Validation of N Protein Antibodies to Diagnose Previous SARS-CoV-2 Infection in a Large Cohort of Healthcare Workers: Use of Roche Elecsys® Immunoassay in the S Protein Vaccination Era
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. SARS-CoV-2 Infection Criteria
2.3. RT-PCR
2.4. Commercial Immunoassays to Detect Antibodies against SARS-CoV-2
2.5. Validation Specificity of Immunoassays
2.6. Statistical Analysis
3. Results
3.1. Immunoassays’ Specificity
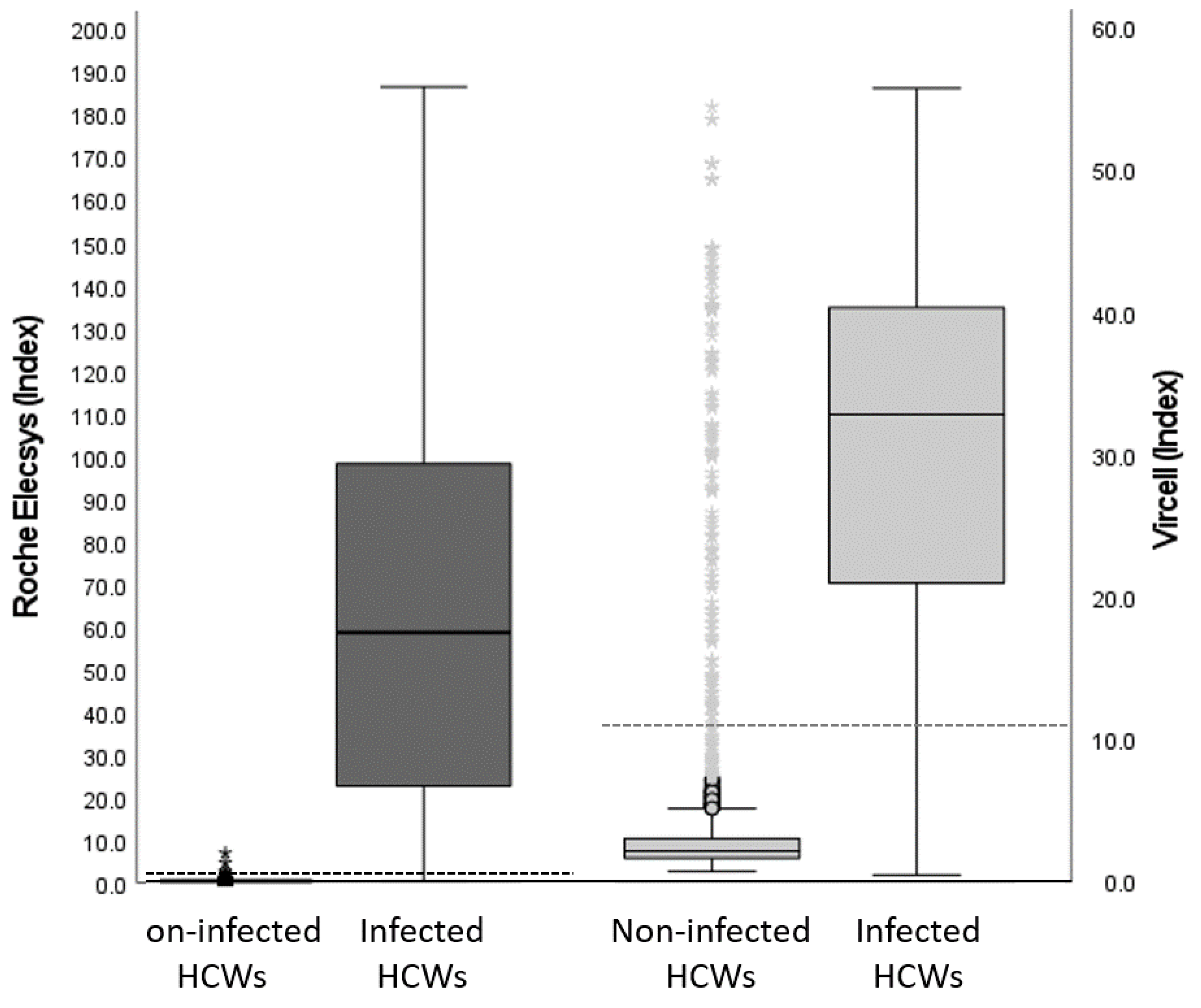
3.2. Immunoassays’ Performance
3.3. RT-PCR Performance
3.4. N protein Antibody Immunoassay Performed to Diagnose SARS-CoV-2 Infection
3.5. Antibodies against SARS-CoV-2 and RT-PCR Association with Clinical Symptoms
3.6. Roche Elecsys® Immunoassay Performance in the Validation Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, H.; Wang, X.; Yuan, X.; Xiao, G.; Wang, C.; Deng, T.; Yuan, Q.; Xiao, X. The Epidemiology and Clinical Information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1011–1019. [Google Scholar] [CrossRef]
- Binnicker, M.J. Challenges and Controversies to Testing for COVID-19. J. Clin. Microbiol. 2020, 58, e01695-20. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.P.; Choy, K.W.; Doerig, C.; Lim, C.X. SARS-CoV-2 Testing Strategies in the Diagnosis and Management of COVID-19 Patients in Low-Income Countries: A Scoping Review. Mol. Diagn. Ther. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Negi, A.; Gopalakrishnan, V.; Panda, S.; Sharma, V.; Sharma, R. COVID-19 Diagnostics: Molecular Biology to Nanomaterials. Clin. Chim. Acta 2023, 538, 139–156. [Google Scholar] [CrossRef]
- Huynh, A.; Arnold, D.M.; Smith, J.W.; Moore, J.C.; Zhang, A.; Chagla, Z.; Harvey, B.J.; Stacey, H.D.; Ang, J.C.; Clare, R.; et al. Characteristics of Anti-SARS-CoV-2 Antibodies in Recovered COVID-19 Subjects. Viruses 2021, 13, 697. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Zhang, Z. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Theel, E.S.; Slev, P.; Wheeler, S.; Couturier, M.R.; Wong, S.J.; Kadkhoda, K. The Role of Antibody Testing for SARS-CoV-2: Is There One? J. Clin. Microbiol. 2020, 58, e00797-20. [Google Scholar] [CrossRef]
- Loeffelholz, M.J. Evaluation of High-Throughput Serological Tests for SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e02179-20. [Google Scholar] [CrossRef]
- Mallano, A.; Ascione, A.; Flego, M. Antibody Response against SARS-CoV-2 Infection: Implications for Diagnosis, Treatment and Vaccine Development. Int. Rev. Immunol. 2022, 41, 393–413. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cobos, A.; Gómez de Frutos, S.; Domingo García, D.; Navarro Lara, E.; Yarci Carrión, A.; Fontán García-Rodrigo, L.; Fraile Torres, A.M.; Cardeñoso Domingo, L. Evaluation of Diagnostic Accuracy of 10 Serological Assays for Detection of SARS-CoV-2 Antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.; Geppert, J.; Dinnes, J.; Scandrett, K.; Bigio, J.; Sulis, G.; Hettiarachchi, D.; Mathangasinghe, Y.; Weeratunga, P.; Wickramasinghe, D.; et al. Antibody Tests for Identification of Current and Past Infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2022, 6, CD013652. [Google Scholar] [CrossRef]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody Response to SARS-CoV-2 Infection in Humans: A Systematic Review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, F.; Cen, Y.; Ye, L.; Shi, X.; Huang, Y.; Fang, S.; Ma, L. Comparative Research on Nucleocapsid and Spike Glycoprotein as the Rapid Immunodetection Targets of COVID-19 and Establishment of Immunoassay Strips. Mol. Immunol. 2021, 131, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Y.; Zhao, R.; Gao, L.-J.; Gao, X.-F.; Wang, D.-P.; Cao, J.-M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Lei, Q.; Lai, D.; Hou, H.; Jiang, H.; Zheng, Y.; Wang, X.; Wu, J.; Ma, M.; et al. Antibody Landscape against SARS-CoV-2 Reveals Significant Differences between Non-Structural/Accessory and Structural Proteins. Cell Rep. 2021, 36, 109391. [Google Scholar] [CrossRef]
- Kritikos, A.; Gabellon, S.; Pagani, J.-L.; Monti, M.; Bochud, P.-Y.; Manuel, O.; Coste, A.; Greub, G.; Perreau, M.; Pantaleo, G.; et al. Anti-SARS-CoV-2 Titers Predict the Severity of COVID-19. Viruses 2022, 14, 1089. [Google Scholar] [CrossRef]
- Tamizuddin, S.; Cham, J.; Ghiasi, Y.; Borroto, L.; Cao, C.; Orendain, N.; Quigley, M.M.; Nicholson, L.J.; Pandey, A.C. Hospitalization Requiring Intensive Care Unit Due to SARS-CoV-2 Infection Correlated with IgM Depression and IgG Elevation. Future Sci. OA 2022, 8, FSO783. [Google Scholar] [CrossRef]
- Shields, A.M.; Faustini, S.E.; Perez-Toledo, M.; Jossi, S.; Allen, J.D.; Al-Taei, S.; Backhouse, C.; Dunbar, L.A.; Ebanks, D.; Emmanuel, B.; et al. Serological Responses to SARS-CoV-2 Following Non-Hospitalised Infection: Clinical and Ethnodemographic Features Associated with the Magnitude of the Antibody Response. BMJ Open Respir. Res. 2021, 8, e000872. [Google Scholar] [CrossRef]
- Markmann, A.J.; Giallourou, N.; Bhowmik, D.R.; Hou, Y.J.; Lerner, A.; Martinez, D.R.; Premkumar, L.; Root, H.; van Duin, D.; Napravnik, S.; et al. Sex Disparities and Neutralizing-Antibody Durability to SARS-CoV-2 Infection in Convalescent Individuals. Msphere 2021, 6, e00275-21. [Google Scholar] [CrossRef] [PubMed]
- Bayarri-Olmos, R.; Idorn, M.; Rosbjerg, A.; Pérez-Alós, L.; Hansen, C.B.; Johnsen, L.B.; Helgstrand, C.; Zosel, F.; Bjelke, J.R.; Öberg, F.K.; et al. SARS-CoV-2 Neutralizing Antibody Responses towards Full-Length Spike Protein and the Receptor-Binding Domain. J. Immunol. 2021, 207, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination with BNT162b2 and MRNA-1273. JAMA 2021, 326, 1533. [Google Scholar] [CrossRef]
- Delgado, J.F.; Berenguer-Llergo, A.; Julià, G.; Navarro, G.; Espasa, M.; Rodríguez, S.; Sánchez, N.; Van Den Eynde, E.; Navarro, M.; Calvet, J.; et al. Antibody Response Induced by BNT162b2 and MRNA-1273 Vaccines against the SARS-CoV-2 in a Cohort of Healthcare Workers. Viruses 2022, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Mansour Ghanaie, R.; Jamee, M.; Khodaei, H.; Shirvani, A.; Amirali, A.; Karimi, A.; Fallah, F.; Azimi, L.; Armin, S.; Fahimzad, S.A.; et al. Assessment of Early and Post COVID-19 Vaccination Antibody Response in Healthcare Workers: A Multicentre Cross-Sectional Study on Inactivated, MRNA and Vector-Based Vaccines. Epidemiol. Infect. 2023, 151, e12. [Google Scholar] [CrossRef]
- Shah, A.; Coiado, O.C. COVID-19 Vaccine and Booster Hesitation around the World: A Literature Review. Front. Med. 2023, 9, 1054557. [Google Scholar] [CrossRef]
- Sheng, W.-H.; Chang, H.-C.; Chang, S.-Y.; Hsieh, M.-J.; Chen, Y.-C.; Wu, Y.-Y.; Pan, S.-C.; Wang, J.-T.; Chen, Y.-C. SARS-CoV-2 Infection among Healthcare Workers Whom Already Received Booster Vaccination during Epidemic Outbreak of Omicron Variant in Taiwan. J. Formos. Med. Assoc. 2022, S0929-6646(22)00442-9. [Google Scholar] [CrossRef]
- Wang, H.; Ai, J.; Loeffelholz, M.J.; Tang, Y.-W.; Zhang, W. Meta-Analysis of Diagnostic Performance of Serology Tests for COVID-19: Impact of Assay Design and Post-Symptom-Onset Intervals. Emerg. Microbes Infect. 2020, 9, 2200–2211. [Google Scholar] [CrossRef]
- Wernike, K.; Keller, M.; Conraths, F.J.; Mettenleiter, T.C.; Groschup, M.H.; Beer, M. Pitfalls in SARS-CoV-2 PCR Diagnostics. Transbound. Emerg. Dis. 2021, 68, 253–257. [Google Scholar] [CrossRef]
- Wikramaratna, P.S.; Paton, R.S.; Ghafari, M.; Lourenço, J. Estimating the False-Negative Test Probability of SARS-CoV-2 by RT-PCR. Eurosurveillance 2020, 25, 2000568. [Google Scholar] [CrossRef]
- Alharbi, S.A.; Almutairi, A.Z.; Jan, A.A.; Alkhalify, A.M. Enzyme-Linked Immunosorbent Assay for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) IgM/IgA and IgG Antibodies Among Healthcare Workers. Cureus 2020, 12, e10285. [Google Scholar] [CrossRef] [PubMed]
- Turbett, S.E.; Anahtar, M.; Dighe, A.S.; Garcia Beltran, W.; Miller, T.; Scott, H.; Durbin, S.M.; Bharadwaj, M.; Thomas, J.; Gogakos, T.S.; et al. Evaluation of Three Commercial SARS-CoV-2 Serologic Assays and Their Performance in Two-Test Algorithms. J. Clin. Microbiol. 2020, 59, e01892-20. [Google Scholar] [CrossRef] [PubMed]
- Dörschug, A.; Schwanbeck, J.; Hahn, A.; Hillebrecht, A.; Blaschke, S.; Mese, K.; Groß, U.; Dierks, S.; Frickmann, H.; Zautner, A.E. Comparison of Five Serological Assays for the Detection of SARS-CoV-2 Antibodies. Diagnostics 2021, 11, 78. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.W.; Imöhl, M.; Kleines, M. Determination of SARS-CoV-2 Antibodies with Assays from Diasorin, Roche and IDvet. J. Virol. Methods 2021, 287, 113978. [Google Scholar] [CrossRef]
- Kukoč, A.; Mihelčić, A.; Miko, I.; Romić, A.; Pražetina, M.; Tipura, D.; Drmić, Ž.; Čučković, M.; Ćurčić, M.; Blagaj, V.; et al. Clinical and Laboratory Predictors at ICU Admission Affecting Course of Illness and Mortality Rates in a Tertiary COVID-19 Center. Heart Lung 2022, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Volpe, M.; Kreutz, R. Hypertension, a Moving Target in COVID-19: Current Views and Perspectives. Circ. Res. 2021, 128, 1062–1079. [Google Scholar] [CrossRef]
- Kreutz, R.; Algharably, E.A.E.-H.; Azizi, M.; Dobrowolski, P.; Guzik, T.; Januszewicz, A.; Persu, A.; Prejbisz, A.; Riemer, T.G.; Wang, J.-G.; et al. Hypertension, the Renin–Angiotensin System, and the Risk of Lower Respiratory Tract Infections and Lung Injury: Implications for COVID-19. Cardiovasc. Res. 2020, 116, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Pignataro, G.; Saviano, A.; Ojetti, V.; Gabrielli, M.; Piccioni, A.; Gullì, A.; Antonelli, M.; Gasbarrini, A.; Franceschi, F. Is BMI Associated with Covid-19 Severity? A Retrospective Observational Study. Curr. Med. Chem. 2023, 30. [Google Scholar] [CrossRef]
- Hancková, M.; Betáková, T. Pandemics of the 21st Century: The Risk Factor for Obese People. Viruses 2021, 14, 25. [Google Scholar] [CrossRef]
- Minnai, F.; De Bellis, G.; Dragani, T.A.; Colombo, F. COVID-19 Mortality in Italy Varies by Patient Age, Sex and Pandemic Wave. Sci. Rep. 2022, 12, 4604. [Google Scholar] [CrossRef]
- Tong, X.; Huang, Z.; Zhang, X.; Si, G.; Lu, H.; Zhang, W.; Xue, Y.; Xie, W. Old Age Is an Independent Risk Factor for Pneumonia Development in Patients with SARS-CoV-2 Omicron Variant Infection and a History of Inactivated Vaccine Injection. Infect. Drug Resist. 2022, 15, 5567–5573. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Tanaka, M.; Kozai, H.; Hotta, K.; Aoyama, Y.; Shigeno, Y.; Aoike, M.; Kawamura, H.; Tsurudome, M.; Ito, M. Antibody Response of Smokers to the COVID-19 Vaccination: Evaluation Based on Cigarette Dependence. Drug Discov. Ther. 2022, 16, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central Obesity, Smoking Habit, and Hypertension Are Associated with Lower Antibody Titres in Response to COVID-19 MRNA Vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef] [PubMed]

| HCW SARS-CoV-2 | HCW without SARS-CoV-2 | p Value | ||
|---|---|---|---|---|
| Infection (n = 563) | Infection (n = 2987) | |||
| Clinical | Age in years (median ± IQR) | 39.0 (29.0–50.0) | 42.0 (33.0–52.0) | <0.001 |
| Female/Male ratio | 3.8 | 3.5 | 0.534 | |
| characteristics | Days after onset symptoms (median ± IQR) | 55.0 (44.0–54.0) | 57.0 (41.0–70.7) | 0.041 |
| Body mass index (median ± IQR) | 23.5 (21.5–26.6) | 23.9 (21.5–26.7) | 0.573 | |
| Overweight (%) | 27.4 | 28.7 | 0.520 | |
| Obese (%) | 9.4 | 10.0 | 0.665 | |
| Smoker (%) | 11.5 | 25.1 | <0.001 | |
| Hospitalization (%) | 4.4 | 0.1 | <0.001 | |
| Symptoms (%) | Vomits | 75.3 | 11.4 | <0.001 |
| Difficulty breathing | 72.4 | 11.1 | <0.001 | |
| Abdominal pain | 71.1 | 8.4 | <0.001 | |
| Sore throat | 58.4 | 6.0 | <0.001 | |
| Nasal congestion | 54.7 | 8.6 | <0.001 | |
| Diarrhea | 54.7 | 7.6 | <0.001 | |
| Dry cough | 41.9 | 6.6 | <0.001 | |
| Fever | 41.0 | 10.4 | <0.001 | |
| Loss of taste | 35.6 | 12.0 | <0.001 | |
| Loss of smell | 34.0 | 11.9 | <0.001 | |
| Chill | 33.4 | 8.4 | <0.001 | |
| Headache | 23.6 | 3.1 | <0.001 | |
| Myalgia | 25.2 | 5.4 | <0.001 | |
| Comorbidities | 16.3 | 19.0 | 0.130 | |
| Fatigue | 12.5 | 2.6 | <0.001 | |
| Arterial hypertension | 6.4 | 7.5 | 0.342 | |
| Asymptomatic | 30.4 | 84.5 | <0.001 |
| Manufacturer | Roche Concordance Kappa [95% CI] | Vircell IgG Concordance Kappa [95% CI] | Infection Concordance Kappa [95% CI] |
|---|---|---|---|
| Roche Elecsys® | - | −0.32 [−0.45–(−0.18)] | 0.73 [0.58–0.87] |
| Vircell IgG | −0.32 [−0.45–(−0.18)] | - | −0.27 [−0.38–(−0.15)] |
| Diasorin IgG | 0.58 [0.39–0.76] | −0.18 [−0.28–(−0.08)] | 0.81 [0.68–0.94] |
| Euroimmun IgG | 0.61 [0.39–0.82] | 0.12 [0.04–0.20] | 0.73 [0.56–0.89] |
| Immunoassay | RT-PCR Positive HCWs Group (n = 203) | RT-PCR Negative HCWs Group (n = 222) | RT-PCR Kappa [CI] (n = 425) | SARS-CoV-2 Infection Kappa [CI] (n = 425) |
|---|---|---|---|---|
| Roche Elecsys®-Positive | 182 (89.7%) | 67 (30.2%) | 0.59 [0.51–0.66] | 0.89 [0.85–0.94] |
| Roche Elecsys®-Negative | 21 (10.3%) | 155 (69.8%) | ||
| Vircell IgG-Positive | 180 (88.7%) | 71 (32.0%) | 0.56 [0.48–0.64] | 0.84 [0.79–0.89] |
| Vircell IgG-Negative | 23 (11.3%) | 151 (68.0%) | ||
| SARS-CoV-2 Infection | 203 (100.0%) | 67 (30.2%) | 0.68 [0.62–0.75] | - |
| Manufacturer | Sensitivity | Specificity | Positive Predicted Value | Negative Predicted Value | Accuracy (95% CI) | Infection Concordance (Kappa, 95% CI) |
|---|---|---|---|---|---|---|
| Roche Elecsys® | 94.8 | 99.8 | 99.1 | 99.0 | 99.3 (99.8–99.9) | 0.96 (0.95–0.97) |
| Vircell IgG | 92.9 | 95.3 | 77.8 | 98.6 | 96.9 (96.1–97.6) | 0.82 (0.80–0.85) |
| Manufacturer | Sensitivity | Specificity | Positive Predicted Value | Negative Predicted Value | Accuracy (95% CI) | Infection Concordance (Kappa, 95% CI) |
|---|---|---|---|---|---|---|
| Roche Elecsys | 95.3 | 99.7 | 98.6 | 99.1 | 96.9 (95.3–98.5) | 0.96 (0.95–0.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, J.F.; Vidal, M.; Julià, G.; Navarro, G.; Serrano, R.M.; van den Eynde, E.; Navarro, M.; Calvet, J.; Gratacós, J.; Espasa, M.; et al. Validation of N Protein Antibodies to Diagnose Previous SARS-CoV-2 Infection in a Large Cohort of Healthcare Workers: Use of Roche Elecsys® Immunoassay in the S Protein Vaccination Era. Viruses 2023, 15, 930. https://doi.org/10.3390/v15040930
Delgado JF, Vidal M, Julià G, Navarro G, Serrano RM, van den Eynde E, Navarro M, Calvet J, Gratacós J, Espasa M, et al. Validation of N Protein Antibodies to Diagnose Previous SARS-CoV-2 Infection in a Large Cohort of Healthcare Workers: Use of Roche Elecsys® Immunoassay in the S Protein Vaccination Era. Viruses. 2023; 15(4):930. https://doi.org/10.3390/v15040930
Chicago/Turabian StyleDelgado, Juan Francisco, Mònica Vidal, Germà Julià, Gema Navarro, Rosa María Serrano, Eva van den Eynde, Marta Navarro, Joan Calvet, Jordi Gratacós, Mateu Espasa, and et al. 2023. "Validation of N Protein Antibodies to Diagnose Previous SARS-CoV-2 Infection in a Large Cohort of Healthcare Workers: Use of Roche Elecsys® Immunoassay in the S Protein Vaccination Era" Viruses 15, no. 4: 930. https://doi.org/10.3390/v15040930
APA StyleDelgado, J. F., Vidal, M., Julià, G., Navarro, G., Serrano, R. M., van den Eynde, E., Navarro, M., Calvet, J., Gratacós, J., Espasa, M., & Peña, P. (2023). Validation of N Protein Antibodies to Diagnose Previous SARS-CoV-2 Infection in a Large Cohort of Healthcare Workers: Use of Roche Elecsys® Immunoassay in the S Protein Vaccination Era. Viruses, 15(4), 930. https://doi.org/10.3390/v15040930






