Diagnosis of Acute Myocarditis Following mRNA Vaccines against SARS-CoV-2: A Methodological Review
Abstract
1. Introduction
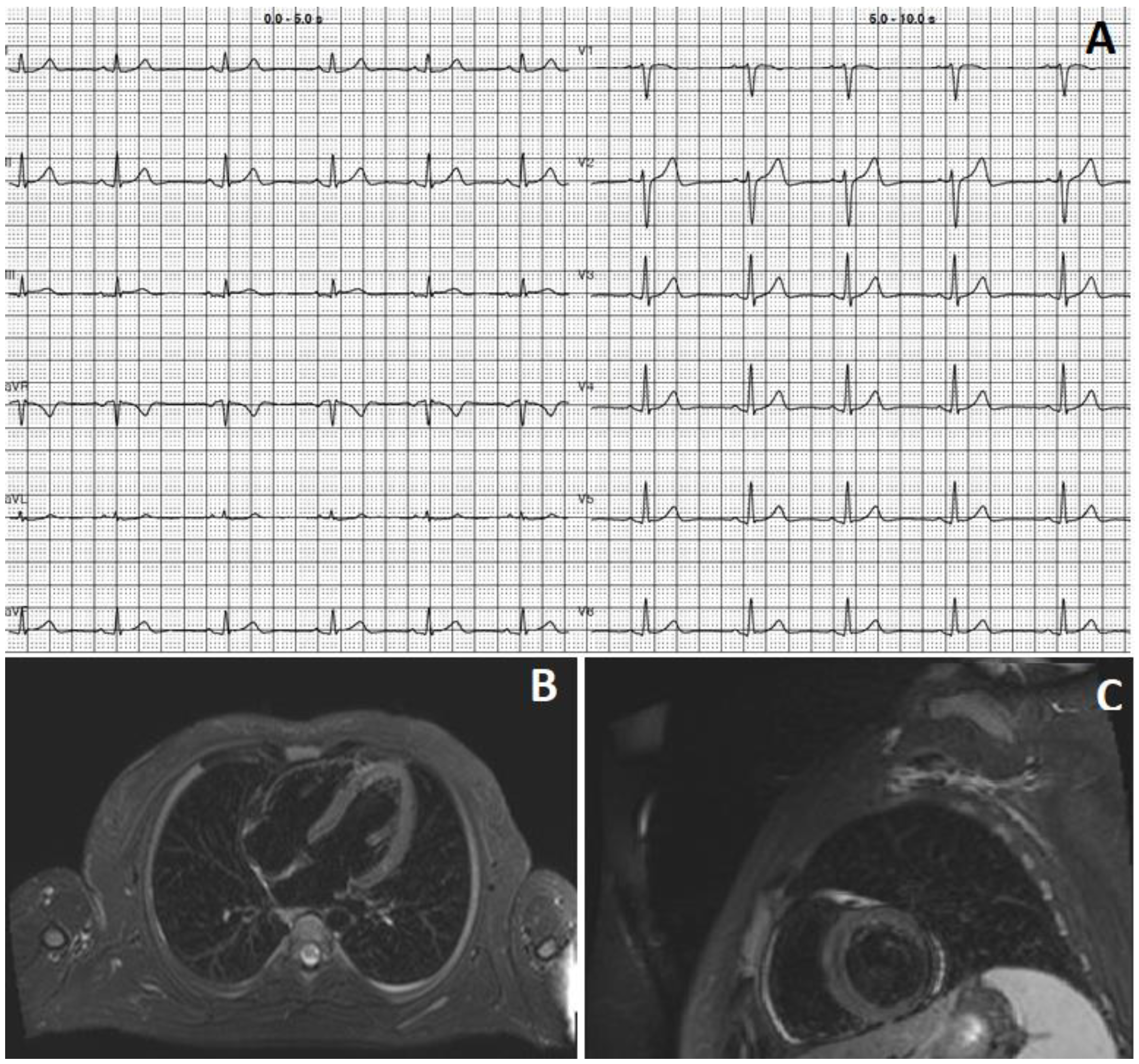
2. Case 1
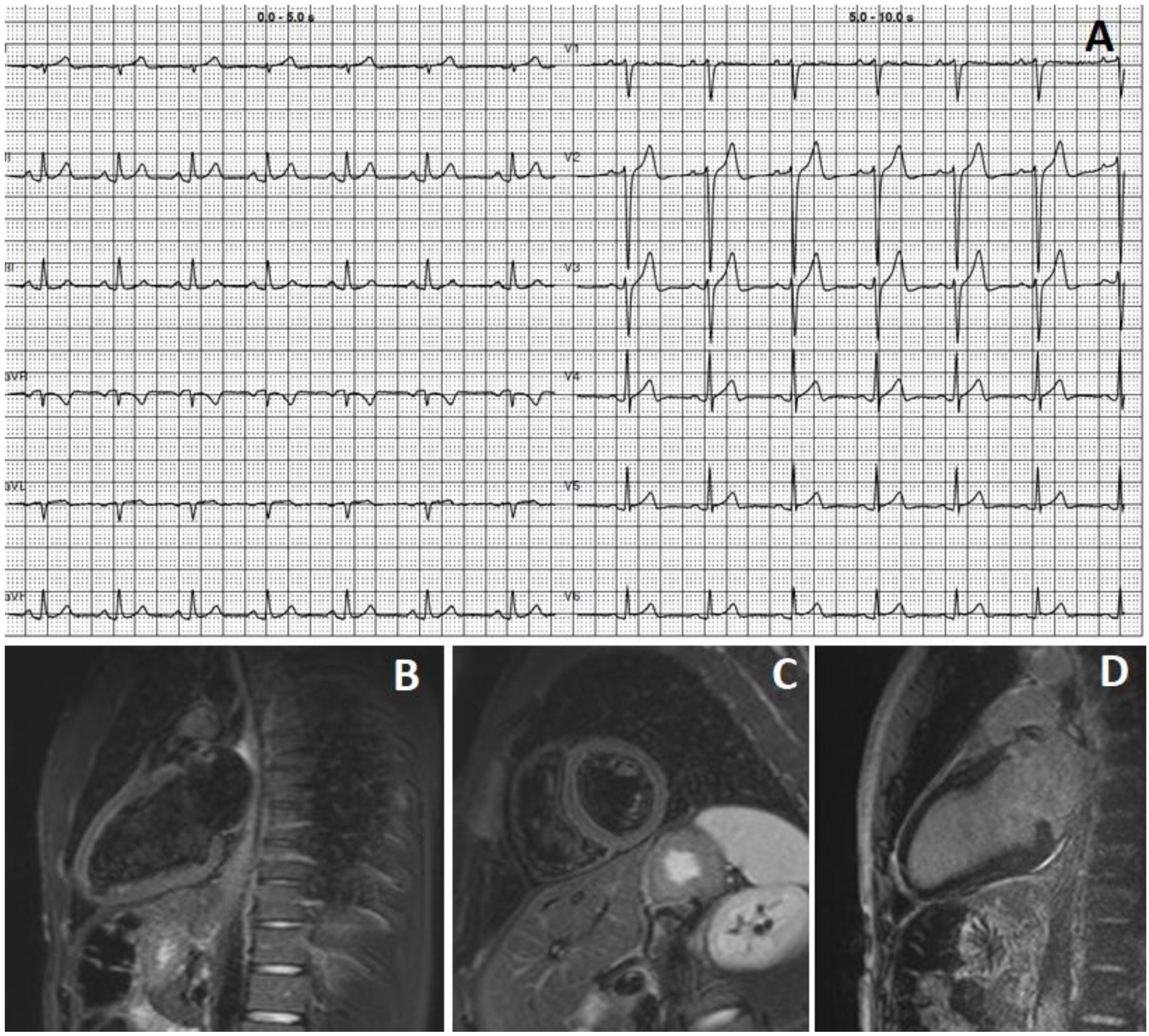
3. Case 2
4. Pathophysiological Aspects
5. Epidemiological Aspects
6. Comorbidities and Clinical Presentation
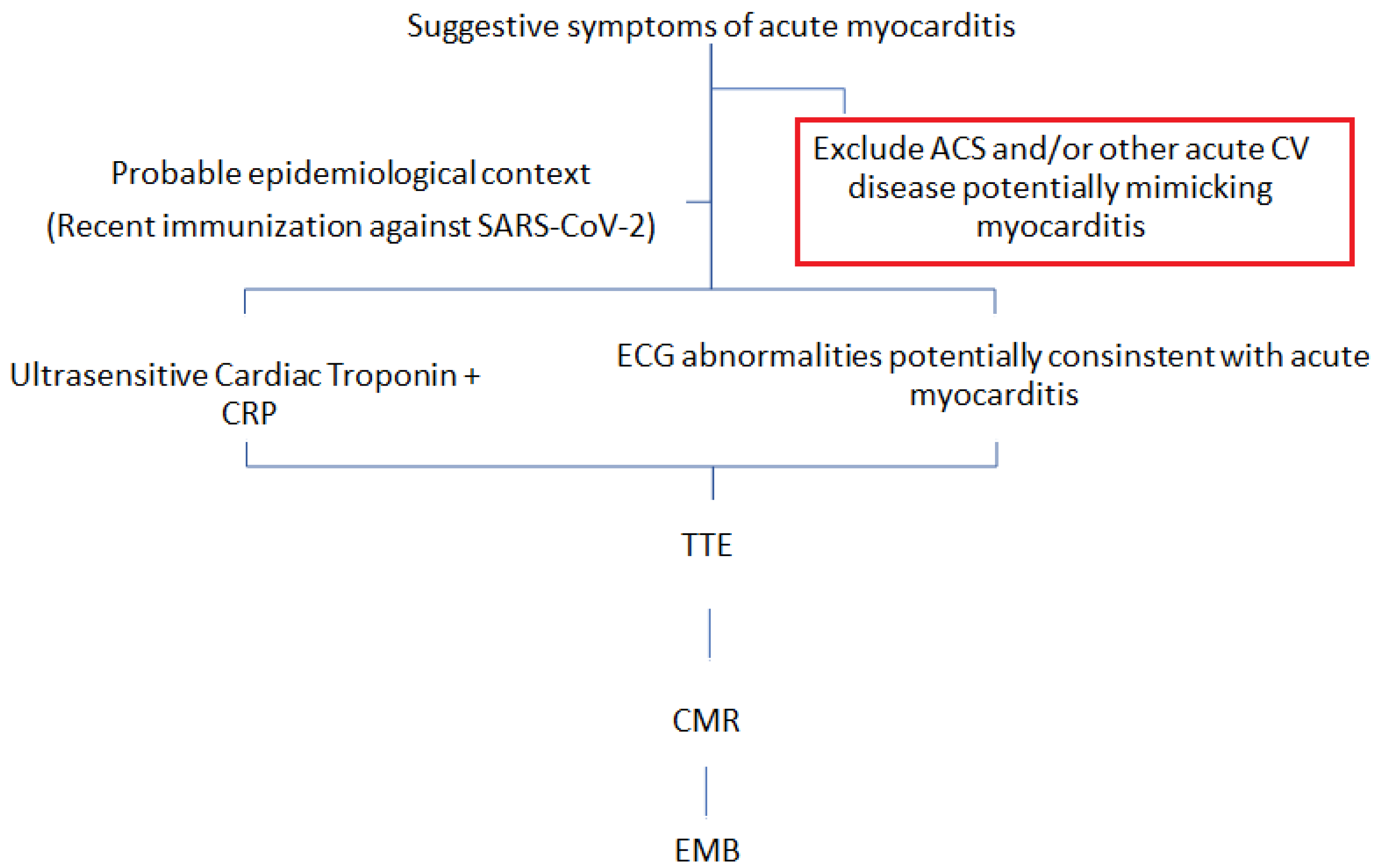
7. Investigations
8. Differential Diagnosis
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 2023, CD015477. [Google Scholar] [CrossRef]
- Ling, Y.; Zhong, J.; Luo, J. Safety and effectiveness of SARS-CoV-2 vaccines: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 6486–6495. [Google Scholar] [CrossRef] [PubMed]
- Voleti, N.; Reddy, S.P.; Ssentongo, P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 951314. [Google Scholar] [CrossRef]
- Karlstad, Ø.; Hovi, P.; Husby, A.; Härkänen, T.; Selmer, R.M.; Pihlström, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundström, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600. [Google Scholar] [CrossRef]
- Goyal, M.; Ray, I.; Mascarenhas, D.; Kunal, S.; Sachdeva, R.A.; Ish, P. Myocarditis post-SARS-CoV-2 vaccination: A systematic review. QJM Int. J. Med. 2022, 116, 7–25. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Basso, C.; Calabrese, F.; Angelini, A.; Carturan, E.; Thiene, G. Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Fail. Rev. 2012, 18, 673–681. [Google Scholar] [CrossRef]
- Schauer, J.; Buddhe, S.; Gulhane, A.; Sagiv, E.; Studer, M.; Colyer, J.; Chikkabyrappa, S.M.; Law, Y.; Portman, M.A. Persistent Cardiac Magnetic Resonance Imaging Findings in a Cohort of Adolescents with Post-Coronavirus Disease 2019 mRNA Vaccine Myopericarditis. J. Pediatr. 2022, 245, 233–237. [Google Scholar] [CrossRef]
- Samimisedeh, P.; Afshar, E.J.; Hassani, N.S.; Rastad, H. Cardiac MRI Findings in COVID -19 Vaccine-Related Myocarditis: A Pooled Analysis of 468 Patients. J. Magn. Reson. Imaging 2022, 56, 971–982. [Google Scholar] [CrossRef]
- Shiyovich, A.; Plakht, Y.; Witberg, G.; Aviv, Y.; Shafir, G.; Kornowski, R.; Hamdan, A. Myocarditis Following COVID-19 Vaccination: A Follow-up Magnetic Resonance Imaging Study. JACC Cardiovasc. Imaging 2022, 15, 2006–2007. [Google Scholar] [CrossRef]
- Shiyovich, A.; Witberg, G.; Aviv, Y.; Eisen, A.; Orvin, K.; Wiessman, M.; Grinberg, T.; Porter, A.; Kornowski, R.; Hamdan, A. Myocarditis following COVID-19 vaccination: Magnetic resonance imaging study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1075–1082. [Google Scholar] [CrossRef]
- Özen, S.; Gül, A.E.K.; Gülhan, B.; Özbülbül, N.I.; Yüksek, S.K.; Terin, H.; Mustafaoğlu, Ö.; Bayraktar, P.; Ece, I.; Çetin, I.I.; et al. Admission and follow-up cardiac magnetic resonance imaging findings in BNT162b2 Vaccine-Related myocarditis in adolescents. Infect. Dis. 2023, 55, 199–206. [Google Scholar] [CrossRef]
- Patel, Y.R.; Shah, N.R.; Lombardi, K.; Agarwal, S.; Salber, G.; Patel, R.; Poppas, A.; Atalay, M.K. Follow-Up Cardiovascular Magnetic Resonance Findings in Patients With COVID-19 Vaccination-Associated Acute Myocarditis. JACC Cardiovasc. Imaging 2022, 15, 2007–2010. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: An expert consensus document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Porcari, A.; Merlo, M.; Roncon, L.; Sinagra, G. One-Year Risk of Myocarditis After COVID-19 Infection: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Hajra, A.; Gupta, M.; Ghosh, B.; Ashish, K.; Patel, N.; Manek, G.; Rai, D.; Sreenivasan, J.; Goel, A.; Lavie, C.J.; et al. Proposed Pathogenesis, Characteristics, and Management of COVID-19 mRNA Vaccine-Related Myopericarditis. Am. J. Cardiovasc. Drugs 2022, 22, 9–26. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331. [Google Scholar] [CrossRef]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID-19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of The European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar]
- Husby, A.; Gulseth, H.L.; Hovi, P.; Hansen, J.V.; Pihlström, N.; Gunnes, N.; Härkänen, T.; Dahl, J.; Karlstad, Ø.; Heliö, T.; et al. Clinical outcomes of myocarditis after SARS-CoV-2 mRNA vaccination in four Nordic countries: Population based cohort study. BMJ Med. 2023, 2, e000373. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA vaccine against covid19 in Israel. N. Engl. J. Med. 2021, 385, 2140e9. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M. The effect of aging of the immune system on vaccination responses. Hum. Vaccines Immunother. 2013, 9, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T. The Quest for New Approaches in Myocarditis and Inflammatory Car-diomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364. [Google Scholar] [CrossRef] [PubMed]
- Saputra, P.B.T.; Kurniawan, R.B.; Trilistyoati, D.; Al Farabi, M.J.; Susilo, H.; Alsagaff, M.Y.; Oktaviono, Y.H.; Sutanto, H.; Gusnanto, A.; Wungu, C.D.K. Myocarditis and coronavirus disease 2019 vaccination: A systematic review and meta-summary of cases. Biomol. Biomed. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cushion, S.; Arboleda, V.; Hasanain, Y.; Beckler, M.D.; Hardigan, P.; Kesselman, M.M. Comorbidities and Symptomatology of SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2)-Related Myocarditis and SARS-CoV-2 Vaccine-Related Myocarditis: A Review. Cureus 2022, 14, e24084. [Google Scholar] [CrossRef]
- Abu Mouch, S.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef]
- Patel, P.; Desai, D.; Ganta, N.; Tadepalli, S.; Kata, P.; Kanukuntla, A.; Schoenfeld, M.; Sathya, B.; Okere, A. Symptomatic Myocarditis Post COVID-19 Vaccination. Cureus 2022, 14, e24052. [Google Scholar] [CrossRef]
- Sinagra, G.; Porcari, A.; Merlo, M.; Fabris, E.; Imazio, M.; Barillà, F.; Basso, C.; Ciccone, M.M.; Curcio, A.; Mancone, M.; et al. Update 2022 dell’Expert Opinion della Società Italiana di Cardiologia su miocarditi, pericarditi e vaccini contro il COVID-19 [2022 Update on myocarditis and pericarditis following COVID-19 vaccination. Expert Opinion of the Italian Society of Cardiology]. G. Ital. Di Cardiol. 2022, 23, 408–413. [Google Scholar]
- Patrignani, A.; Schicchi, N.; Calcagnoli, F.; Falchetti, E.; Ciampani, N.; Argalia, G.; Mariani, A. Acute myocarditis following Comirnaty vaccination in a healthy man with previous SARS-CoV-2 infection. Radiol. Case Rep. 2021, 16, 3321–3325. [Google Scholar] [CrossRef]
- Park, D.Y.; An, S.; Kaur, A.; Malhotra, S.; Vij, A. Myocarditis after COVID-19 mRNA vaccination: A systematic review of case reports and case series. Clin. Cardiol. 2022, 45, 691–700. [Google Scholar] [CrossRef]
- Rosner, C.M.; Atkins, M.; Saeed, I.M.; de Lemos, J.A.; Khera, A.; Maghsoudi, A.; Min, J.; Tehrani, B.N.; O’Connor, C.M.; Defilippi, C.R. Patients with Myocarditis Associated With COVID-19 Vaccination. J. Am. Coll. Cardiol. 2022, 79, 1317–1319. [Google Scholar] [CrossRef]
- Zeng, J.-H.; Liu, Y.-X.; Yuan, J.; Wang, F.-X.; Wu, W.-B.; Li, J.-X.; Wang, L.-F.; Gao, H.; Wang, Y.; Dong, C.-F.; et al. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection 2020, 48, 773–777. [Google Scholar] [CrossRef]
- Murase, H.; Zhu, Y.; Sakaida, K.; Mizuno, H.; Mori, H.; Iwayama, H.; Suzuki, N.; Nagai, N.; Okumura, A. Case report: Five patients with myocarditis after mRNA COVID-19 vaccination. Front. Pediatr. 2022, 10, 977476. [Google Scholar] [CrossRef]
- Chiu, S.-N.; Chen, Y.-S.; Hsu, C.-C.; Hua, Y.-C.; Tseng, W.-C.; Lu, C.-W.; Lin, M.-T.; Chen, C.-A.; Wu, M.-H.; Chen, Y.-T.; et al. Changes of ECG parameters after BNT162b2 vaccine in the senior high school students. Eur. J. Pediatr. 2023, 182, 1155–1162. [Google Scholar] [CrossRef]
- Mansanguan, S.; Charunwatthana, P.; Piyaphanee, W.; Dechkhajorn, W.; Poolcharoen, A.; Mansanguan, C. Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop. Med. Infect. Dis. 2022, 7, 196. [Google Scholar] [CrossRef]
- Lu, Z.A.; Aubry, M.C.; Fallon, J.T.; Fishbein, M.C.; Giordano, C.; Klingel, K.; Leone, O.; Rizzo, S.; Veinot, J.P.; Halushka, M.K. Myocarditis and endomyocardial biopsy: Achieving consensus diagnosis on 100 cases. Cardiovasc. Pathol. 2023, 62, 107492. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tajiri, K.; Ayuzawa, S.; Ieda, M. Pathological findings of clinically suspected myocarditis temporally associated with COVID-19 vaccination. Eur. J. Heart Fail. 2022, 24, 1132–1138. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Viroj, W. Cardiac magnetic resonance imaging findings in COVID-19 vaccine-related myocarditis. Infect. Dis. 2023; ahead of print. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Lai, F.T.T.; Chan, E.W.W.; Huang, L.; Cheung, C.L.; Chui, C.S.L.; Li, X.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Prognosis of Myocarditis Developing After mRNA COVID-19 Vaccination Compared With Viral Myocarditis. J. Am. Coll. Cardiol. 2022, 80, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Clinical Considerations: Myocarditis and Pericarditis after Receipt of COVID-19 Vaccines Among Adolescents and Young Adults. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (accessed on 28 February 2023).
- Pomara, C.; Sessa, F.; Ciaccio, M.; Dieli, F.; Esposito, M.; Giammanco, G.M.; Garozzo, S.F.; Giarratano, A.; Prati, D.; Rappa, F.; et al. COVID-19 Vaccine and Death: Causality Algorithm According to the WHO Eligibility Diagnosis. Diagnostics 2021, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- WHO. Causality Assessment of an Adverse Event Following Immunization, 2nd ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241513654.
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 2020, 183, 1479–1495.e20. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, V.R.; Baumgartner, A.; Molani, S.; Hernandez, P.V.; Yuan, D.; Roper, R.T.; Matos, W.F.; Robinson, M.; Su, Y.; Subramanian, N.; et al. Angiotensin-Converting Enzyme (ACE) Inhibitors May Moderate COVID-19 Hyper-inflammatory Response: An Observational Study with Deep Immunophenotyping. Health Data Sci. 2022, 2022, 002. [Google Scholar] [CrossRef]
- Lee, J.W.; Su, Y.; Baloni, P.; Chen, D.; Pavlovitch-Bedzyk, A.J.; Yuan, D.; Duvvuri, V.R.; Ng, R.H.; Choi, J.; Xie, J.; et al. Integrated analysis of plasma and single immune cells uncovers metabolic changes in individuals with COVID-19. Nat. Biotechnol. 2021, 40, 110–120. [Google Scholar] [CrossRef]
- Krämer, B.; Knoll, R.; Bonaguro, L.; ToVinh, M.; Raabe, J.; Astaburuaga-García, R.; Schulte-Schrepping, J.; Kaiser, K.M.; Rieke, G.J.; Bischoff, J.; et al. Early IFN-α signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity 2021, 54, 2650–2669.e14. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Li, J.; Zaslavsky, M.; Su, Y.; Guo, J.; Sikora, M.J.; van Unen, V.; Christophersen, A.; Chiou, S.H.; Chen, L.; Li, J.; et al. KIR+CD8+T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 2022, 376, eabi9591. [Google Scholar] [CrossRef]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef]
| Fever | Aspecific symptoms |
| Myalgia | |
| Headache | |
| Malaise | |
| Nausea | |
| Vomiting | |
| Diarrhoea | |
| Arthralgia | |
| Shortness of breath | Cardiovascular symptoms |
| Chest pain (with or without radiation) | |
| Palpitation | |
| Edema of lower limb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuin, M.; Zimelli, E.; Dalla Valle, C.; Cavedon, S.; Rigatelli, G.; Bilato, C. Diagnosis of Acute Myocarditis Following mRNA Vaccines against SARS-CoV-2: A Methodological Review. Viruses 2023, 15, 929. https://doi.org/10.3390/v15040929
Zuin M, Zimelli E, Dalla Valle C, Cavedon S, Rigatelli G, Bilato C. Diagnosis of Acute Myocarditis Following mRNA Vaccines against SARS-CoV-2: A Methodological Review. Viruses. 2023; 15(4):929. https://doi.org/10.3390/v15040929
Chicago/Turabian StyleZuin, Marco, Emma Zimelli, Chiara Dalla Valle, Stefano Cavedon, Gianluca Rigatelli, and Claudio Bilato. 2023. "Diagnosis of Acute Myocarditis Following mRNA Vaccines against SARS-CoV-2: A Methodological Review" Viruses 15, no. 4: 929. https://doi.org/10.3390/v15040929
APA StyleZuin, M., Zimelli, E., Dalla Valle, C., Cavedon, S., Rigatelli, G., & Bilato, C. (2023). Diagnosis of Acute Myocarditis Following mRNA Vaccines against SARS-CoV-2: A Methodological Review. Viruses, 15(4), 929. https://doi.org/10.3390/v15040929









