A Rice Receptor-like Protein Negatively Regulates Rice Resistance to Southern Rice Black-Streaked Dwarf Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Subcellular Localization Methods
2.3. Multiple Sequence Alignment and Phylogenetic Analysis
2.4. Isolation and Identification of Mutant Plants
2.5. Insect Vector and Virus Inoculation Assays
2.6. Total RNA Extraction and RT-qPCR Assays
2.7. Protein Extraction and Western Blotting Analysis
2.8. RNA Library Construction and Sequencing
2.9. Statistical Analysis
3. Results
3.1. A Gene Encoding a Receptor-like Protein OsBAP1 Was Up-Regulated in Response to SRBSDV Infection
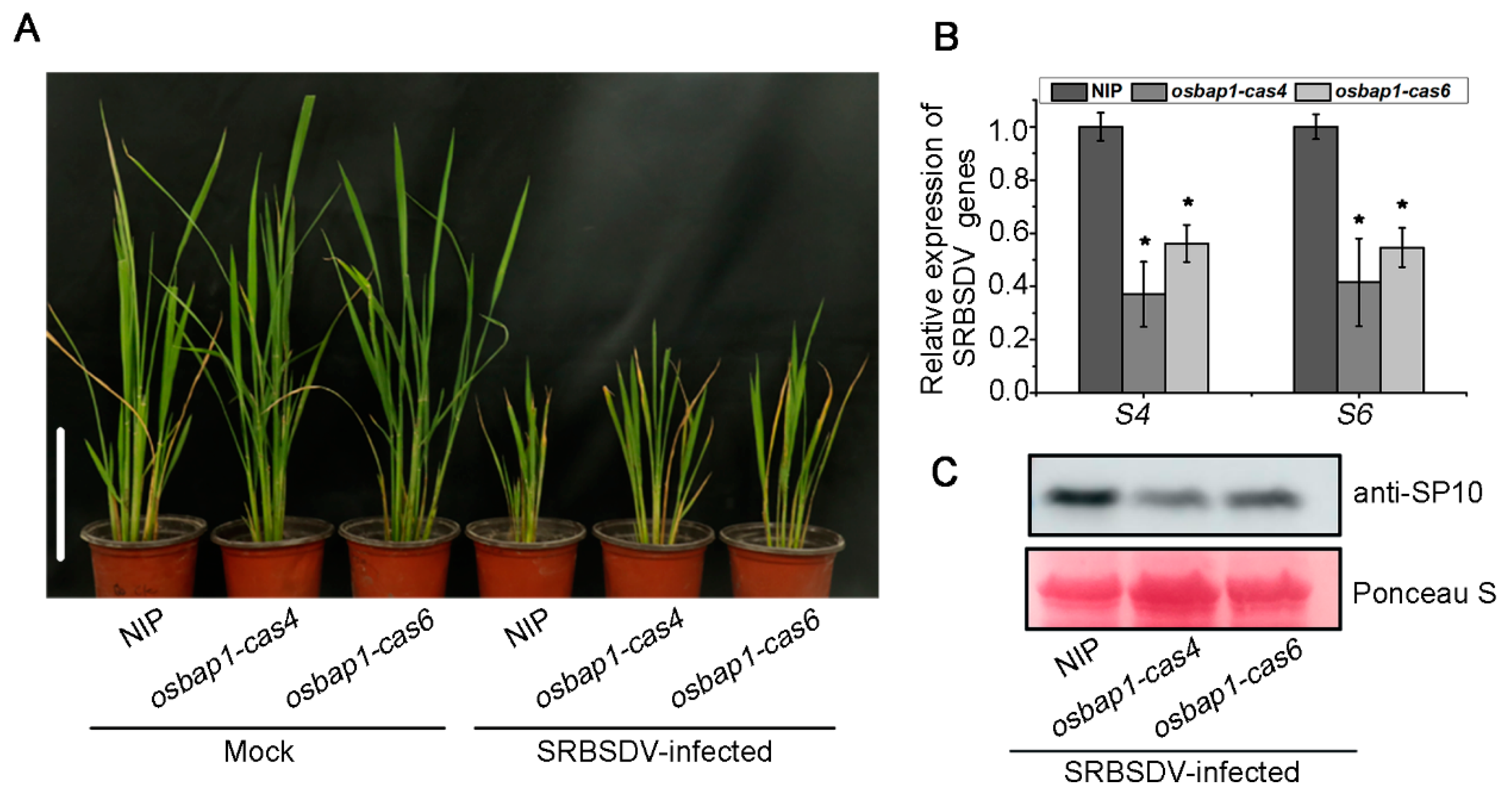
3.2. OsBAP1 Negatively Regulates Resistance to SRBSDV Infection in Rice
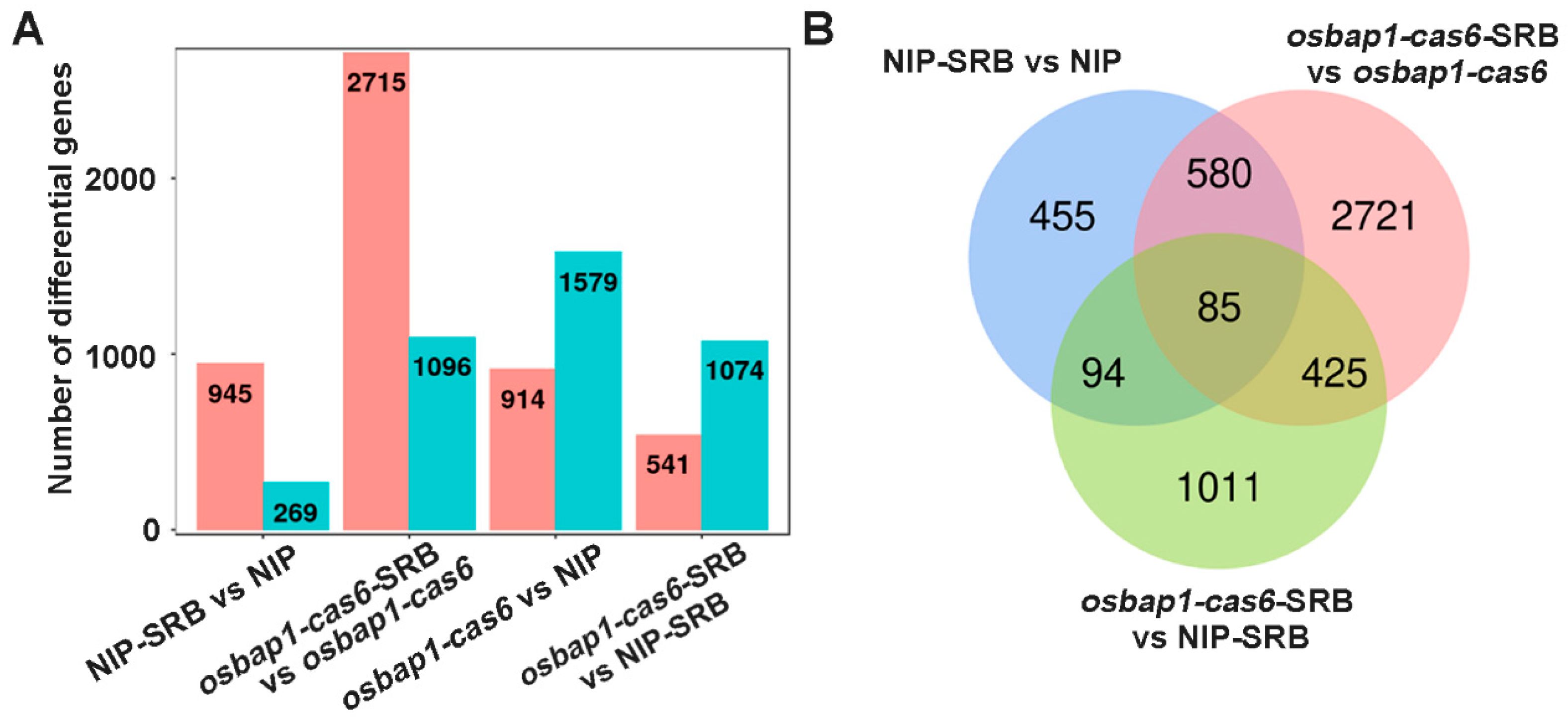
3.3. Differentially Expressed Genes in OsBAP1 Mutant and NIP Plants in Response to SRBSDV Infection
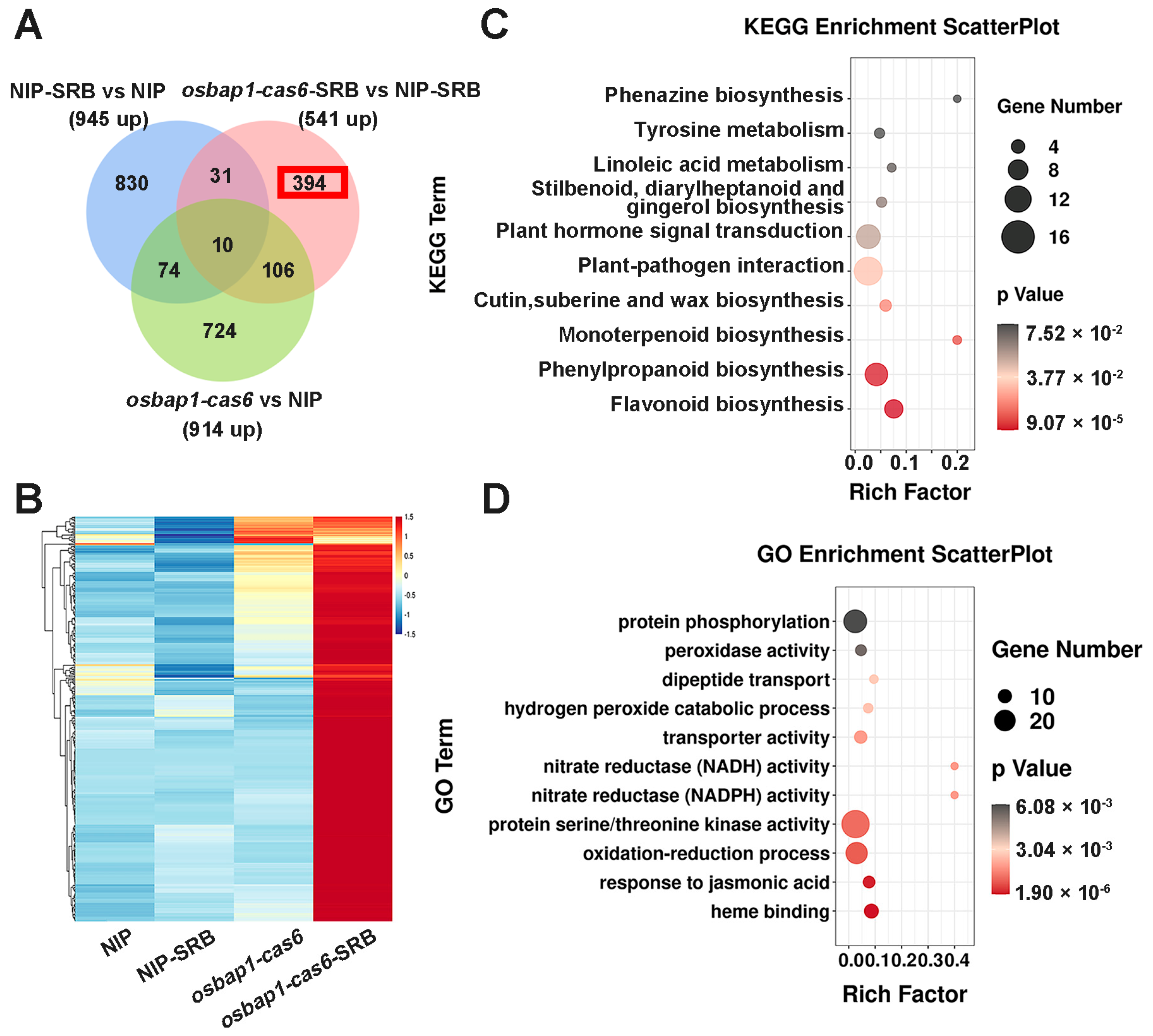
3.4. Analysis of DEGs Induced in OsBAP1 Mutants under SRBSDV Inoculation
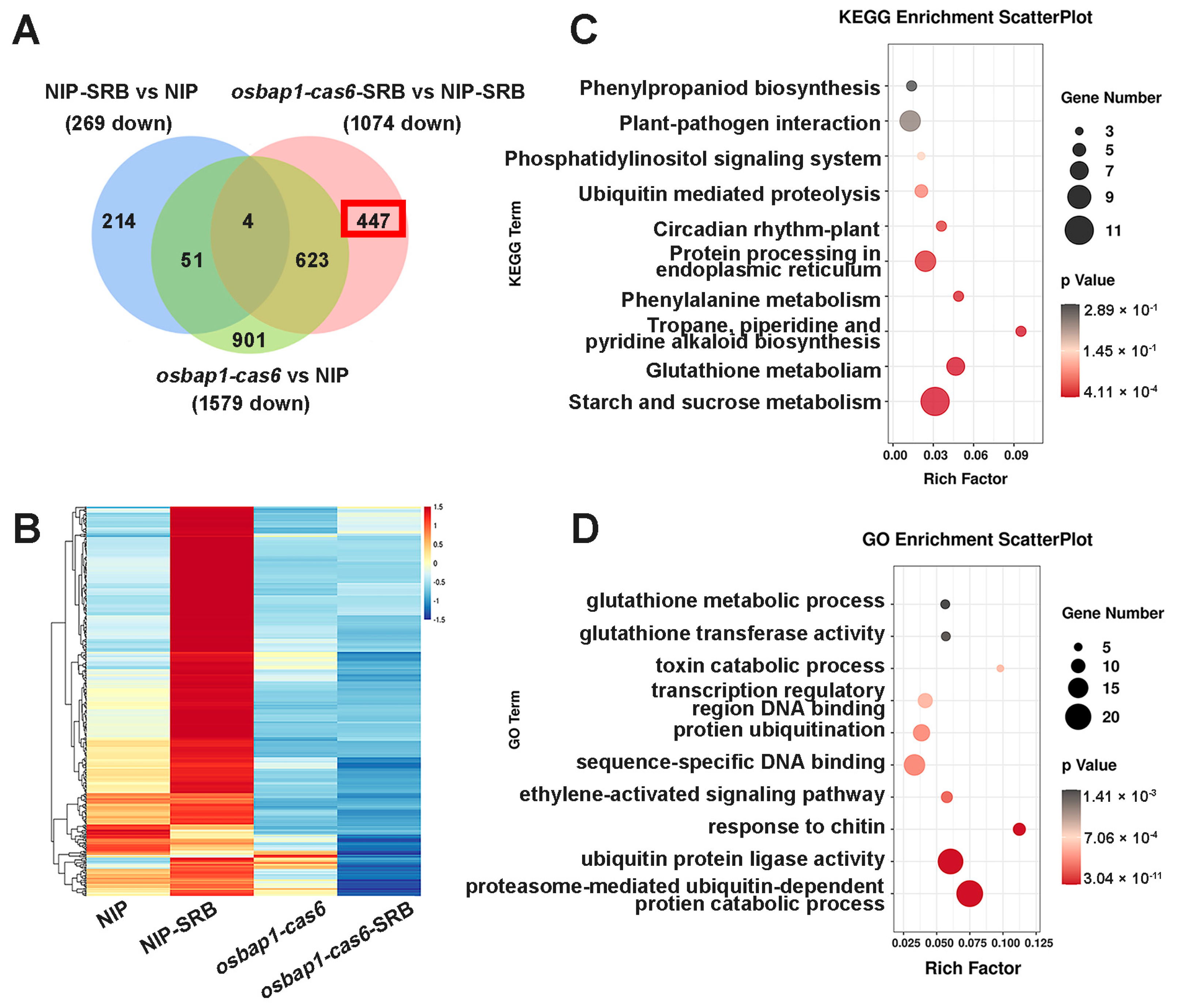
3.5. Analysis of DEGs Suppressed in OsBAP1 Mutants under SRBSDV Inoculation
3.6. RT-qPCR Validation of the Expression Profiles of Some Defense Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthayya, S.; Sugimoto, J.; Montgomery, S.; Maberly, G. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, Y.; Li, J. Towards molecular breeding and improvement of rice in China. Trends Plant Sci. 2005, 10, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wen, J.; Cai, D.; Li, P.; Xu, D.; Zhang, S. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chin. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Wu, J.; Yang, G.; Zhao, S.; Zhang, S.; Qin, B.; Zhu, Y.; Xie, H.; Chang, Q.; Wang, L.; Hu, J.; et al. Current rice production is highly vulnerable to insect-borne viral diseases. Natl. Sci. Rev. 2022, 9, nwac131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, C.; Peng, Y.; Ding, C.; Li, Q.; Wang, D.; Yuan, X. Close evolutionary relationship between rice black-streaked dwarf virus and southern rice black-streaked dwarf virus based on analysis of their bicistronic RNAs. Virol. J. 2019, 16, 53. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Zhou, G.; Zhang, H.; Chen, J.; Adams, M. The complete genome sequence of two isolates of southern rice black-streaked dwarf virus, a new member of the genus Fijivirus. J. Phytopathol. 2010, 158, 733–737. [Google Scholar] [CrossRef]
- Cuong, H.; Hai, N.; Man, V.; Matsumoto, M. Rice dwarf disease in north vietnam in 2009 is caused by southern rice black-streaked dwarf virus (SRBSDV). Bull. Inst. Trop. Agric. Kyushu Univ. 2009, 32, 85–92. [Google Scholar]
- Jia, D.; Chen, H.; Zheng, A.; Chen, Q.; Liu, Q.; Xie, L.; Wu, Z.; Wei, T. Development of an insect vector cell culture and RNA interference system to investigate the functional role of fijivirus replication protein. J. Virol. 2012, 86, 5800–5807. [Google Scholar] [CrossRef]
- Pu, L.; Xie, G.; Ji, C.; Ling, B.; Zhang, M.; Xu, D.; Zhou, G. Transmission characteristics of Southern rice black-streaked dwarf virus by rice planthoppers. Crop Prot. 2012, 41, 71–76. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, D.; Xu, D.; Zhang, M. Southern rice black-streaked dwarf virus: A white-backed planthopper-transmitted fijivirus threatening rice production in Asia. Front. Microbiol. 2013, 4, 270. [Google Scholar] [CrossRef]
- Tang, J.; Hu, B.; Wang, J. Outbreak analysis of rice migratory pests in China and management strategies recommended. Acta Ecol. Sin. 1996, 16, 167–173. [Google Scholar]
- Nürnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol. Rev. 2004, 198, 249–264. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J. Plant immunity triggered by microbial molecular signatures. Mol. Plant 2010, 3, 783–793. [Google Scholar] [CrossRef]
- Jones, J.; Dangl, J. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Zipfe, C.; Oldroyd, G. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, J. Receptor-like cytoplasmic kinases: Central players in plant receptor kinase-mediated signaling. Annu. Rev. Plant Biol. 2018, 69, 267–299. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Li, L.; Tan, X.; Wei, Z.; Li, Y.; Li, J.; Yan, F.; Chen, J.; Sun, Z. A rice LRR receptor-like protein associates with its adaptor kinase OsSOBIR1 to mediate plant immunity against viral infection. Plant Biotechnol. J. 2021, 19, 2319–2332. [Google Scholar] [CrossRef]
- Rafiqi, M.; Bernoux, M.; Ellis, J.; Dodds, P. In the trenches of plant pathogen recognition: Role of NB-LRR proteins. Semin. Cell Dev. Biol. 2009, 20, 1017–1024. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef]
- Jamieson, P.; Shan, L.; He, P. Plant cell surface molecular cypher: Receptor-like proteins and their roles in immunity and development. Plant Sci. 2018, 274, 242–251. [Google Scholar] [CrossRef]
- Wang, G.; Long, Y.; Thomma, B.; de Wit, P.; Angenent, G.; Fiers, M. Functional analyses of the CLAVATA2-Like proteins and their domains that contribute to CLAVATA2 specificity. Plant Physiol. 2010, 152, 320–331. [Google Scholar] [CrossRef]
- Fritz-Laylin, L.; Krishnamurthy, N.; Tör, M.; Sjölander, K.; Jones, J. Phylogenomic analysis of the receptor-like proteins of Rice and Arabidopsis. Plant Physiol. 2005, 138, 611–623. [Google Scholar] [CrossRef]
- Gao, M.; Wang, X.; Wang, D.; Xu, F.; Ding, X.; Zhang, Z.; Bi, D.; Cheng, Y.; Chen, S.; Li, X.; et al. Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 2009, 6, 34–44. [Google Scholar] [CrossRef]
- Postma, J.; Liebrand, T.; Bi, G.; Evrard, A.; Bye, R.; Mbengue, M.; Kuhn, H.; Joosten, M.; Robatzek, S. Avr4 promotes Cf-4 receptor-like protein association with the BAK1/SERK3 receptor-like kinase to initiate receptor endocytosis and plant immunity. New Phytol. 2016, 210, 627–642. [Google Scholar] [CrossRef]
- Van der Burgh, A.; Postma, J.; Robatzek, S.; Joosten, M. Kinase activity of SOBIR1 and BAK1 is required for immune signalling. Mol. Plant Pathol. 2019, 20, 410–422. [Google Scholar] [CrossRef]
- Jones, D.; Jones, J. The role of leucine-rich repeat proteins in plant defences. Adv. Bot. Res. 1997, 24, 89–167. [Google Scholar]
- Jones, D.; Thomas, C. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 1994, 266, 789–793. [Google Scholar] [CrossRef]
- Thomas, C.; Jones, D.; Parniske, M.; Harrison, K.; Balint-Kurti, P.; Hatzixanthis, K.; Jones, J. Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. Plant Cell 1997, 9, 2209–2224. [Google Scholar]
- Kawchuk, L.; Hachey, J.; Lynch, D.; Kulcsar, F.; van Rooijen, G.; Waterer, D.; Robertson, A.; Kokko, E.; Byers, R.; Howard, R.; et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 6511–6515. [Google Scholar] [CrossRef]
- Fradin, E.; Zhang, Z.; Juarez Ayala, J.; Castroverde, C.; Nazar, R.; Robb, J.; Liu, C.; Thomma, B. Genetic dissection of verticillium wilt resistance mediated by Tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef]
- Fradin, E.; Abd-El-Haliem, A.; Masini, L.; Van den Berg, G.; Joosten, M.; Thomma, B. Interfamily transfer of Tomato Ve1 mediates verticillium resistance in Arabidopsis. Plant Physiol. 2011, 156, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ellendorff, U.; Kemp, B.; Mansfield, J.; Forsyth, A.; Mitchell, K.; Bastas, K.; Liu, C.; Woods-Tör, A.; Zipfel, C.; et al. A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol. 2008, 147, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Ramonell, K.; Berrocal-Lobo, M.; Koh, S.; Wan, J.; Edwards, H.; Stacey, G.; Somerville, S. Loss-of-function mutations in chitin responsive genes show increased susceptibility to the rowdery mildew pathogen Erysiphe cichoracearum. Plant Physiol. 2005, 138, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ge, S.; Xing, L.; Han, D.; Kang, Z.; Zhang, G.; Wang, X.; Wang, X.; Chen, P.; Cao, A. RLP1.1, a novel wheat receptor-like protein gene, is involved in the defence response against Puccinia striiformis f. sp. tritici. J. Exp. Bot. 2013, 64, 3735–3746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; He, Y.; Qin, Q.; Chen, C.; Wei, Z.; Tan, X.; Xie, K.; Zhang, R.; Hong, G.; et al. Distinct modes of manipulation of rice auxin response factor OsARF17 by different plant RNA viruses for infection. Proc. Natl. Acad. Sci. USA 2020, 117, 9112–9121. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Yang, Z.; Wang, C.; Li, S.; Cao, C.; Yao, T.; Wei, Z.; Li, Y.; Chen, J.; et al. Independently evolved viral effectors convergently suppress DELLA protein SLR1-mediated broad-spectrum antiviral immunity in rice. Nat. Commun. 2022, 13, 6920. [Google Scholar] [CrossRef]
- Sun, Z.; He, Y.; Li, J.; Wang, X.; Chen, J. Genome-wide characterization of rice black streaked dwarf virus-responsive microRNAs in rice leaves and roots by small RNA and degradome sequencing. Plant Cell Physiol. 2015, 56, 688–699. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, X.; He, Y.; Xie, K.; Li, L.; Wang, R.; Hong, G.; Li, J.; Li, J.; Taliansky, M.; et al. Rice black-streaked dwarf virus P10 acts as either a synergistic or antagonistic determinant during superinfection with related or unrelated virus. Mol. Plant Pathol. 2019, 20, 641–655. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.; Sun, Z.; Li, J.; Hong, G.; Zhu, Q.; Zhou, X.; MacFarlane, S.; Yan, F.; Chen, J. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to Rice black streaked dwarf virus infection in rice. New Phytol. 2017, 214, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, X.; Li, L.; He, Y.; Hong, G.; Li, J.; Lin, L.; Cheng, Y.; Yan, F.; Chen, J.; et al. Suppression of auxin signalling promotes rice susceptibility to Rice black streaked dwarf virus infection. Mol. Plant Pathol. 2019, 20, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hong, G.; Zhang, H.; Tan, X.; Li, L.; Kong, Y.; Sang, T.; Xie, K.; Wei, J.; Li, J.; et al. The OsGSK2 Kinase Integrates Brassinosteroid and Jasmonic Acid Signaling by Interacting with OsJAZ4. Plant Cell 2020, 32, 2806–2822. [Google Scholar] [CrossRef]
- Xie, K.; Li, L.; Zhang, H.; Wang, R.; Tan, X.; He, Y.; Hong, G.; Li, J.; Ming, F.; Yao, X.; et al. Abscisic acid negatively modulates plant defence against rice black-streaked dwarf virus infection by suppressing the jasmonate pathway and regulating reactive oxygen species levels in rice. Plant Cell Environ. 2018, 41, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, H.; Yang, Z.; Wei, Z.; Li, Y.; Chen, J.; Sun, Z. NF-YA transcription factors suppress jasmonic acid-mediated antiviral defense and facilitate viral infection in rice. PLoS Pathog. 2022, 18, e1010548. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Chen, C.; Huang, H.; Tan, X.; Wei, Z.; Li, J.; Yan, F.; Zhang, C.; Chen, J.; et al. A class of independently evolved transcriptional repressors in plant RNA viruses facilitates viral infection and vector feeding. Proc. Natl. Acad. Sci. USA 2021, 118, e2016673118. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.; Dixon, R. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Thomas, C. Molecular interactions between tomato and the leaf mold pathogen Cladosporium fulvum. Annu. Rev. Phytopathol. 2005, 43, 395–436. [Google Scholar] [CrossRef]
- Monaghan, J.; Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Schwessinger, B.; Roux, M.; Kadota, Y.; Ntoukakis, V.; Sklenar, J.; Jones, A.; Zipfel, C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011, 7, e1002046. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Song, W.; Huang, C.; Wei, Z.; Li, Y.; Chen, J.; Zhang, H.; Sun, Z. A Rice Receptor-like Protein Negatively Regulates Rice Resistance to Southern Rice Black-Streaked Dwarf Virus Infection. Viruses 2023, 15, 973. https://doi.org/10.3390/v15040973
Wang F, Song W, Huang C, Wei Z, Li Y, Chen J, Zhang H, Sun Z. A Rice Receptor-like Protein Negatively Regulates Rice Resistance to Southern Rice Black-Streaked Dwarf Virus Infection. Viruses. 2023; 15(4):973. https://doi.org/10.3390/v15040973
Chicago/Turabian StyleWang, Fengmin, Weiqi Song, Chaorui Huang, Zhongyan Wei, Yanjun Li, Jianping Chen, Hehong Zhang, and Zongtao Sun. 2023. "A Rice Receptor-like Protein Negatively Regulates Rice Resistance to Southern Rice Black-Streaked Dwarf Virus Infection" Viruses 15, no. 4: 973. https://doi.org/10.3390/v15040973
APA StyleWang, F., Song, W., Huang, C., Wei, Z., Li, Y., Chen, J., Zhang, H., & Sun, Z. (2023). A Rice Receptor-like Protein Negatively Regulates Rice Resistance to Southern Rice Black-Streaked Dwarf Virus Infection. Viruses, 15(4), 973. https://doi.org/10.3390/v15040973






