Diversity of Anal HPV and Non-HPV Sexually Transmitted Infections and Concordance with Genital Infections in HIV-Infected and HIV-Uninfected Women in the Tapajós Region, Amazon, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Issues
2.2. Study Design and Population
2.3. Anal and Cervical Samples
2.4. DNA Extraction and HPV DNA Detection
2.5. HPV Genotyping and Multiple HPV Types
2.6. Non-HPV STI Molecular Detection
2.7. Statistical Analysis
3. Results
3.1. Main Sociodemographic Characteristics of the Participants
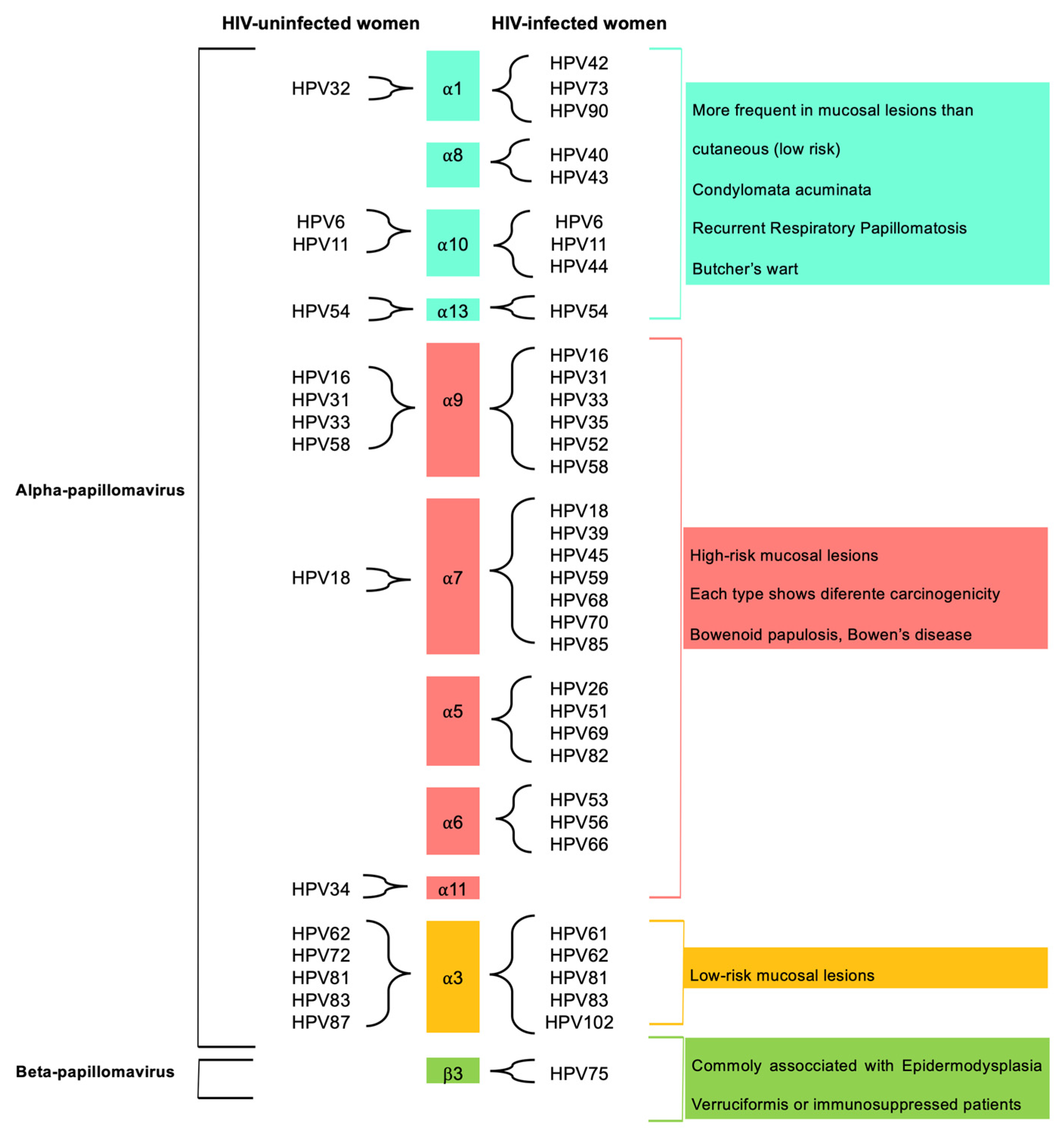
3.2. Anal HPV Infection and Diversity
3.3. Anal hrHPV and Sociodemographic Characteristics of HIV-Uninfected and HIV-Infected Women in the Tapajós Region, Amazon, Brazil
3.4. Single and Multiple Anal HPV Infections and HPV Types in HIV-Uninfected and HIV-Infected Women
3.5. Anal Non-HPV STIs in HIV-Uninfected and HIV-Infected Women
3.6. Concordance between Anal and Genital HPV and Non-HPV STIs in HIV-Uninfected and HIV-Infected Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, C.; Slama, J.; Gonzalez, P.; Goodman, M.T.; Xia, N.; Kreimer, A.R.; Wu, T.; Hessol, N.A.; Shvetsov, Y.; Ortiz, A.P.; et al. Cervical determinants of anal HPV infection and high-grade anal lesions in women: A collaborative pooled analysis. Lancet Infect. Dis. 2019, 19, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.; Hashim, P.W.; Reddy, V.B.; Einarsdottir, H.; Longo, W.E. Sexually transmitted infections of the anus and rectum. World J. Gastroenterol. 2014, 20, 15262–15268. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.-B.; Schiffman, M.; Burchell, A.; Albero, G.; Giuliano, A.R.; Goodman, M.T.; Kjaer, S.K.; Palefsky, J. Updating the Natural History of Human Papillomavirus and Anogenital Cancers. Vaccine 2012, 30, F24–F33. [Google Scholar] [CrossRef]
- van Eer, K.; Laâbi, I.; van Benthem, B.H.B.; Steenbergen, R.D.M.; King, A.J.; Adema, D.; Buist-Arkema, R.; Beerens, A.; Luijt, D.; Meijer, S.; et al. The association between viral load and concurrent human papillomavirus infection at the genital and anal sites of young women and the impact of vaccination. Tumour Virus Res. 2022, 13, 200233. [Google Scholar] [CrossRef]
- Morhason-Bello, I.O.; Baisley, K.; Pavon, M.A.; Adewole, I.F.; Bakare, R.; de Sanjosé, S.; Francis, S.C.; Watson-Jones, D. Prevalence and genotype specific concordance of oro-genital and anal human papillomavirus infections among sexually active Nigerian women. Infect. Agents Cancer 2021, 16, 59. [Google Scholar] [CrossRef]
- Crawford, R.; Grignon, A.-L.; Kitson, S.; Winder, D.M.; Ball, S.L.R.; Vaughan, K.; Stanley, M.A.; Sterling, J.C.; Goon, P.K.C. High prevalence of HPV in non-cervical sites of women with abnormal cervical cytology. BMC Cancer 2011, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Xia, N.; Ocampo, R.; Goodman, M.T.; Hessol, N.A.; Grinsztejn, B.; Ortiz, A.P.; Zhao, F.; Kojic, E.M.; Kaul, R.; et al. Age-Specific Prevalence of Anal and Cervical Human Papillomavirus Infection and High-Grade Lesions in 11 177 Women by Human Immunodeficiency Virus Status: A Collaborative Pooled Analysis of 26 Studies. J. Infect. Dis. 2022, 227, 488–497. [Google Scholar] [CrossRef]
- Rodrigues, L.L.S.; Morgado, M.G.; Sahasrabuddhe, V.V.; De Paula, V.S.; Oliveira, N.S.; Chavez-Juan, E.; Da Silva, D.M.; Kast, W.M.; Nicol, A.F.; Pilotto, J.H. Cervico-vaginal self-collection in HIV-infected and uninfected women from Tapajós region, Amazon, Brazil: High acceptability, hrHPV diversity and risk factors. Gynecol. Oncol. 2018, 151, 102–110. [Google Scholar] [CrossRef]
- Rodrigues, L.L.S.; Pilotto, J.H.; Lima, L.R.P.; Gaydos, C.A.; Hardick, J.; Morgado, M.G.; Martinelli, K.G.; de Paula, V.S.; Nicol, A.F. Self-collected versus clinician-collected samples for HSV-2 and HSV-2/HPV screening in HIV-infected and -uninfected women in the Tapajós region, Amazon, Brazil. Int. J. STD AIDS 2019, 30, 1055–1062. [Google Scholar] [CrossRef]
- Rodrigues, L.L.S.; Hardick, J.; Nicol, A.F.; Morgado, M.G.; Martinelli, K.G.; de Paula, V.S.; Pilotto, J.H.; Gaydos, C.A. Sexually transmitted infections among HIV-infected and HIV-uninfected women in the Tapajós region, Amazon, Brazil: Self-collected vs. clinician-collected samples. PLoS ONE 2019, 14, e0215001. [Google Scholar] [CrossRef]
- Cornall, A.M.; Poljak, M.; Garland, S.M.; Phillips, S.; Tan, J.H.; Machalek, D.A.; Quinn, M.A.; Tabrizi, S.N. Anyplex II HPV28 detection and Anyplex II HPV HR detection assays are highly concordant with other commercial assays for detection of high-risk HPV genotypes in women with high grade cervical abnormalities. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 545–551. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30 (Suppl. 5), F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Dondog, B.; Waterboer, T.; Pawlita, M.; Tommasino, M.; Gheit, T. Abundance of Multiple High-Risk Human Papillomavirus (HPV) Infections Found in Cervical Cells Analyzed by Use of an Ultrasensitive HPV Genotyping Assay. J. Clin. Microbiol. 2010, 48, 143–149. [Google Scholar] [CrossRef]
- Gheit, T.; Billoud, G.; de Koning, M.N.C.; Gemignani, F.; Forslund, O.; Sylla, B.S.; Vaccarella, S.; Franceschi, S.; Landi, S.; Quint, W.G.V.; et al. Development of a Sensitive and Specific Multiplex PCR Method Combined with DNA Microarray Primer Extension to Detect Betapapillomavirus Types. J. Clin. Microbiol. 2007, 45, 2537–2544. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Colpani, V.; Falcetta, F.S.; Bidinotto, A.B.; Kops, N.L.; Falavigna, M.; Hammes, L.S.; Benzaken, A.S.; Maranhão, A.G.K.; Domingues, C.M.A.S.; Wendland, E.M. Prevalence of human papillomavirus (HPV) in Brazil: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0229154. [Google Scholar] [CrossRef] [PubMed]
- Nasioutziki, M.; Chatzistamatiou, K.; Loufopoulos, P.-D.; Vavoulidis, E.; Tsampazis, N.; Pratilas, G.-C.; Liberis, A.; Karpa, V.; Parcharidis, E.; Daniilidis, A.; et al. Cervical, anal and oral HPV detection and HPV type concordance among women referred for colposcopy. Infect. Agents Cancer 2020, 15, 22–29. [Google Scholar] [CrossRef]
- Goodman, M.T.; Shvetsov, Y.B.; McDuffie, K.; Wilkens, L.R.; Zhu, X.; Thompson, P.J.; Ning, L.; Killeen, J.; Kamemoto, L.; Hernandez, B.Y. Sequential Acquisition of Human Papillomavirus (HPV) Infection of the Anus and Cervix: The Hawaii HPV Cohort Study. J. Infect. Dis. 2010, 201, 1331–1339. [Google Scholar] [CrossRef]
- Goodman, M.T.; McDuffie, K.; Hernandez, B.Y.; Wilkens, L.R.; Zhu, X.; Thompson, P.J.; Killeen, J.; Kamemoto, L.; Shvetsov, Y.B. The Influence of Multiple Human Papillomavirus Types on the Risk of Genotype-Concordant Incident Infections of the Anus and Cervix: The Hawaii HPV Cohort Study. J. Infect. Dis. 2011, 203, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.M.R.L.; Eleutério Junior, J.; Peixoto, R.A.C.; Rebouças, K.C.F.; Eleutério, R.M.N. Anal High-risk HPV and Liquid-based Cytology of Immunocompetent Brazilian Women with Genital High-risk HPV. Rev. Bras. De Ginecol. E Obstet. 2022, 44, 280–286. [Google Scholar] [CrossRef]
- Bregar, A.J.; Cronin, B.; Luis, C.; DiSilvestro, P.; Schechter, S.; Pisharodi, L.; Raker, C.; Clark, M.; Robison, K. Anal and Cervical High-Risk Human Papillomavirus Genotyping in Women with and without Genital Neoplasia. J. Low. Genit. Tract Dis. 2018, 22, 115–119. [Google Scholar] [CrossRef]
- Burger, E.A.; De Kok, I.M.C.M.; Groene, E.; Killen, J.; Canfell, K.; Kulasingam, S.; Kuntz, K.M.; Matthijsse, S.; Regan, C.; Simms, K.T.; et al. Estimating the Natural History of Cervical Carcinogenesis Using Simulation Models: A CISNET Comparative Analysis. J. Natl. Cancer Inst. 2020, 112, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, E.A.K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.J.; Palefsky, J.M. HPV-Associated Anal Cancer in the HIV/AIDS Patient. Cancer Treat. Res. 2019, 177, 183–209. [Google Scholar] [CrossRef]
- Abdelsamed, H.; Peters, J.; Byrne, G.I. Genetic variation in Chlamydia trachomatis and their hosts: Impact on disease severity and tissue tropism. Future Microbiol. 2013, 8, 1129–1146. [Google Scholar] [CrossRef]
- Juliana, N.C.A.; Omar, A.M.; Pleijster, J.; Aftab, F.; Uijldert, N.B.; Ali, S.M.; Ouburg, S.; Sazawal, S.; Morré, S.A.; Deb, S.; et al. The Natural Course of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium in Pregnant and Post-Delivery Women in Pemba Island, Tanzania. Microorganisms 2021, 9, 1180. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Taeko, D.; Chaves, T.A.; Amorim, L.D.D.C.; Murari, R.S.W.; Miranda, A.E.; Chen, Z.; Burk, R.D.; Ferreira, L.C.L. HPV Infection and Cervical Screening in Socially Isolated Indigenous Women Inhabitants of the Amazonian Rainforest. PLoS ONE 2015, 10, e0133635. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.V.; Vieira, R.C.; de Brito, E.B.; Pinheiro, M.D.C.N.; Monteiro, J.D.S.V.; Valente, M.D.R.; Ishikawa, E.A.Y.; Fuzii, H.T.; de Sousa, M.S. Prevalence of Human Papillomavirus Infection and Cervical Cancer Screening among Riverside Women of the Brazilian Amazon. Rev. Bras. De Ginecol. E Obs. RBGO Gynecol. Obstet. 2017, 39, 350–357. [Google Scholar] [CrossRef]
- McCloskey, J.C.; Martin Kast, W.; Flexman, J.P.; McCallum, D.; French, M.A.; Phillips, M. Syndemic synergy of HPV and other sexually transmitted pathogens in the development of high-grade anal squamous intraepithelial lesions. Papillomavirus Res. 2017, 4, 90–98. [Google Scholar] [CrossRef]
- Dube Mandishora, R.S.; Gjøtterud, K.S.; Lagström, S.; Stray-Pedersen, B.; Duri, K.; Chin’Ombe, N.; Nygård, M.; Christiansen, I.K.; Ambur, O.H.; Chirenje, M.Z.; et al. Intra-host sequence variability in human papillomavirus. Papillomavirus Res. 2018, 5, 180–191. [Google Scholar] [CrossRef]
- Ranjeva, S.L.; Baskerville, E.B.; Dukic, V.; Villa, L.L.; Lazcano-Ponce, E.; Giuliano, A.R.; Dwyer, G.; Cobey, S. Recurring infection with ecologically distinct HPV types can explain high prevalence and diversity. Proc. Natl. Acad. Sci. USA 2017, 114, 13573–13578. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Jalil, E.M.; Wilson, E.C.; Monteiro, L.; de Velasque, L.S.; Cristina Ferreira, A.G.; Nazer, S.C.; Friedman, R.K.; Veloso, V.G.; Eduardo Levi, J.; Grinsztejn, B. High prevalence of anal high-risk HPV infection among transwomen: Estimates from a Brazilian RDS study. J. Int. AIDS Soc. 2021, 24, e25691. [Google Scholar] [CrossRef] [PubMed]


| Variables | HIV-Uninfected n = 112 (%) | HIV-Infected n = 41 (%) | Total N = 153 (%) | p-Value * |
|---|---|---|---|---|
| Age range (years) | 0.242 | |||
| ≤20 | 08 (7.1) | 05 (12.2) | 13 (8.5) | |
| 21–30 | 36 (32.1) | 08 (19.5) | 44 (28.8) | |
| ≥31 | 68 (60.7) | 28 (68.3) | 96 (62.7) | |
| Marital status | 0.006 | |||
| Single | 36 (32.7) | 23 (57.5) | 59 (39.3) | |
| Married/living together | 74 (67.3) | 17 (42.5) | 91 (60.7) | |
| Number of pregnancies | 3.0 (±2.3) | 4.3 (± 5.7) | 3.4 (± 3.5) | 0.050 # |
| Age at first sexual intercourse (years) | 0.052 | |||
| ≤17 | 71 (64.0) | 33 (80.5) | 104 (68.4) | |
| ≥18 | 40 (36.0) | 08 (19.5) | 48 (31.6) | |
| Number of sexual partners | 0.012 | |||
| 1–3 | 57 (51.4) | 11 (28.2) | 68 (45.3) | |
| ≥4 | 54 (48.6) | 28 (71.8) | 82 (54.7) | |
| Regular use of condoms | 0.010 | |||
| Yes | 13 (11.7) | 12 (29.3) | 25 (16.4) | |
| No | 98 (88.3) | 29 (70.7) | 127 (83.6) | |
| Anal sex practice | 0.457 | |||
| Yes | 67 (60.4) | 22 (53.7) | 89 (58.6) | |
| No | 44 (39.6) | 19 (46.3) | 63 (41.4) |
| Variables | Anal hrHPV | |||
|---|---|---|---|---|
| Yes N (%) | No N (%) | Total (%) | p-Value * | |
| HIV-uninfected (n = 112) | ||||
| Age range (years) | 0.510 | |||
| ≤20 | 0 (0.0) | 08 (8.2) | 08 (7.1) | |
| 21–30 | 05 (33.3) | 31 (32.0) | 36 (32.1) | |
| ≥31 | 10 (66.7) | 58 (59.8) | 68 (60.7) | |
| Marital status | 0.560 | |||
| Single | 06 (40.0) | 30 (31.6) | 36 (32.7) | |
| Married/ living together | 09 (60.0) | 65 (68.4) | 74 (67.3) | |
| Age of first sexual intercourse (years) | 0.164 | |||
| ≤17 | 12 (80.0) | 59 (61.5) | 71 (64.0) | |
| ≥18 | 03 (20.0) | 37 (38.5) | 40 (36.0) | |
| Number of sexual partners | 0.040 | |||
| 1–3 | 04 (26.7) | 53 (55.2) | 57 (51.4) | |
| ≥4 | 11 (73.3) | 43 (44.8) | 54 (48.6) | |
| Regular use of condoms | 0.050 | |||
| Yes | 04 (26.7) | 09 (9.4) | 13 (11.7) | |
| No | 11 (73.3) | 87 (90.6) | 98 (88.3) | |
| Anal sex practice | 0.591 | |||
| Yes | 10 (66.7) | 57 (59.4) | 67 (60.4) | |
| No | 05 (33.3) | 39 (40.6) | 44 (39.6) | |
| Cervical hrHPV | 0.045 | |||
| Yes | 06 (40.0) | 17 (17.5) | 23 (20.5) | |
| No | 09 (60.0) | 80 (82.5) | 89 (79.5) | |
| HIV-infected (n = 41) | ||||
| Age range (years) | 0.916 | |||
| ≤20 | 04 (13.3) | 01 (9.1) | 05 (12.2) | |
| 21–30 | 06 (20.0) | 02 (18.2) | 08 (19.5) | |
| ≥31 | 20 (66.7) | 08 (72.7) | 28 (68.3) | |
| Marital status | 0.730 | |||
| Single | 16 (55.2) | 07 (63.6) | 23 (57.5) | |
| Married/ living together | 13 (44.8) | 04 (36.4) | 17 (42.5) | |
| Age of first sexual intercourse (years) | 0.412 | |||
| ≤17 | 23 (76.7) | 10 (90.9) | 33 (80.5) | |
| ≥18 | 07 (23.3) | 01 (9.1) | 08 (19.5) | |
| Number of sexual partners | 0.935 | |||
| 1–3 | 08 (28.6) | 03 (27.3) | 68 (45.3) | |
| ≥4 | 20 (71.4) | 08 (72.7) | 82 (54.7) | |
| Regular use of condoms | 0.701 | |||
| Yes | 08 (26.7) | 04 (36.4) | 12 (29.3) | |
| No | 22 (73.3) | 07 (63.6) | 29 (70.7) | |
| Anal sex practice | 0.524 | |||
| Yes | 17 (56.7) | 05 (45.5) | 22 (53.7) | |
| No | 13 (43.3) | 06 (54.5) | 19 (46.3) | |
| Cervical hrHPV | 0.001 | |||
| Yes | 27 (90.0) | 04 (36.4) | 31 (75.6) | |
| No | 03 (10.0) | 07 (63.6) | 10 (24.4) | |
| STIs | HPV | CT | NG | MG | HSV-2 | TV |
|---|---|---|---|---|---|---|
| All participants (N = 153) | ||||||
| Agreement | 66.01% | 84.96% | 99.34% | 96.07% | 95.42% | - * |
| Positive | 101/153 | 130/153 | 152/153 | 147/153 | 146/153 | - * |
| Kappa (95% CI) | 0.44 (0.33–0.56) | 0.26 (0.05–0.47) | 0.85 (0.45–1.00) | 0.23 (−0.03–0.66) | 0.35 (0.00–0.72) | - * |
| p-value | <0.001 | 0.001 | <0.001 | 0.003 | <0.001 | - * |
| HIV-uninfected (n = 112) | - * | |||||
| Agreement | 61.61% | 84.82% | 99.10% | 95.53% | 94.64% | - * |
| Positive | 69/112 | 95/112 | 111/112 | 107/112 | 106/112 | - * |
| Kappa (95% CI) | 0.22 (0.07–0.37) | 0.29 (0.02–0.51) | 0.66 (0.04–1.00) | 0.26 (−0.04–0.66) | 0.24 (0.00–0.66) | - * |
| p-value | 0.002 | 0.002 | <0.001 | 0.005 | <0.001 | - * |
| HIV-infected (n = 41) | - * | |||||
| Agreement | 78.05% | 85.36% | 100% | - * | 97.56% | - * |
| Positive | 32/41 | 35/41 | 41/41 | - * | 40/41 | - * |
| Kappa (95% CI) | 0.45 (0.16–0.72) | 0.22 (0.0–0.64) | 1.00 | - * | 0.65 (0.00–1.00) | - * |
| p-value | 0.001 | 0.026 | <0.001 | - * | <0.001 | - * |
| The most frequent anal hrHPV types | HPV16 | HPV18 | HPV31 | HPV51 | HPV58 | HPV59 |
| All participants (N = 153) | ||||||
| Agreement | 88.23% | 93.46% | 92.81% | 96.73% | 94.77% | 93.46% |
| Positive | 135/153 | 143/153 | 142/153 | 148/153 | 145/153 | 143/153 |
| Kappa (95% CI) | 0.18 (−0.57–0.44) | 0.34 (−0.03–0.61) | 0.38 (0.06–0.65) | 0.78 (0.56–0.94) | 0.57 (0.25–0.81) | 0.41 (0.07–0.68) |
| p-value | 0.018 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HIV-uninfected (n = 112) | ||||||
| Agreement | 89.28% | 94,64% | ||||
| Positive | 100/112 | 106/112 | ||||
| Kappa (95% CI) | −0.05 (−0.08–0.16) | 0.37 (−0.03–0.78) | ||||
| p-value | 0.555 | <0.001 | ||||
| HIV-infected (n = 41) | ||||||
| Agreement | 80.48% | 87.80% | 90.24% | 75.60% | ||
| Positive | 33/41 | 36/41 | 37/41 | 31/41 | ||
| Kappa (95% CI) | 0.32 (−0.84–0.66) | 0.71 (0.43–0.93) | 0.69 (0.32–0.93) | 0.29 (−0.09–0.62) | ||
| p-value | 0.028 | <0.001 | <0.001 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.L.S.; Pilotto, J.H.; Martinelli, K.G.; Nicol, A.F.; De Paula, V.S.; Gheit, T.; Oliveira, N.S.C.; Silva-de-Jesus, C.; Sahasrabuddhe, V.V.; Da Silva, D.M.; et al. Diversity of Anal HPV and Non-HPV Sexually Transmitted Infections and Concordance with Genital Infections in HIV-Infected and HIV-Uninfected Women in the Tapajós Region, Amazon, Brazil. Viruses 2023, 15, 1328. https://doi.org/10.3390/v15061328
Rodrigues LLS, Pilotto JH, Martinelli KG, Nicol AF, De Paula VS, Gheit T, Oliveira NSC, Silva-de-Jesus C, Sahasrabuddhe VV, Da Silva DM, et al. Diversity of Anal HPV and Non-HPV Sexually Transmitted Infections and Concordance with Genital Infections in HIV-Infected and HIV-Uninfected Women in the Tapajós Region, Amazon, Brazil. Viruses. 2023; 15(6):1328. https://doi.org/10.3390/v15061328
Chicago/Turabian StyleRodrigues, Luana Lorena Silva, José Henrique Pilotto, Katrini Guidolini Martinelli, Alcina F. Nicol, Vanessa Salete De Paula, Tarik Gheit, Nathália Silva Carlos Oliveira, Carlos Silva-de-Jesus, Vikrant V. Sahasrabuddhe, Diane M. Da Silva, and et al. 2023. "Diversity of Anal HPV and Non-HPV Sexually Transmitted Infections and Concordance with Genital Infections in HIV-Infected and HIV-Uninfected Women in the Tapajós Region, Amazon, Brazil" Viruses 15, no. 6: 1328. https://doi.org/10.3390/v15061328
APA StyleRodrigues, L. L. S., Pilotto, J. H., Martinelli, K. G., Nicol, A. F., De Paula, V. S., Gheit, T., Oliveira, N. S. C., Silva-de-Jesus, C., Sahasrabuddhe, V. V., Da Silva, D. M., Kast, W. M., Hardick, J., Gaydos, C. A., & Morgado, M. G. (2023). Diversity of Anal HPV and Non-HPV Sexually Transmitted Infections and Concordance with Genital Infections in HIV-Infected and HIV-Uninfected Women in the Tapajós Region, Amazon, Brazil. Viruses, 15(6), 1328. https://doi.org/10.3390/v15061328









