, Hua-Ji Qiu, and Lian-Feng Li. 2023. "The Host Cytoskeleton Functions as a Pleiotropic Scaffold: Orchestrating Regulation of the Viral Life Cycle and Mediating Host Antiviral Innate Immune Responses" Viruses 15, no. 6: 1354. https://doi.org/10.3390/v15061354
APA StyleLi, M., Peng, D., Cao, H., Yang, X., Li, S., Qiu, H.-J., & Li, L.-F. (2023). The Host Cytoskeleton Functions as a Pleiotropic Scaffold: Orchestrating Regulation of the Viral Life Cycle and Mediating Host Antiviral Innate Immune Responses. Viruses, 15(6), 1354. https://doi.org/10.3390/v15061354









