An Investigation of the Antiviral Potential of Phytocompounds against Avian Infectious Bronchitis Virus through Template-Based Molecular Docking and Molecular Dynamics Simulation Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ligand Preparation
2.2. Protein Preparation
2.3. Determining the Target Sites
2.4. Pharmacokinetic Assessment
2.5. Virtual Screening
2.6. Molecular Dynamics
3. Results
3.1. Virtual Screening of Ligand Libraries
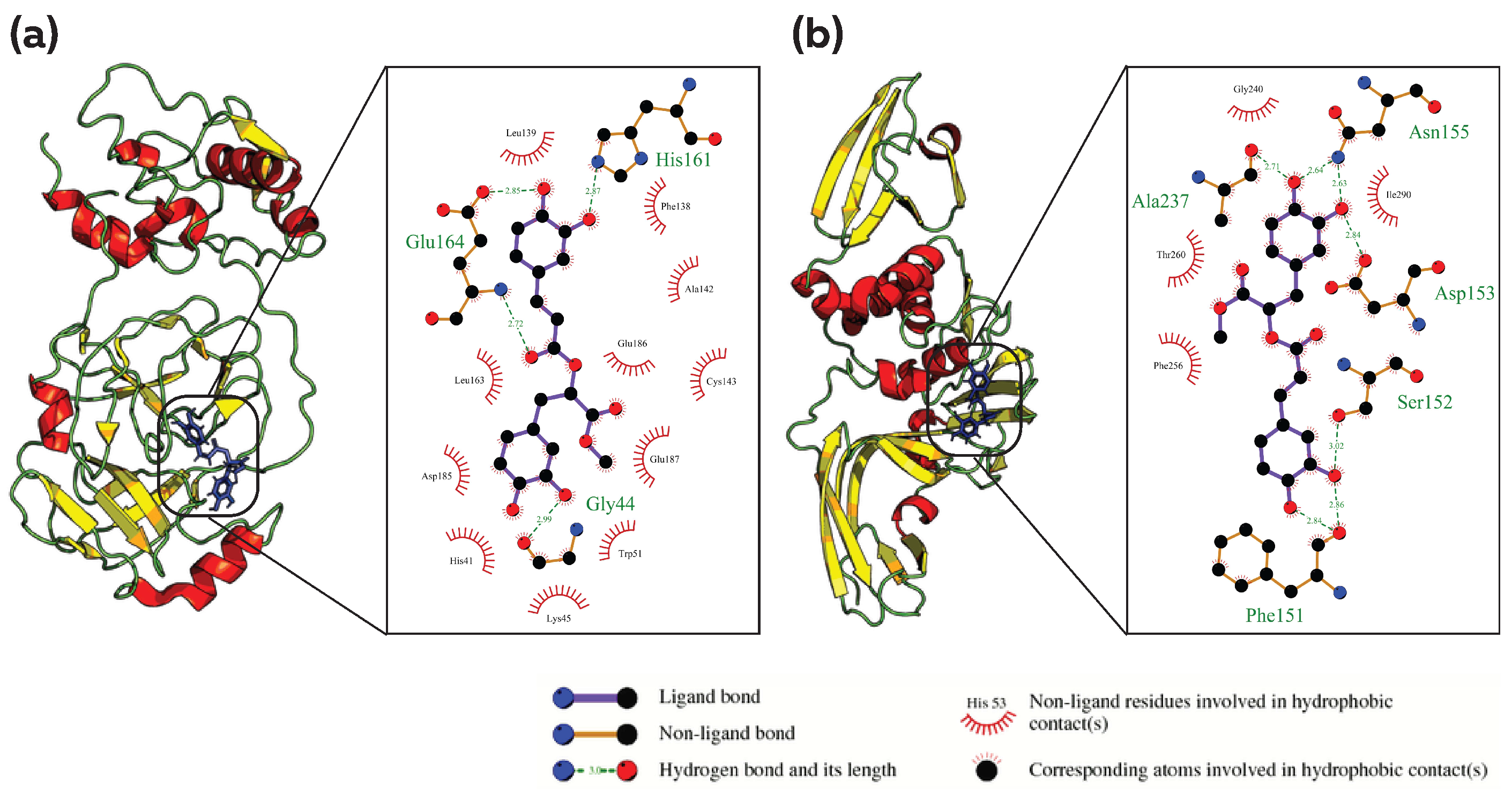
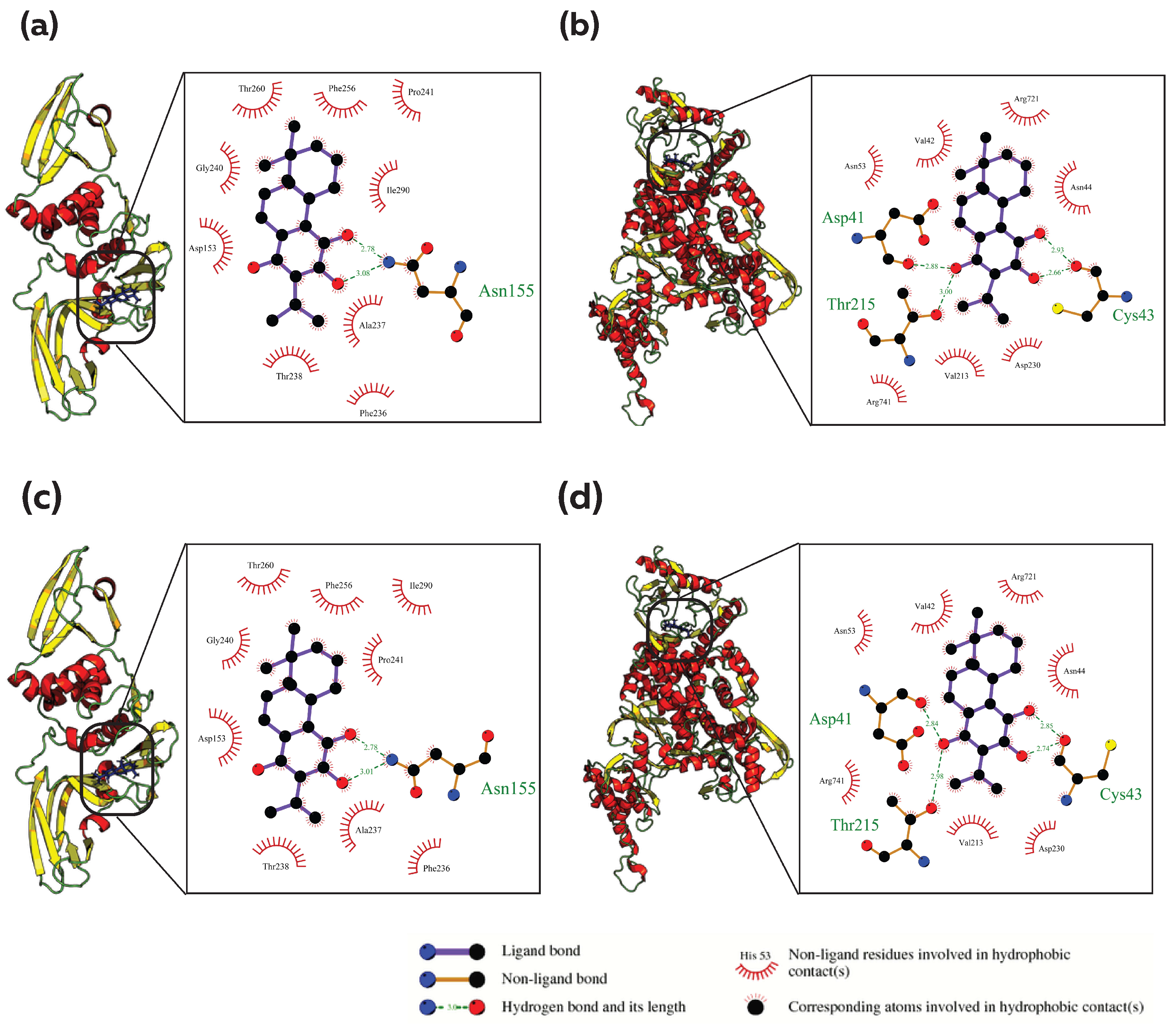
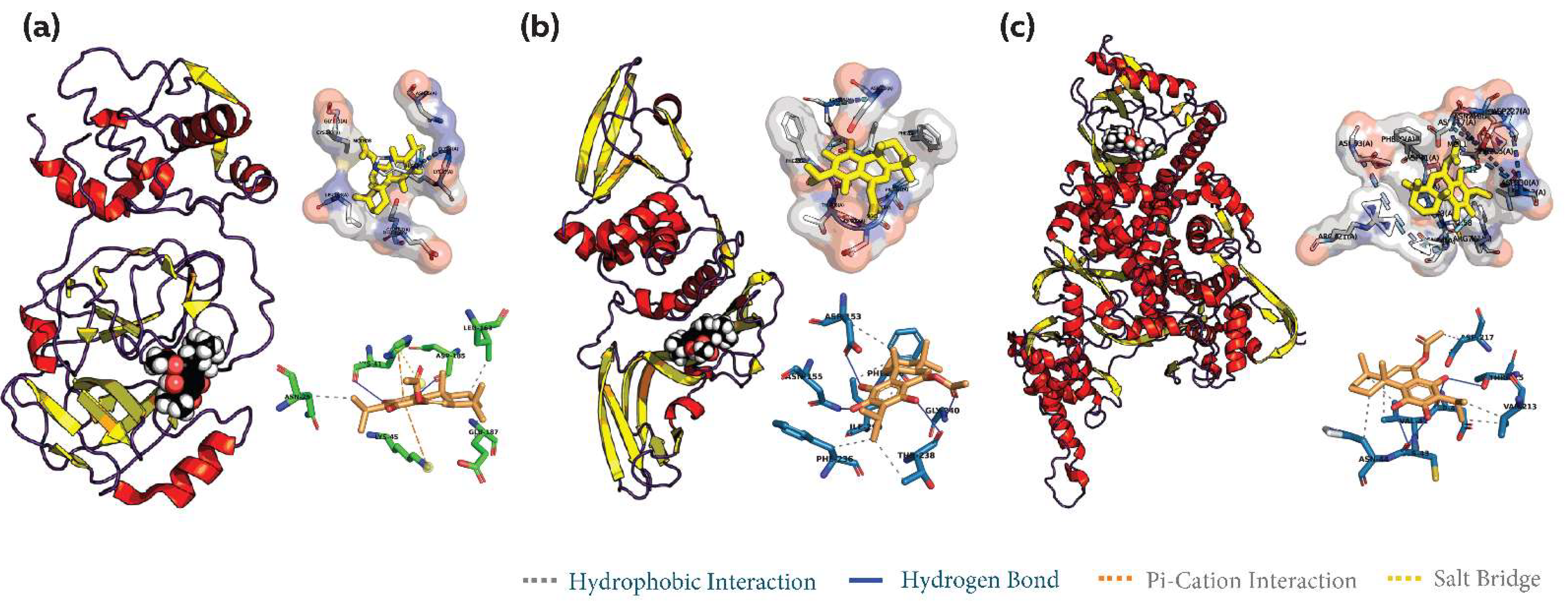
3.2. Common Phytocompound Inhibitor against Mpro and Plpro
3.3. Common Phytocompound Inhibitor against Mpro and RdRp
3.4. Common Phytocompound Inhibitor against PLpro and RdRp
3.5. Common Phytocompound Inhibitor against Mpro, PLpro and RdRp
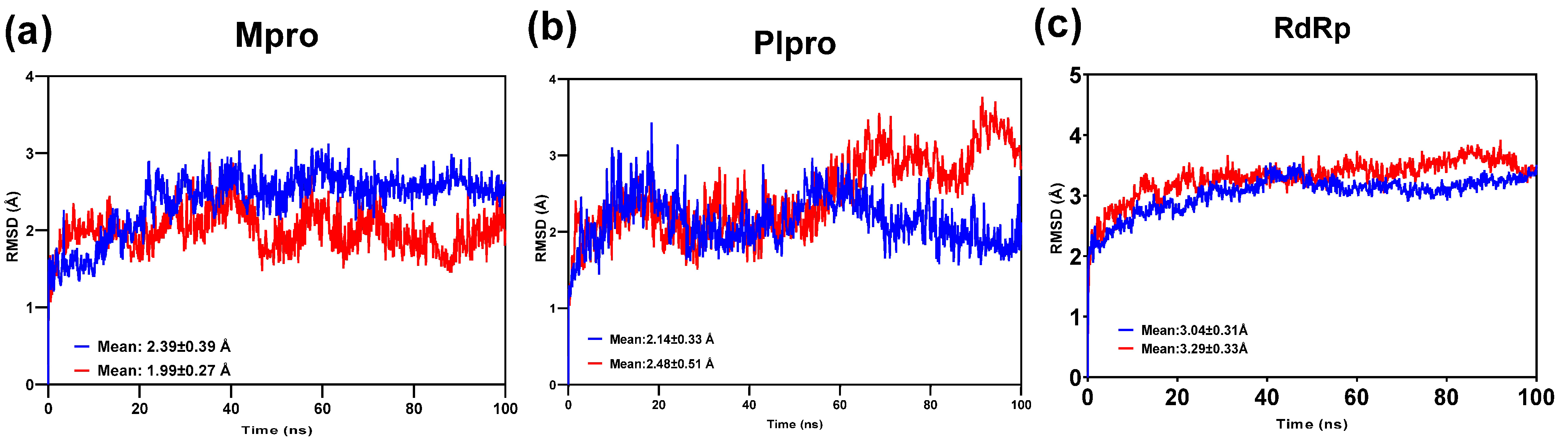
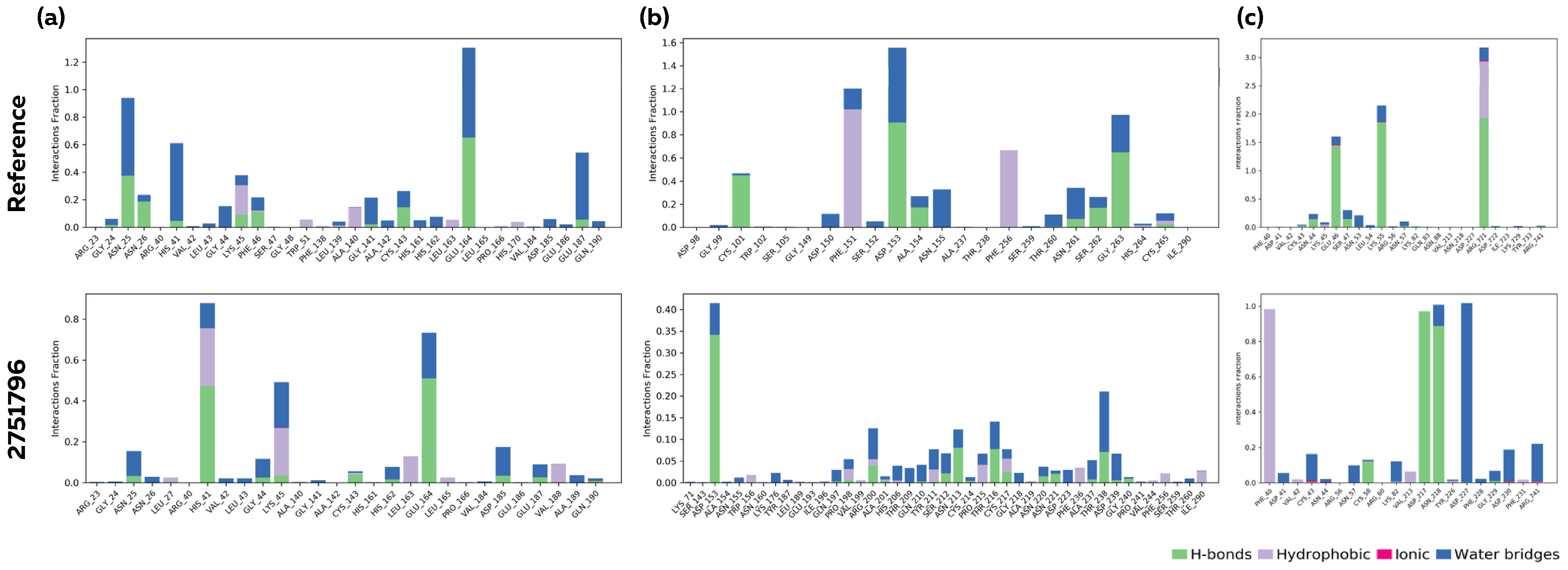
3.6. Molecular Dynamics Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schalk, A.F. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931, 78, 413–423. [Google Scholar]
- Cavanagh, D. Coronaviruses in poultry and other birds. Avian Pathol. 2005, 34, 439–448. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Hall, D.; Handel, A. Molecular evolution and emergence of avian gammacoronaviruses. Infect. Genet. Evol. 2012, 12, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, J.; Ashton, D.F.; Reece, R.; Scott, P.; Hooper, P. Pathogenicity of Australian strains of avian infectious bronchitis virus. J. Comp. Pathol. 2002, 126, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Seifi, S.; Asasi, K.; Mohammadi, A. Natural co-infection caused by avian influenza H9 subtype and infectious bronchitis viruses in broiler chicken farms. Vet. Arhiv 2010, 80, 269–281. [Google Scholar]
- Hopkins, S.R.; Yoder, H.W., Jr. Increased incidence of airsacculitis in broilers infected with Mycoplasma synoviae and chicken-passaged infectious bronchitis vaccine virus. Avian Dis. 1984, 28, 386–396. [Google Scholar] [CrossRef]
- Matthijs, M.G.R.; Van Eck, J.H.H.; Landman, W.J.M.; Stegeman, J.A. Ability of Massachusetts-type infectious bronchitis virus to increase colibacillosis susceptibility in commercial broilers: A comparison between vaccine and virulent field virus. Avian Pathol. 2003, 32, 473–481. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Gomez-Puertas, P.; Cavanagh, D.; Gorbalenya, A.E.; Enjuanes, L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 2003, 148, 2207–2235. [Google Scholar] [CrossRef]
- Boursnell, M.E.G.; Brown, T.D.K.; Foulds, I.J.; Green, F.; Tomley, F.M.; Binns, M.M. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J. Gen. Virol. 1987, 68, 57–77. [Google Scholar] [CrossRef]
- Ziebuhr, J.; Snijder, E.J.; Gorbalenya, A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000, 81, 853–879. [Google Scholar] [CrossRef]
- Mo, M.; Huang, B.; Wei, P.; Wei, T.; Chen, Q.; Wang, X.; Li, M.; Fan, W. Complete genome sequences of two Chinese virulent avian coronavirus infectious bronchitis virus variants. Am. Soc. Microbiol. 2012, 86, 19. [Google Scholar] [CrossRef] [PubMed]
- Van Hemert, M.J.; Van Den Worm, S.H.; Knoops, K.; Mommaas, A.M.; Gorbalenya, A.E.; Snijder, E.J. SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog. 2008, 4, e1000054. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M.; Cavanagh, D. The molecular biology of coronaviruses. Adv. Virus Res. 1997, 48, 1–100. [Google Scholar]
- Pasternak, A.O.; Spaan, W.J.; Snijder, E.J. Nidovirus transcription: How to make sense…? J. Gen. Virol. 2006, 87, 1403–1421. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yu, H.; Yang, H.; Xue, F.; Wu, Z.; Shen, W.; Li, J.; Zhou, Z.; Ding, Y.; Zhao, Q.; et al. Structures of two coronavirus main proteases: Implications for substrate binding and antiviral drug design. J. Virol. 2008, 82, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef]
- Bedford, M. Removal of antibiotic growth promoters from poultry diets: Implications and strategies to minimise subsequent problems. World’s Poult. Sci. J. 2000, 56, 347–365. [Google Scholar] [CrossRef]
- Naqi, S.; Thompson, G.; Bauman, B.; Mohammed, H. The exacerbating effect of infectious bronchitis virus infection on the infectious bursal disease virus-induced suppression of opsonization by Escherichia coil antibody in chickens. Avian Dis. 2001, 45, 52–60. [Google Scholar] [CrossRef]
- de Wit, J.J.; Cook, J.K.A. Spotlight on avian pathology: Infectious bronchitis virus. Avian Pathol. 2019, 48, 393–395. [Google Scholar] [CrossRef]
- Ashour, E.A.; Abd El-Hack, M.E.; Swelum, A.A.; Osman, A.O.; Taha, A.E.; Alhimaidi, A.R.; Ismail, I.E. Does the dietary graded levels of herbal mixture powder impact growth, carcass traits, blood indices and meat quality of the broilers? Ital. J. Anim. Sci. 2020, 19, 1228–1237. [Google Scholar] [CrossRef]
- Oliveira, N.A.; Gonçalves, B.L.; Lee, S.H.I.; Oliveira, C.A.F.; Corassin, C.H. Use of antibiotics in animal production and its impact on human health. J. Food Chem. Nanotechnol. 2020, 6, 40–47. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Shaheen, H.M.; Abdel-Latif, M.A.; Noreldin, A.E.; Patra, A.K. The usefulness of oregano and its derivatives in poultry nutrition. World’s Poult. Sci. J. 2018, 74, 463–474. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; Abd El-Hack, M.E.; Alagawany, M.; Naiel, M.A.; Mahgoub, S.A.; Badr, M.M.; Hussein, E.O.; Alowaimer, A.N.; Swelum, A.A. Paulownia leaves as a new feed resource: Chemical composition and effects on growth, carcasses, digestibility, blood biochemistry, and intestinal bacterial populations of growing rabbits. Animals 2019, 9, 95. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alaidaroos, B.A.; Farsi, R.M.; Abou-Kassem, D.E.; El-Saadony, M.T.; Saad, A.M.; Shafi, M.E.; Albaqami, N.M.; Taha, A.E.; Ashour, E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals 2021, 11, 1878. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Lillehoj, H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed. Sci. Technol. 2019, 250, 41–50. [Google Scholar] [CrossRef]
- Arif, M.; Iram, A.; Bhutta, M.A.; Naiel, M.A.; Abd El-Hack, M.E.; Othman, S.I.; Allam, A.A.; Amer, M.S.; Taha, A.E. The biodegradation role of Saccharomyces cerevisiae against harmful effects of mycotoxin contaminated diets on broiler performance, immunity status, and carcass characteristics. Animals 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- El-Shall, N.A.; Awad, A.M.; El-Hack, M.E.A.; Naiel, M.A.; Othman, S.I.; Allam, A.A.; Sedeik, M.E. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals 2019, 10, 70. [Google Scholar] [CrossRef]
- Hussein, E.O.; Ahmed, S.H.; Abudabos, A.M.; Aljumaah, M.R.; Alkhlulaifi, M.M.; Nassan, M.A.; Suliman, G.M.; Naiel, M.A.; Swelum, A.A. Effect of antibiotic, phytobiotic and probiotic supplementation on growth, blood indices and intestine health in broiler chicks challenged with Clostridium perfringens. Animals 2020, 10, 507. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin dietary supplementation advances growth performance, gut microbiota, and intestinal mrna expression genes in broiler chickens. Animals 2021, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Nahed, A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical control of poultry coccidiosis: A review. Poult. Sci. 2022, 101, 101542. [Google Scholar]
- Abbas, G.; Yu, J.; Li, G. Novel and Alternative Therapeutic Strategies for Controlling Avian Viral Infectious Diseases: Focus on Infectious Bronchitis and Avian Influenza. Front. Vet. Sci. 2022, 9, 933274. [Google Scholar] [CrossRef] [PubMed]
- Lelešius, R.; Karpovaitė, A.; Mickienė, R.; Drevinskas, T.; Tiso, N.; Ragažinskienė, O.; Kubilienė, L.; Maruška, A.; Šalomskas, A. In vitro antiviral activity of fifteen plant extracts against avian infectious bronchitis virus. BMC Vet. Res. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, I.; Paunova-Krasteva, T.; Petrova, Z.; Grozdanov, P.; Nikolova, N.; Tsonev, G.; Triantafyllidis, A.; Andreev, S.; Trepechova, M.; Milkova, V.; et al. Bulgarian Medicinal Extracts as Natural Inhibitors with Antiviral and Antibacterial Activity. Plants 2022, 11, 1666. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Koonin, E.V.; Donchenko, A.P.; Blinov, V.M. Coronavirus genome: Prediction of putative functional domains in the non-structural polyprotein by comparative amino acid sequence analysis. Nucleic Acids Res. 1989, 17, 4847–4861. [Google Scholar] [CrossRef]
- Yang, H.; Yang, M.; Ding, Y.; Liu, Y.; Lou, Z.; Zhou, Z.; Sun, L.; Mo, L.; Ye, S.; Pang, H.; et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA 2003, 100, 13190–13195. [Google Scholar] [CrossRef]
- Wang, H.; Xue, S.; Yang, H.; Chen, C. Recent progress in the discovery of inhibitors targeting coronavirus proteases. Virol. Sin. 2016, 31, 24–30. [Google Scholar] [CrossRef]
- Pluskota-Karwatka, D.; Hoffmann, M.; Barciszewski, J. Reducing SARS-CoV-2 pathological protein activity with small molecules. J. Pharm. Anal. 2021, 11, 383–397. [Google Scholar] [CrossRef]
- Amin, S.A.; Banerjee, S.; Ghosh, K.; Gayen, S.; Jha, T. Protease targeted COVID-19 drug discovery and its challenges: Insight into viral main protease (Mpro) and papain-like protease (PLpro) inhibitors. Bioorganic Med. Chem. 2021, 29, 115860. [Google Scholar] [CrossRef]
- Elfiky, A.A. Zika viral polymerase inhibition using anti-HCV drugs both in market and under clinical trials. J. Med. Virol. 2016, 88, 2044–2051. [Google Scholar] [CrossRef]
- Ganesan, A.; Barakat, K. Applications of computer-aided approaches in the development of hepatitis C antiviral agents. Expert Opin. Drug Discov. 2017, 12, 407–425. [Google Scholar] [CrossRef]
- Ribavirin, E.A.; Remdesivir, S. Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar]
- Norouzi, B.; Qotbi, A.A.A.; Seidavi, A.; Schiavone, A.; Marín, A.L.M. Effect of Different Dietary Levels of Rosemary (Rosmarinus Officinalis) and Yarrow (Achillea Millefolium) on the Growth Performance, Carcass Traits and Ileal Micro-biota of Broilers. Italian Journal of Animal Science. Ital. J. Anim. Sci. 2015, 14, 3930. [Google Scholar] [CrossRef]
- Cross, D.E.; McDevitt, R.M.; Acamovic, T. Herbs, thyme essential oil and condensed tannin extracts as dietary supplements for broilers, and their effects on performance, digestibility, volatile fatty acids and organoleptic properties. Br. Poult. Sci. 2011, 52, 227–237. [Google Scholar] [CrossRef]
- Cross, D.E.; McDevitt, R.M.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Horton, G.M.J.; Fennell, M.J.; Prasad, B.M. Effect of dietary garlic (Allium sativum) on performance, carcass composition and blood chemistry changes in broiler chickens. Can. J. Anim. Sci. 1991, 71, 939–942. [Google Scholar] [CrossRef]
- Pistova, V.; ARPÁŠOVÁ, H.; HRNČÁR, C. The effect of the humic acid and garlic (Allium sativum L.) on performance parameters and carcass characteristic of broiler chicken. J. Cent. Eur. Agric. 2016, 17, 1168–1178. [Google Scholar] [CrossRef]
- Sheoran, N.; Kumar, R.; Kumar, A.; Batra, K.; Sihag, S.; Maan, S.; Maan, N.S. Nutrigenomic evaluation of garlic (Allium sativum) and holy basil (Ocimum sanctum) leaf powder supplementation on growth performance and immune characteristics in broilers. Vet. World 2017, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Ogunlesi, O.O.; Oladele, O.A.; Aina, O.O.; Esan, O.O. Effects of dietary garlic (Allium sativum) meal on skin thickness and fat deposition in commercial broiler chickens. Bulg. J. Vet. Med. 2017, 20, 118–124. [Google Scholar] [CrossRef]
- Ibrahim, D.K.; Salman, K.A.; Al-Khilani, F.M. Effect of supplementation aqueous extract of borage (Borago officinalis) and Melilotus (Melilotus officinalis) to drinking water on production performance of broiler during summer season. Iraqi Poult. Sci. J. 2016, 10, 13–23. [Google Scholar]
- Boruta, A.; Niemiec, J.; Marcin, L. The effect of borage seeds in hens diet on fatty acids composition in egg yolk. In Proceedings of the XVII European Symposium on the Quality of Poultry Meat and XI European Symposium on the Quality of Eggs and Egg Products, Doorwerth, The Netherlands, 23–26 May 2005; Golden Tulip Parkhotel: Doorwerth, The Netherlands, 2005. [Google Scholar]
- Mueller, K.; Blum, N.M.; Kluge, H.; Mueller, A.S. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic-and antioxidant enzymes in broiler chickens. Br. J. Nutr. 2012, 108, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Poursina, B.; Roudi, P.S.; Sedghi, M.; Taibipour, A. Effect of peppermint (Mentha piperita L.), thyme (Thymus vulgaris L.) and chicory (Chicorium intybus L.) on performance and intestine morphology of broilers. Iran. J. Med. Aromat. Plants 2016, 31, 1035–1045. [Google Scholar]
- Liu, H.Y.; Ivarsson, E.; Jönsson, L.; Holm, L.; Lundh, T.; Lindberg, J.E. Growth performance, digestibility, and gut development of broiler chickens on diets with inclusion of chicory (Cichorium intybus L.). Poult. Sci. 2011, 90, 815–823. [Google Scholar] [CrossRef]
- Ocak, N.; Erener, G.; Burak Ak, F.; Sungu, M.; Altop, A.; Ozmen, A. Performance of broilers fed diets supplemented with dry peppermint (Mentha piperita L.) or thyme (Thymus vulgaris L.) leaves as growth promoter source. Czech J. Anim. Sci. 2008, 53, 169. [Google Scholar] [CrossRef]
- Cetin, E.; Yibar, A.R.T.U.N.; Yesilbag, D.; Cetin, I.; Cengiz, S.S. The effect of volatile oil mixtures on the performance and ilio-caecal microflora of broiler chickens. Br. Poult. Sci. 2016, 57, 780–787. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Abbas, R.J. The effect of using fennel seeds (Foeniculum vulgare L.) on productive performance of broiler chickens. Int. J. Poult. Sci. 2009, 8, 642–644. [Google Scholar] [CrossRef]
- Kwiecieñ, M.; Winiarska-Mieczan, A. Effect of addition of herbs on body weight and assessment of physical and chemical alterations in the tibia bones of broiler chickens. J. Elem. 2009, 14, 705–715. [Google Scholar] [CrossRef]
- Umar, S.; Rehman, A.; Younus, M.; Ali, A.; Shahzad, M.; Shah, M.A.A.; Munir, M.T.; Aslam, H.B.; Yaqoob, M. Effects of Nigella sativa on immune responses and pathogenesis of avian influenza (H9N2) virus in turkeys. J. Appl. Poult. Res. 2016, 25, 95. [Google Scholar] [CrossRef]
- Al-Hothaify, S.A.; Al-Sanabani, M.A. The effects of supplementation Nigella sativa seeds as a natural substance on growth rate, some serum indices, carcass quality and antibody titers of broiler birds. Am. J. Res. Commun. 2016, 4, 43–51. [Google Scholar]
- Guler, T.; Ertas, O.N.; Kizil, M.; Dalkýlýc, B.; Ciftci, M. Effect of dietary supplemental black cumin seeds on antioxidant activity in broilers. Med. Weter. 2007, 63, 1060–1063. [Google Scholar]
- Franciosini, M.P.; Casagrande-Proietti, P.; Forte, C.; Beghelli, D.; Acuti, G.; Zanichelli, D.; dal Bosco, A.; Castellini, C.; Trabalza-Marinucci, M. Effects of oregano (Origanum vulgare L.) and rosemary (Rosmarinus officinalis L.) aqueous extracts on broiler performance, immune function and intestinal microbial population. J. Appl. Anim. Res. 2016, 44, 474–479. [Google Scholar] [CrossRef]
- Ghazi, S.; Amjadian, T.; Norouzi, S. Single and combined effects of vitamin C and oregano essential oil in diet, on growth performance, and blood parameters of broiler chicks reared under heat stress condition. Int. J. Biometeorol. 2015, 59, 1019–1024. [Google Scholar] [CrossRef]
- Shariatmadari, F.; Shariatmadari, R. Sumac (Rhus coriaria) supplementation in poultry diet. World’s Poult. Sci. J. 2020, 76, 358–364. [Google Scholar] [CrossRef]
- Azizi, M.; Passantino, G.; Akter, Y.; Javandel, F.; Seidavi, A.; Bahar, B.; O’Shea, C.J.; Tufarelli, V.; Laudadio, V. Effect of incremental levels of sumac (Rhus coriaria L.) seed powder on growth, carcass traits, blood parameters, immune system and selected ileal microorganisms of broilers. Vet. Ital. 2020, 56, 185–192. [Google Scholar]
- Schrödinger Release 2022-1: Maestro; Schrödinger, LLC: New York, NY, USA, 2021.
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, K.; Karthikeyan, B.S.; Vivek-Ananth, R.P.; Chand, R.B.; Aparna, S.R.; Mangalapandi, P.; Samal, A. IMPPAT: A curated database of Indian medicinal plants, phytochemistry and therapeutics. Sci. Rep. 2018, 8, 4329. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Cheeseright, T.; Mackey, M.; Rose, S.; Vinter, A. Molecular field extrema as descriptors of biological activity: Definition and validation. J. Chem. Inf. Model. 2006, 46, 665–676. [Google Scholar] [CrossRef]
- Bauer, M.R.; Mackey, M.D. Electrostatic complementarity as a fast and effective tool to optimize binding and selectivity of protein–ligand complexes. J. Med. Chem. 2019, 62, 3036–3050. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Firth-Clark, S.; Tosco, P.; Mey, A.S.; Mackey, M.; Michel, J. Assessment of Binding Affinity via Alchemical Free-Energy Calculations. J. Chem. Inf. Model. 2020, 60, 3120–3130. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Shaw, N.; Yan, L.; Lou, Z.; Rao, Z. Structural view and substrate specificity of papain-like protease from avian infectious bronchitis virus. J. Biol. Chem. 2015, 290, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384–W388. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Chistyakov, V.V.; Thornton, J.M. PDBsum more: New summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Res. 2005, 33 (Suppl. 1), D266–D268. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Jabłońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018, 27, 129–134. [Google Scholar] [CrossRef]
- Peng, S.; Fang, C.; He, H.; Song, X.; Zhao, X.; Zou, Y.; Li, L.; Jia, R.; Yin, Z. Myricetin exerts its antiviral activity against infectious bronchitis virus by inhibiting the deubiquitinating activity of papain-like protease. Poult. Sci. 2022, 101, 101626. [Google Scholar] [CrossRef]
- Osipiuk, J.; Azizi, S.A.; Dvorkin, S.; Endres, M.; Jedrzejczak, R.; Jones, K.A.; Kang, S.; Kathayat, R.S.; Kim, Y.; Lisnyak, V.G.; et al. Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat. Commun. 2021, 12, 743. [Google Scholar] [CrossRef]
- Shannon, A.; Fattorini, V.; Sama, B.; Selisko, B.; Feracci, M.; Falcou, C.; Gauffre, P.; El Kazzi, P.; Delpal, A.; Decroly, E.; et al. A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat. Commun. 2022, 13, 621. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FA, USA, 11–17 November 2006. [Google Scholar]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug. Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Suriyarak, S.; Bayrasy, C.; Schmidt, H.; Villeneuve, P.; Weiss, J. Impact of fatty acid chain length of rosmarinate esters on their antimicrobial activity against Staphylococcus carnosus LTH1502 and Escherichia coli K-12 LTH4263. J. Food Prot. 2013, 76, 1539–1548. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.F.; Yang, S.X.; Qiu, Y.; Yuan, K. Chemical constituents of Hyptis rhomboidea and their antifungal activity. Zhongguo Zhong Yao Za Zhi=Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica. 2014, 39, 2284–2288. [Google Scholar]
- Lin, L.; Dong, Y.; Zhao, H.; Wen, L.; Yang, B.; Zhao, M. Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (MAXIM.) HARA as inhibitors of tyrosinase and α-glucosidase. Food Chem. 2011, 129, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, W.; Wang, L.; Shan, L.; Li, H.; Huang, J.; Sun, Q.; Zhang, W. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1). Eur. J. Med. Chem. 2013, 62, 148–157. [Google Scholar] [CrossRef]
- Woo, E.R.; Piao, M.S. Antioxidative constituents from Lycopus lucidus. Arch. Pharm. Res. 2004, 27, 173–176. [Google Scholar] [CrossRef]
- Srivastava, R.; Tripathi, S.; Unni, S.; Hussain, A.; Haque, S.; Dasgupta, N.; Singh, V.; Mishra, B.N. Silybin B and Cianidanol Inhibit M(pro) and Spike Protein of SARS-CoV-2: Evidence from in silico Molecular Docking Studies. Curr. Pharm. Des. 2021, 27, 3476–3489. [Google Scholar] [CrossRef]
- Pár, A.; Szekeres-Bartho, J.; Pácsa, S.; Jávor, T. Effect of cianidanol on natural killer cell activity in patients with chronic B virus hepatitis. Int. J. Clin. Pharmacol. Res. 1987, 7, 301–306. [Google Scholar]
- Suzuki, H.; Yamamoto, S.; Hirayama, C.; Takino, T.; Fujisawa, K.; Oda, T. Cianidanol therapy for HBe-antigen-positive chronic hepatitis: A multicentre, double-blind study. Liver 1986, 6, 35–44. [Google Scholar] [CrossRef]
- Garcia, C.; Isca, V.M.; Pereira, F.; Monteiro, C.M.; Ntungwe, E.; Sousa, F.; Dinic, J.; Holmstedt, S.; Roberto, A.; Díaz-Lanza, A.; et al. Royleanone Derivatives from Plectranthus spp. as a Novel Class of P-Glycoprotein Inhibitors. Front. Pharmacol. 2020, 11, 557789. [Google Scholar] [CrossRef] [PubMed]
- Fronza, M.; Lamy, E.; Günther, S.; Heinzmann, B.; Laufer, S.; Merfort, I. Abietane diterpenes induce cytotoxic effects in human pancreatic cancer cell line MIA PaCa-2 through different modes of action. Phytochemistry 2012, 78, 107–119. [Google Scholar] [CrossRef] [PubMed]


| Pubchem CID | Mpro | Plpro | RdRp | Phytochemical Name | Botanical |
|---|---|---|---|---|---|
| Reference | −9.698 | −7.672 | −2.265 | - | - |
| 2751796 | −8.608 | −8.703 | −8.9 | ||
| 7alpha-Acetoxyroyleanone | Rosmarinus officinalis | ||||
| 6479915 | −10.135 | −8.688 | - | ||
| Methyl rosmarinate | Mentha piperita | ||||
| 23243692 | −8.966 | −8.864 | |||
| - | 7-O-Methylrosmanol | Rosmarinus officinalis | |||
| 23243694 | −8.945 | −8.773 | |||
| - | Epirosmanol | Rosmarinus officinalis | |||
| 46883407 | −8.759 | −8.49 | |||
| - | Rosmaquinone β | Rosmarinus officinalis | |||
| 442084 | −8.794 | −7.961 | |||
| - | Royleanone | Rosmarinus officinalis | |||
| 2751794 | −8.28 | −8.117 | |||
| - | 6,7-Dehydroroyleanone | Rosmarinus officinalis | |||
| 75552 | −7.916 | −8.077 | |||
| - | Diallyl tetrasulfide | Allium Sativum | |||
| 9064 | −9.497 | - | −9.389 | ||
| Cianidanol | Ocimum sanctum | ||||
| 13820511 | −9.191 | - | −8.154 | ||
| Isorosmanol | Rosmarinus officinalis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, I.; Hassan, A.; Haq, E.; Ahmad, S.M.; Shah, R.A.; Ganai, N.A.; Chikan, N.A.; Abdul-Careem, M.F.; Shabir, N. An Investigation of the Antiviral Potential of Phytocompounds against Avian Infectious Bronchitis Virus through Template-Based Molecular Docking and Molecular Dynamics Simulation Analysis. Viruses 2023, 15, 847. https://doi.org/10.3390/v15040847
Gul I, Hassan A, Haq E, Ahmad SM, Shah RA, Ganai NA, Chikan NA, Abdul-Careem MF, Shabir N. An Investigation of the Antiviral Potential of Phytocompounds against Avian Infectious Bronchitis Virus through Template-Based Molecular Docking and Molecular Dynamics Simulation Analysis. Viruses. 2023; 15(4):847. https://doi.org/10.3390/v15040847
Chicago/Turabian StyleGul, Irfan, Amreena Hassan, Ehtishamul Haq, Syed Mudasir Ahmad, Riaz Ahmad Shah, Nazir Ahmad Ganai, Naveed Anjum Chikan, Mohamed Faizal Abdul-Careem, and Nadeem Shabir. 2023. "An Investigation of the Antiviral Potential of Phytocompounds against Avian Infectious Bronchitis Virus through Template-Based Molecular Docking and Molecular Dynamics Simulation Analysis" Viruses 15, no. 4: 847. https://doi.org/10.3390/v15040847
APA StyleGul, I., Hassan, A., Haq, E., Ahmad, S. M., Shah, R. A., Ganai, N. A., Chikan, N. A., Abdul-Careem, M. F., & Shabir, N. (2023). An Investigation of the Antiviral Potential of Phytocompounds against Avian Infectious Bronchitis Virus through Template-Based Molecular Docking and Molecular Dynamics Simulation Analysis. Viruses, 15(4), 847. https://doi.org/10.3390/v15040847






