The Bovine Herpesvirus 1 Latency-Reactivation Cycle, a Chronic Problem in the Cattle Industry
Abstract
1. BoHV-1 Is a World-Wide Pathogen That Causes Several Diseases
2. BoHV-1 Is an Important Pathogen in Cattle
3. BoHV-1 Acute Infection
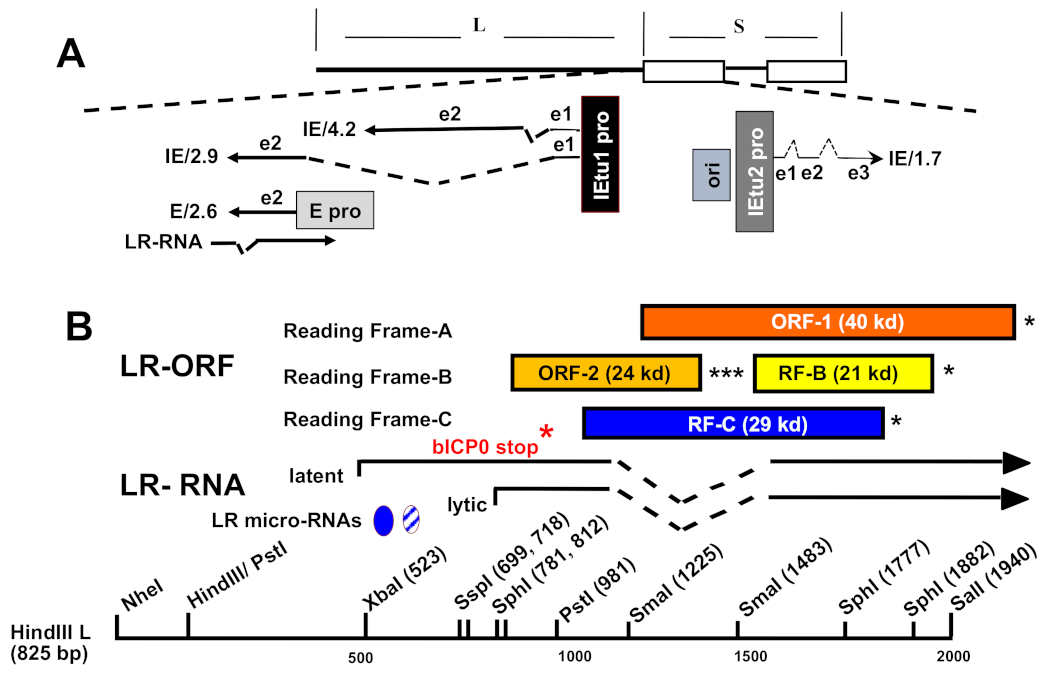
4. BoHV-1 Establishes Life-Long Latency in Neurons and Lymphoid Tissue
5. LR Gene Products Modulate the Latency-Reactivation Cycle
5.1. Identification and Characterization of Viral Gene Products Expressed during Latency
5.2. DEX Promotes BoHV-1 Productive Infection and Consistently Initiates Reactivation from Latency
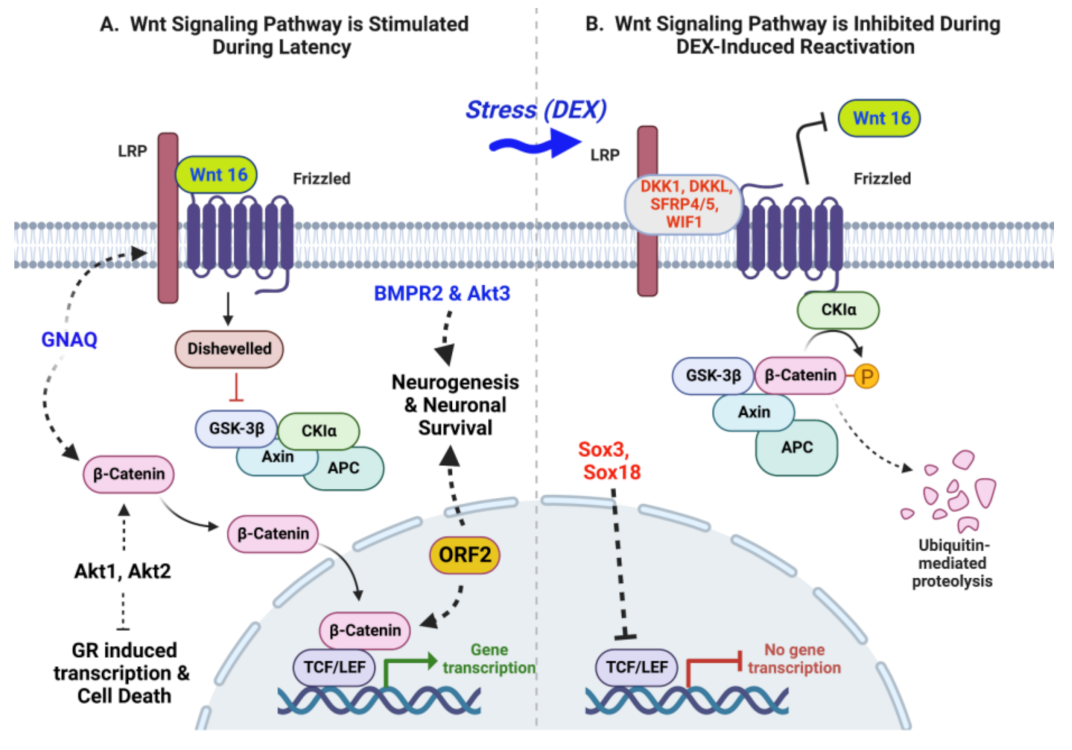
6. Regulation of Wnt/β-Catenin Signaling Pathway during Latency and DEX-Induced Reactivation
7. GR and Stress-Induced Transcription Factors Stimulate Key Viral Promoters
8. Androgen and Progesterone Receptors Stimulate IE and E Promoters Cooperatively with KLF4 and KLF15
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roizman, B.; Knipe, D.M. Herpes Simplex Viruses and Their Replication. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams & Wilkins: New York, NY, USA, 2001; Volume 2, pp. 2399–2459. [Google Scholar]
- Brown, F. The classification and nomenclature of viruses: Summary of results of meetings of the International Committee on Taxonomy of Viruses in Edmonton, Canada 1987. Intervirology 1989, 30, 181–186. [Google Scholar] [CrossRef]
- Plummer, G.; Goodheart, C.R.; Henson, D.; Bowling, C.P. A comparative study of the DNA density and behavior in tissue cultures of fourteen different herpesviruses. Virology 1969, 39, 134–137. [Google Scholar] [CrossRef]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef]
- Biswas, S.; Bandyopadhyay, S.; Dimri, U.; Patra, P.H. Bovine herpesvirus-1 (BHV-1)—A re-emerging concern in livestock: A revisit to its biology, epidemiology, diagnosis, and prophylaxis. Vet. Q. 2013, 33, 68–81. [Google Scholar] [CrossRef]
- Robinson, K.E.; Meers, J.; Gravel, J.L.; McCarthy, F.M.; Mahony, T.J. The essential and non-essential genes of Bovine herpesvirus 1. J. Gen. Virol. 2008, 89, 2851–2863. [Google Scholar] [CrossRef]
- Metzler, A.E.; Matile, H.; Gassmann, U.; Engels, M.; Wyler, R. European isolates of bovine herpesvirus 1: A comparison of restriction endonuclease sites, polypeptides, and reactivity with monoclonal antibodies. Arch. Virol. 1985, 85, 57–69. [Google Scholar] [CrossRef]
- van Oirschot, J.T. Bovine herpesvirus 1 in semen of bulls and the risk of transmission: A brief review. Vet. Q. 1995, 17, 29–33. [Google Scholar] [CrossRef]
- D’Arce, R.C.; Almeida, R.S.; Silva, T.C.; Franco, A.C.; Spilki, F.; Roehe, P.M.; Arns, C.W. Restriction endonuclease and monoclonal antibody analysis of Brazilian isolates of bovine herpesviruses types 1 and 5. Vet. Microbiol. 2002, 88, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Seal, B.S.; St Jeor, S. Isolation of infectious bovine rhinotracheitis virus from the soft-shelled tick, Ornithodoros coriaceus. Science 1982, 216, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Mars, M.H.; de Jong, M.C.; van Maanen, C.; Hage, J.J.; van Oirschot, J.T. Airborne transmission of bovine herpesvirus 1 infections in calves under field conditions. Vet. Microbiol. 2000, 76, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmied, H.U.; Kihm, U.; Bachmann, P.; Muller, K.H.; Ackermann, M. Transmission of IBR/IPV virus in bovine semen: A case report. Theriogenology 1986, 25, 439–443. [Google Scholar] [CrossRef]
- Jones, C.; Chowdhury, S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex, and development of improved vaccines. Adv. Anim. Health 2007, 8, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Chowdhury, S. Bovine herpesvirus type 1 (BHV-1) is an important cofactor in the bovine respiratory disease complex. In Veterinary Clinics of North America, Food Animal Practice, Bovine Respiratory Disease; Broderson, B., Cooper, V., Eds.; Elsevier: New York, NY, USA, 2010; Volume 26, pp. 303–321. [Google Scholar]
- Chase, C.; Fulton, R.W.; O’Toole, D.; Gillette, B.; Daly, R.F.; Perry, G.; Clement, T. Bovine herpesvirus 1 modified live vaccines for cattle reproduction: Balancing protection with undesired effects. Vet. Microbiol. 2017, 206, 69–77. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Miller, M.M.; Cavender, J.; Cornish, T. Pathology in Practice. Vet. Med. Today 2012, 241, 189–191. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Van Campen, H. Abortifacient vaccines and bovine herpesvirus-1. J. Am. Vet. Med. Assoc. 2010, 237, 259–260. [Google Scholar]
- Miller, J.; Van der Maaten, M.J. Early embryonic death in heifers after inoculation with bovine herpesvirus-1 and reactivation of latency virus in reproductive tissues. Am. J. Vet. Res. 1987, 48, 1555–1558. [Google Scholar]
- Perry, G.; Zimmerman, A.D.; Daly, R.F.; Butterbaugh, R.E.; Rhoades, J.; Schultz, D.; Harmon, A.; Chase, C. The effects of vaccination on serum hormone concentrations and conception rates in synchronized naive beef heifers. Theriongenology 2013, 79, 200–205. [Google Scholar] [CrossRef]
- Johnson, K.; Pendell, D.L. Market impacts of reducing the prevalence of bovine respiratory disease in United States beef cattle feedlots. Front. Vet. Sci. 2017, 4, 189. [Google Scholar] [CrossRef]
- Edwards, A.J. Respiratory diseases of feedlot cattle in teh central USA. Bov. Pract. 1996, 30, 5–7. [Google Scholar]
- Griffin, D. Economic impact associated with respiratory disease in beef cattle. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 367–377. [Google Scholar] [CrossRef]
- Kapil, S.; Basaraba, R.J. Infectious bovine rhinotracheitis, parainfluenza-3, and respiratory coronavirus. Bovine respiratory disease update. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 455–461. [Google Scholar] [CrossRef] [PubMed]
- NASS. Agricultural Statistics Board; U.S. Department of Agriculture: Washington, DC, USA, 1996.
- Songer, J.G.; Post, K.W. (Eds.) The Genera Mannheimia and Pasteurella; Elsevier Saunders: St. Louis, MO, USA, 2005. [Google Scholar]
- Frank, G. Bacteria as etiologic agents in bovine respiratory disease. In Proceedings of Bovine Respiratory Disease: A Symposium; Texas A&M University Press: College Station, TX, USA, 2010; Volume 2, pp. 381–394. [Google Scholar]
- Rice, J.A.; Carrasco-Medina, L.; Hodgins, D.; Shewen, P. Mannheimia haemolytica and bovine respiratory disease. Anim. Health Res. Rev. 2008, 8, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Highlander, S.K.; Fedorova, N.D.; Dusek, D.M.; Panciera, R.; Alvarez, L.E.; Renehart, C. Inactivation of Pasteurella (Mannheimia) haemolytica leukotoxin causes partial attenuation of virulence in a calf challenge model. Infect. Immun. 2000, 68, 3916–3922. [Google Scholar] [CrossRef] [PubMed]
- Highlander, S.K. Molecular genetic analysis of virulence in Mannheimia (Pasteurella) haemolytica. Front. Biosci. 2001, D1128–D1150. [Google Scholar] [CrossRef]
- Zecchinon, L.; Fett, T.; Desmecht, D. How Mannheimia haemolytica defeats host defense through a kiss of death mechanism. Vet. Res. 2005, 36, 133–156. [Google Scholar] [CrossRef]
- Hodgins, D.C.; Shewen, P.E. (Eds.) Pneumonic Pasteurellosis of Cattle; Oxford University Press: Cape Town, South Africa, 2004; Volume 3, pp. 1677–1684. [Google Scholar]
- Hodgson, P.D.; Aich, P.; Manuja, A.; Hokamp, K.; Roche, F.M.; Brinkman, F.S.L.; Potter, A.; Babiuk, L.A.; Griebel, P.J. Effect of stress on viral-bacterial synergy in bovine respiratoryt disease: Novel mechanisms to regulate inflammation. Comp. Funct. Genom. 2005, 6, 244–250. [Google Scholar] [CrossRef]
- Yates, W.D.; Babiuk, L.A.; Jericho, K.W. Viral-bacterial pneumonia in calves: Duration of the interaction between bovine herpesvirus 1 and Pasteurella haemolytica. Can. J. Comp. Med. 1983, 47, 257–264. [Google Scholar]
- Rivera-Rivas, J.J.; Kisiela, D.; Czuprynski, C.J. Bovine herpesvirus type 1 infection of bovine bronchial epithelial cells increases neutrophil adhesion and activation. Vet. Immunol. Immunopathol. 2009, 131, 167–176. [Google Scholar] [CrossRef]
- Leite, F.; Kuckleburg, C.; Atapattu, D.; Schulz, R.; Czuprynski, C.J. BHV-1 infection and inflammatory cytokines amplify the interaction between Mannheimia haemolytica lukotoxin with bovine peripheral blood mononuclear cells in vitro. Vet. Immunol. Immunopathol. 2004, 99, 193–202. [Google Scholar] [CrossRef]
- Carter, J.J.; Weinberg, A.D.; Pollard, A.; Reeves, R.; Magnuson, J.A.; Magnuson, N.S. Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovine herpesvirus 1. J. Virol. 1989, 63, 1525–1530. [Google Scholar] [CrossRef]
- Griebel, P.; Ohmann, H.B.; Lawman, M.J.; Babiuk, L.A. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J. Gen. Virol. 1990, 71, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.; Qualtiere, L.; Davis, W.C.; Gee, A.; Ohmann, H.B.; Lawman, M.J.; Babiuk, L.A. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1987, 1, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.J.; Qualtiere, L.; Davis, W.C.; Lawman, M.J.; Babiuk, L.A. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1987, 1, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Koppers-Lalic, E.A.; Reits, E.A.J.; Ressing, M.E.; Lipinska, A.D.; Abele, R.; Koch, J.; Rezende, M.M.; Admiraal, P.; van Leeuwen, D.; Bienkowsaka-Szewczyc, K.; et al. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc. Natl. Acad. Sci. USA 2005, 102, 5144–5149. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, C.; Eidmann, S.; Hariharan, M.J.; Sur, J.H.; Perry, G.A.; Srikumaran, S. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 1997, 10, 21–34. [Google Scholar] [CrossRef]
- Hariharan, M.J.; Nataraj, C.; Srikumaran, S. Down regulation of murine MHC class I expression by bovine herpesvirus 1. Viral Immunol. 1993, 6, 273–284. [Google Scholar] [CrossRef]
- Hinkley, S.; Hill, A.B.; Srikumaran, S. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res. 1998, 53, 91–96. [Google Scholar] [CrossRef]
- Neibergs, H.L.; Seabury, C.M.; Wojtowicz, A.J.; Wang, Z.; Scraggs, E.; Kiser, J.N.; Neupane, M.; Womack, J.E.; Van Eenennaam, A.; Hagevortm, G.R.; et al. Susceptibility loci revealed for bovine respiratory disease complex in pre-weaned holstein calves. BMC Genom. 2014, 15, 1164. [Google Scholar] [CrossRef]
- Wirth, U.; Vogt, B.; Schwyzer, M. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J. Virol. 1991, 65, 195–205. [Google Scholar] [CrossRef]
- Wirth, U.V.; Fraefel, C.; Vogt, B.; Vlcek, C.; Paces, V.; Schwyzer, M. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3’ coterminal and encode a putative zinc finger transactivator protein. J. Virol. 1992, 66, 2763–2772. [Google Scholar] [CrossRef]
- Fraefel, C.; Zeng, J.; Choffat, Y.; Engels, M.; Schwyzer, M.; Ackermann, M. Identification and zinc dependence of the bovine herpesvirus 1 transactivator protein BICP0. J. Virol. 1994, 68, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.; Bratanich, A.C.; Carpenter, D.; O’Hare, P. Protein and DNA elements involved in transactivation of the promoter of the bovine herpesvirus (BHV) 1 IE-1 transcription unit by the BHV alpha gene trans-inducing factor. J. Virol. 1994, 68, 4898–4909. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.; Walker, S.; Hayes, S.; O’Hare, P. The bovine herpesvirus alpha gene trans-inducing factor activates transcription by mechanisms different from those of its herpes simplex virus type 1 counterpart VP16. J. Virol. 1995, 69, 5209–5216. [Google Scholar] [CrossRef] [PubMed]
- Wirth, U.; Gunkel, K.; Engels, M.; Schwyzer, M. Spatial and temporal distribution of bovine herpesvirus 1 transcripts. J. Virol. 1989, 63, 4882–4889. [Google Scholar] [CrossRef]
- Jones, C. Alphaherpesvirus Latency: Its Role in Disease and Survival of the Virus in Nature. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Shatkin, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1998; Volume 51, pp. 81–133. [Google Scholar]
- Jones, C. Herpes Simplex Virus Type 1 and Bovine Herpesvirus 1 Latency. Clin. Microbiol. Rev. 2003, 16, 79. [Google Scholar] [CrossRef]
- Jones, C.; Geiser, V.; Henderson, G.; Jiang, Y.; Meyer, F.; Perez, S.; Zhang, Y. Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency. Vet. Micro 2006, 113, 199–210. [Google Scholar] [CrossRef]
- Jones, C. Regulation of innate immune responses by bovine herpesvirus 1 and infected cell protein 0. Viruses 2009, 1, 255–275. [Google Scholar] [CrossRef]
- Schang, L.; Jones, C. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J. Virol. 1997, 71, 6786–6795. [Google Scholar] [CrossRef]
- Jones, C. Latency of Bovine Herpesvirus 1 (BoHV-1) in Sensory Neurons. In Herpesviridae; InTech: Budapest, Hungary, 2016. [Google Scholar] [CrossRef]
- Inman, M.; Lovato, L.; Doster, A.; Jones, C. A Mutation in the Latency-Related Gene of Bovine Herpesvirus 1 Disrupts the Latency Reactivation Cycle in Calves. J. Virol. 2002, 76, 6771. [Google Scholar] [CrossRef]
- Winkler, M.T.C.; Doster, A.; Jones, C. Persistence and reactivation of bovine herpesvirus-1 (BHV-1) in tonsils of latently infected cattle. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J. Virol. 2000, 74, 5337–5346. [Google Scholar] [CrossRef]
- Mweene, A.S.; Okazaki, K.; Kida, H. Detection of viral genome in non-neural tissues of cattle experimentally infected with bovine herpesvirus 1. Jpn. J. Res. 1996, 44, 165–174. [Google Scholar]
- Perez, S.; Inman, M.; Doster, A.; Jones, C. Latency-related gene encoded by bovine herpesvirus 1 promotes virus growth and reactivation from latency in tonsils of infected calves. J. Clin. Micro 2005, 43, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.T.; Doster, A.; Jones, C. Bovine herpesvirus 1 can infect CD4(+) T lymphocytes and induce programmed cell death during acute infection of cattle. J. Virol. 1999, 73, 8657–8668. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K. Investigation of pseudorabies virus DNA and RNA in trigeminal ganglia and tonsil tissues of latently infected swine. Am. J. Vet. Res. 1995, 56, 45–50. [Google Scholar] [PubMed]
- Sabo, A.; Rajanci, A. Latent pseudorabies virus infection in pigs. Acta Virol. 1976, 20, 208–214. [Google Scholar]
- Borchers, K.; Wolfinger, U.; Ludwig, H. Latency-associated transcript of equine herpesvirus 4 in trigeminal ganglia of naturally infected horses. J. Gen. Virol. 1999, 80, 2165–2171. [Google Scholar] [CrossRef]
- Miyoshi, M.; Ishii, Y.; Takiguchi, M.; Takada, A.; Yasuda, J.; Hashimoto, A.; Okasaki, K.; Kida, H. Detection of canine herpesvirus DNA in ganglionic neurons and the lymph node lymphocytes of latently infected dogs. J. Vet. Med. Sci. 1999, 61, 375–379. [Google Scholar] [CrossRef]
- Sahin, F.; Gerceker, D.; Karasartova, D.; Ozsan, T.M. Detection of herpes simplex virus type 1 in addition to Epstein–Bar virus in tonsils using a new multiplex polymerase chain reaction assay. Diagn. Microbiol. Infect. Dis. 2007, 57, 47–51. [Google Scholar] [CrossRef]
- Kutish, G.; Mainprize, T.; Rock, D. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J. Virol. 1990, 64, 5730–5737. [Google Scholar] [CrossRef]
- Rock, D.; Lokensgard, J.; Lewis, T.; Kutish, G. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J. Virol. 1992, 66, 2484–2490. [Google Scholar] [CrossRef]
- Rock, D.L.; Beam, S.L.; Mayfield, J.E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J. Virol. 1987, 61, 3827–3831. [Google Scholar] [CrossRef] [PubMed]
- Rock, D.L.; Nesburn, A.B.; Ghiasi, H.; Ong, J.; Lewis, T.L.; Lokensgard, J.R.; Wechsler, S.L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 1987, 61, 3820–3826. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; da Silva, L.F.; Sinani, D. Regulation of the latency-reactivation cycle by products encoded by the bovine herpesvirus 1 (BHV-1) latency-related gene. J. Neurovirol. 2011, 17, 535–545. [Google Scholar] [CrossRef]
- Devireddy, L.R.; Jones, C. Alternative splicing of the latency-related transcript of bovine herpesvirus 1 yields RNAs containing unique open reading frames. J. Virol. 1998, 72, 7294–7301. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Schang, L.M.; Jones, C. Identification of gene products encoded by the latency-related gene of bovine herpesvirus 1. J. Virol. 1995, 69, 5345–5352. [Google Scholar] [CrossRef] [PubMed]
- Bratanich, A.C.; Hanson, N.D.; Jones, A.C. The latency-related gene of bovine herpesvirus 1 inhibits the activity of immediate-early transcription unit 1. Virology 1992, 191, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Bratanich, A.C.; Jones, C.J. Localization of cis-acting sequences in the latency-related promoter of bovine herpesvirus 1 which are regulated by neuronal cell type factors and immediate-early genes. J. Virol. 1992, 66, 6099–6106. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Delhon, G.; Bratanich, A.; Kutish, G.; Rock, D. Analysis of the transcriptional promoter which regulates the latency- related transcript of bovine herpesvirus 1. J. Virol. 1990, 64, 1164–1170. [Google Scholar] [CrossRef]
- Liu, Y.; Jones, C. Regulation of Notch-mediated transcription by a bovine herpesvirus 1 encoded protein (ORF2) that is expressed in latently infected sensory neurons. J. Neurovirol. 2016, 22, 518–528. [Google Scholar] [CrossRef]
- Devireddy, L.; Zhang, Y.; Jones, C. Cloning and initial characterization of an alternatively spliced transcript encoded by the bovine herpes virus 1 latency related (LR) gene. J. Neurovirol. 2003, 9, 612–622. [Google Scholar] [CrossRef]
- Shen, W.; Jones, C. Open reading frame 2, encoded by the latency-related gene of bovine herpesvirus 1, has antiapoptotic activity in transiently transfected neuroblastoma cells. J. Virol. 2008, 82, 10940–10945. [Google Scholar] [CrossRef]
- Workman, A.; Sinani, D.; Pittayakhajonwut, D.; Jones, C. A Protein (ORF2) Encoded by the Latency Related Gene of Bovine Herpesvirus 1 Interacts with Notch1 and Notch3. J. Virol. 2011, 85, 2536–2546. [Google Scholar] [CrossRef]
- Workman, A.L.; Zhu, L.; Keel, B.N.; Smith, T.P.L.; Jones, C. The Wnt signaling pathway is differentially expressed during the bovine herpesvirus 1 latency-reactivation cycle: Evidence that two proteinkinases associated with neuronal survival, Akt3 and BMPR2, are expressed at higher levels during latency. J. Virol. 2018, 92, e01937-17. [Google Scholar] [CrossRef]
- Zhu, L.; Workman, A.; Jones, C. A potential role for a beta-catenin coactivator (high mobility group AT-hook 1 protein) during the latency-reactivation cycle of bovine herpesvirus 1. J. Virol. 2017, 91, e02132-16. [Google Scholar] [CrossRef]
- Meyer, F.; Jones, C. The cellular transcription factor, CCAAT enhancer-binding protein alpa (C/EBP-a) has the potential to activate the bovine herpesvirus 1 immediate early transcription unit 1 promoter. J. Neurovirol. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Meyer, F.; Perez, S.; Geiser, V.; Sintek, M.; Inman, M.; Jones, C. A protein encoded by the bovine herpes virus 1 (BHV-1) latency related gene interacts with specific cellular regulatory proteins, including the CCAAT enhancer binding protein alpha (C/EBP-a). J. Virol. 2007, 81, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.; Perez, S.; Doster, A.; Jones, C. Dexamethasone treatment of calves latently infected with bovine herpesvirus 1 (BHV-1) leads to activation of the bICP0 early promoter, in part by the cellular transcription factor C/EBP-alpha. J. Virol. 2009, 83, 8800–8809. [Google Scholar] [CrossRef] [PubMed]
- Sinani, D.; Jones, C. Localization of sequences in a protein (ORF2) encoded by the latency-related gene of bovine herpesvirus 1 that inhibits apoptosis and interferes with Notch1-mediated trans-activation of the bICP0 promoter. J. Virol. 2011, 85, 12124–12133. [Google Scholar] [CrossRef] [PubMed]
- Sinani, D.; Frizzo da Silva, L.; Jones, C. A bovine herpesvirus 1 protein expressed in latently infected neurons (ORF2) promotes neurite sprouting in the presence of activated Notch1 or Notch3. J. Virol. 2013, 87, 1183–1192. [Google Scholar] [CrossRef]
- Jaber, T.; Workman, A.; Jones, C. Small noncoding RNAs encoded within the bovine herpesvirus 1 latency-related gene can reduce steady-state levels of infected cell protein 0 (bICP0). J. Virol. 2010, 84, 6297–6307. [Google Scholar] [CrossRef]
- Jiang, Y.; Inman, M.; Zhang, Y.; Posadas, N.A.; Jones, C. A mutation in the latency related gene of bovine herpesvirus 1 (BHV-1) inhibits protein expression of a protein from open reading frame 2 (ORF-2) and an adjacent reading frame during productive infection. J. Virol. 2004, 78, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Perez, S.; Jiang, Y.; Zhou, Y.; Henderson, G.; Jones, C. Identification of a novel protein encoded by the latency-related gene of bovine herpesvirus 1. J. Neurovirol. 2007, 13, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Lovato, L.; Inman, M.; Henderson, G.; Doster, A.; Jones, C. Infection of cattle with a bovine herpesvirus 1 (BHV-1) strain that contains a mutation in the latency related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency. J. Virol. 2003, 77, 4848–4857. [Google Scholar] [CrossRef] [PubMed]
- Perng, G.-C.; Maguen, B.; Jin, L.; Mott, K.R.; Osorio, N.; Slanina, S.M.; Yukht, A.; Ghiasi, H.; Nesburn, A.B.; Inman, M.; et al. Wechsler. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 2002, 76, 1224–1235. [Google Scholar] [CrossRef]
- Mott, K.R.; Osorio, N.; Jin, L.; Brick, D.J.; Naito, J.; Cooper, J.; Henderson, G.; Inman, M.; Jones, C.; Wechsler, S.L.; et al. The bovine herpesvirus-1 LR ORF2 is critical for this gene’s ability to restore the high wild-type reactivation phenotype to a herpes simplex virus-1 LAT null mutant. J. Gen. Virol. 2003, 84, 2975–2985. [Google Scholar] [CrossRef]
- Barsam, C.A.; Brick, D.J.; Jones, C.; Wechsler, S.L.; Perng, G.C. A viral model for corneal scarring and neovascularization following ocular infection of rabbits with a herpes simplex virus type 1 (HSV-1) mutant. Cornea 2005, 24, 460–466. [Google Scholar] [CrossRef]
- Jones, C.; Newby, T.J.; Holt, T.; Doster, A.; Stone, M.; Ciacci-Zanella, J.; Webster, C.J.; Jackwood, M.W. Analysis of latency in cattle after inoculation with a temperature sensitive mutant of bovine herpesvirus 1 (RLB106). Vaccine 2000, 18, 3185–3195. [Google Scholar] [CrossRef]
- Workman, A.; Eudy, J.; Smith, L.; da Silva, L.F.; Sinani, D.; Bricker, H.; Cook, E.; Doster, A.; Jones, C. Cellular transcription factors induced in trigeminal ganglia during dexamethasone-induced reactivation from latency stimulate bovine herpesvirus 1 productive infection and certain viral promoters. J. Virol. 2012, 86, 2459–2473. [Google Scholar] [CrossRef]
- Brown, G.; Field, H. Experimental reactivation of bovine herpesvirus 1 (BHV-1) by means of corticosteroids in an intranasal rabbit model. Arch. Virol. 1990, 112, 81–101. [Google Scholar] [CrossRef]
- Ackermann, M.; Peterhans, E.; Wyler, R. DNA of bovine herpesvirus type 1 in the trigeminal ganglia of latently infected calves. Am. J. Vet. Res. 1982, 43, 36–40. [Google Scholar]
- Hage, J.; Glas, R.; Westra, H.; Maris-Veldhuis, M.; Van Oirschot, J.; Rijsewijk, F. Reactivation of latent bovine herpesvirus 1 in cattle seronegative to glycoproteins gB and gE. Vet. Microbiol. 1998, 60, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Homan, E.; Easterday, B. Experimental latent and recrudescent bovine herpesvirus-1 infections in calves. Am. J. Vet. Res. 1983, 44, 309–313. [Google Scholar] [PubMed]
- Sheffy, B.E.; Davies, D.H. Reactivation of a Bovine Herpesvirus After Corticosteroid Treatment. Proc. Soc. Exp. Biol. Med. 1972, 140, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Smoak, K.L.J.; Cidlowski, A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Aging Dev. 2004, 125, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Kook, I.; Henley, C.; Meyer, F.; Hoffmann, F.G.; Jones, C. Bovine herpesvirus 1 productive infection and immediate early transcription unit 1 promoter are stimulated by the synthetic corticosteroid dexamethasone. Virology 2015, 484, 377–385. [Google Scholar] [CrossRef]
- El-mayet, F.; Sawant, L.; Thunuguntla, P.; Zhao, J.; Jones, C. Two pioneer transcription factors, Krüppel-like transcription factor 4 and glucocorticoid receptor, cooperatively transactivate the bovine herpesvirus 1 ICP0 early promoter and stimulate productive infection. J. Virol. 2020, 94, e01670-19. [Google Scholar] [CrossRef]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef]
- Kook, I.; Jones, C. The serum and glucocorticoid-regulated protein kinases (SGK) stimulate bovine herpesvirus 1 and herpes simplex virus 1 productive infection. Virus Res. 2016, 222, 106–112. [Google Scholar] [CrossRef]
- Frizzo da Silva, L.I.K.; Doster, A.; Jones, C. Bovine herpesvirus 1 regulatory proteins, bICP0 and VP16, are readily detected in trigeminal ganglionic neurons expressing the glucocorticoid receptor during the early stages of reactivation from latency. J. Virol. 2013, 87, 11214–11222. [Google Scholar] [CrossRef]
- Kook, I.; Doster, A.; Jones, C. Bovine herpesvirus 1 regulatory proteins are detected in trigeminal ganglionic neurons during the early stages of stress-induced escape from latency. J. Neurovirol. 2015, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Q.; Jones, C. The bovine herpesvirus 1 regulatory proteins, bICP4 and bICP22, are expressed during the escape from latency. J. Neuovirol. 2019, 25, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Toomer, G.; Workman, A.; Harrison, K.S.; Stayton, E.; Hoyt, P.R.; Jones, C. Stress Triggers Expression of Bovine Herpesvirus 1 Infected Cell Protein 4 (bICP4) RNA during Early Stages of Reactivation from Latency in Pharyngeal Tonsil. J. Virol. 2022, in press. [CrossRef]
- Liu, T.; Liu, X.; Wang, H.; Moon, R.T.; Malbon, C.C. Activation of a rat frizzled-1 promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Galpha(q) and Galpha(o) function. J. Biol. Chem. 1999, 274, 33539–33544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Robbins, J.S.; Kimmel, A.R. Rapid, Wnt-induced changes in GSK3beta association that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr. Biol. 2005, 15, 1989–1997. [Google Scholar] [CrossRef]
- Guha, U.; Gomes, W.; Samantra, J.; Guptam, M.; Rice, F.; Kessler, J. Target-derived BMP signaling limits sensory neuron number and the extent of peripheral innervation in vivo. Development 2004, 131, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Hodge, L.; Klassen, M.; Han, B.; Yiu, G.; Hurrel, J.; Howell, A.; Rosseau, G.; Lemaigre, F.; Tressier-Lavigne, M.; Wang, F. Retrograde BMP signaling regulates trigeminal sensory neuron identitities and the formation of precise face maps. Neuron 2007, 55, 572–586. [Google Scholar] [CrossRef]
- Farkas, L.; Jasai, J.; Unsicker, K.; Krieglstein, K. Characterization of bone morphogenetic protein family members as neurotropic factors for cultured sensory neurons. Neuroscience 1999, 92, 227–235. [Google Scholar] [CrossRef]
- Diez, H.; Garrido, J.J.; Wandosell, F. Specific Roles of Akt iso forms in apoptosis and axon growth regulation in nuerons. PLoS ONE 2012, 74, e32715. [Google Scholar]
- Xie, R.; Cheng, M.; Li, M.; Xiong, X.; Daadi, M.; Sapolsky, R.; Zhao, H. Akt isoforms differentially protect against stroke-induced neuronal injury by regulating mTOR activities. J. Cereb. Blood Flow Metab. 2013, 33, 1875–1885. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/Β−catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.C. Wnt signaling in the vertebrate central nervous system: From axon guidance to synaptic function. Cold Spring Harb. Perpect. Biol. 2012, 4, a008003. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clever, H. Wnt/beta-catenin signaling, diseases, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Cisternas, P.; Inestrosa, N.C. Role of Wnt signaling in central nervous system injury. Mol. Neurobiol. 2016, 53, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Rosso, S.B.; Inestrosa, N.C. Wnt signaling in neuronal maturation and synaptogenesis. Cell. Neurosci. 2013, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, L.; Wijesekera, N.; Jones, C. Specific Akt family members impair stress mediated transactivation of viral promoters and enhance neuronal differentiation: Important functions for maintaining latency. J. Virol. 2020, 94, e00901–e00920. [Google Scholar] [CrossRef]
- Liu, Y.; Hancock, M.; Workman, A.; Doster, A.; Jones, C. Beta-catenin, a transcription factor activated by canonical Wnt signaling, is expressed in sensory neurons of calves latently infected with bovine herpesvirus 1. J. Virol. 2016, 90, 3148–3159. [Google Scholar] [CrossRef]
- Zhao, J.; Wijesekera, N.; Jones, C. Inhibition of Stress-Induced Viral Promoters by a Bovine Herpesvirus 1 Non-Coding RNA and the Cellular Transcription Factor, beta-Catenin. Int. J. Molec. Sci. 2021, 22, 519. [Google Scholar] [CrossRef]
- Zorn, A.M. Wnt signaling: Antagonistic Dicckopf. Curr. Biol. 2001, 11, R592–R595. [Google Scholar] [CrossRef]
- Niehrs, C. Function and biological roles of the dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef]
- Magana, R.; Giles, N.; Adcroft, K.; Keeney, D.; Wood, F.; Fear, M.; Dharmarajan, A. Secreted frizzled related protein-4 (sFRP4) promotes epidermal differentiation and apoptosis. Biochem. Biophys. Res. Comm. 2008, 377, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanalakshmi, G.; Arfuso, F.; Millward, M.; Dharmarajan, A.; Warrier, S. Secreted frizzled-related protein 4 inhibits glioma stem-like cells by reversing epithelial to mesenchymal transition, inducing apoptosis and decreasing cancer stem cell properties. PLoS ONE 2015, 10, e0127517. [Google Scholar]
- Lacher, M.; Siegenthaler, A.; Jager, R.; Yan, X.; Hett, S.; Xuan, L.; Saurer, S.; Lareu, R.; Dharmarajan, M.; Friis, R. Role of DDC-4/sFRP-4, a secreted frizzled-related protein, as the onser of apoptosis in mammary involution. Cell Death Differ. 2003, 10, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Kormish, J.; Sinner, D.; Zorn, A.M. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn. 2010, 239, 56–68. [Google Scholar] [PubMed]
- Prasad, A.; Remick, J.; Zeichner, S.L. Activation of human herpesvirus replication by apoptosis. J. Virol. 2013, 87, 10641–10650. [Google Scholar] [CrossRef]
- Du, T.; Zhou, G.; Roizman, B. Induction of apoptosis accelerates reactivation from latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc. Natl. Acad. Sci. USA 2012, 109, 14616–14621. [Google Scholar] [CrossRef]
- El-mayet, F.; El-Habbaa, A.S.; El-Bagoury, G.F.; Sharawi, S.S.A.; El-Nahas, E.M.; Jones, C. Transcription Factors Have the Potential to Synergistically Stimulate Bovine Herpesvirus 1 Transcription and Reactivation from Latency. In Transcriptional Regulation; Kais, G., Ed.; INTECH: Rejeka, Croatia, 2018; Volume 1, pp. 36–54. [Google Scholar]
- El-mayet, F.S.; El-Habbaa, A.; D’Offay, J.; Jones, C. Synergistic activation of bovine herpesvirus 1 productive infection and viral regulatory promoters by the progesterone receptor and Krüppel-like transcription factor 15. J. Virol. 2019, 93, e01519-18. [Google Scholar] [CrossRef]
- Ostler, J.B.; Sawant, L.; Harrison, K.S.; Jones, C. Regulation of neurotropic herpesvirus productive infection and latency-reactivation cycle by glucocorticoid receptor and stress-induced transcription factors. In Hormones, Regulators, and Viruses; Litwack, G., Ed.; Elsiever: Amsterdam, The Netherlands, 2021; Volume 117. [Google Scholar]
- Sawant, L.; Ostler, J.B.; Jones, C. A Pioneer Transcription Factor and Type I Nuclear Hormone Receptors Synergistically Activate the Bovine Herpesvirus 1 Infected Cell Protein 0 (ICP0) Early Promoter. J. Virol. 2021, 95, e00768-21. [Google Scholar] [CrossRef]
- El-Mayet, F.S.; Sawant, L.; Thungunutla, P.; Jones, C. Combinatorial effects of the glucocorticoid receptor and Krüppel-like transcription factor 15 on bovine herpesvirus 1 transcription and productive infection. J. Virol. 2017, 91, e00904-17. [Google Scholar] [CrossRef]
- Kaczynski, J.; Cook, T.; Urrutia, R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003, 4, 206.201–206.208. [Google Scholar] [CrossRef]
- Bieker, J.J. Krüppel-like factors: Three fingers in many pies. J. Biol. Chem. 2001, 276, 34355–34358. [Google Scholar] [CrossRef] [PubMed]
- Polman, J.A.E.; Welten, J.E.; Bosch, D.S.; de Jonge, R.T.; Balog, J.; van der Maarel, S.M.; de Kloet, E.R.; Datson, N.A. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci. 2012, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.; Jones, C. Bovine herpesvirus 1 (BoHV1) productive infection and bICP0 early promoter activity are stimulated by E2F1. J. Virol. 2010, 84, 6308–6317. [Google Scholar] [CrossRef] [PubMed]
- Hapgood, J.P.; Avenant, C.; Moliki, J.M. Glucocorticoid-independent modulation of GR activity: Implications for immunotherapy. Pharm. Ther. 2016, 165, 93–113. [Google Scholar] [CrossRef]
- Scheschowitsch, K.; Leite, J.A.; Assreuy, J. New Insights in Glucocorticoid Receptor Signaling-More Than Just a Ligand-Binding Receptor. Front. Endocrinol. 2017, 8, 16. [Google Scholar] [CrossRef]
- Biddie, S.C.; Hager, G.L. Glucocorticoid receptor dynamics and gene regulation. Stress 2009, 12, 193–205. [Google Scholar] [CrossRef]
- Sawant, L.; Thunuguntla, P.; Jones, C. Cooperative activation of bovine herpesvirus 1 productive infection and viral regulatory promoters by androgen receptor and Krüppel-like transcription factors 4 and 15. Virology 2021, 552, 63–72. [Google Scholar] [CrossRef]
- Sawant, L.; Wijesekera, N.; Jones, C. Pioneer transcription factors, progesterone receptor and Krüppel like transcription factor 4, cooperatively stimulate the bovine herpesvirus 1 ICP0 early promoter and productive late protein expression. Virus Res. 2020, 288, 198115. [Google Scholar] [CrossRef]
- Azeez, J.M.; Susmi, T.R.; Remadevi, V.; Ravindran, V.; Sasikumar Sujatha, A.; Ayswarya, R.N.S.; Sreeja, S. New insights into the functions of progesterone receptor (PR) isoforms and progesterone signaling. Am. J. Cancer Res. 2021, 11, 5214–5232. [Google Scholar]
- Claessens, F.; Joniau, S.; Helsen, C. Comparing the rules of engagement of androgen and glucocorticoid receptors. Cell. Mol. Life Sci. 2017, 74, 2217–2228. [Google Scholar] [CrossRef]
- Rundlett, S.E.; Miesfeld, R.L. Quantitative differences in androgen and glucocorticoid receptor DNA binding properties contribute to receptor-selective transcriptional regulation. Mol. Cell. Endocrinol. 1995, 109, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pecci, A.; Ogara, M.F.; Sanz, R.T.; Vicent, G.P. Choosing the right partner in hormone-dependent gene regulation: Glucocorticoid and progesterone receptors crosstalk in breast cancer cells. Front. Endocrinol. 2022, 13, 1037177. [Google Scholar] [CrossRef] [PubMed]
- El-Mayet, F.S.; Toomer, G.; Ostler, J.B.; Harrison, K.S.; Santos, V.C.; Wijesekera, N.; Stayton, E.; Ritchey, J.; Jones, C. Progesterone Sporadically Induces Reactivation from Latency in Female Calves but Proficiently Stimulates Bovine Herpesvirus 1 Productive Infection. J. Virol. 2022, 96, e0213021. [Google Scholar] [CrossRef] [PubMed]
- El-Mayet, F.S.; Sawant, L.; Wijesekera, N.; Jones, C. Progesterone increases the incidence of bovine herpesvirus 1 reactivation from latency and stimulates productive infection. Virus Res. 2020, 276, 197803. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostler, J.B.; Jones, C. The Bovine Herpesvirus 1 Latency-Reactivation Cycle, a Chronic Problem in the Cattle Industry. Viruses 2023, 15, 552. https://doi.org/10.3390/v15020552
Ostler JB, Jones C. The Bovine Herpesvirus 1 Latency-Reactivation Cycle, a Chronic Problem in the Cattle Industry. Viruses. 2023; 15(2):552. https://doi.org/10.3390/v15020552
Chicago/Turabian StyleOstler, Jeffery B., and Clinton Jones. 2023. "The Bovine Herpesvirus 1 Latency-Reactivation Cycle, a Chronic Problem in the Cattle Industry" Viruses 15, no. 2: 552. https://doi.org/10.3390/v15020552
APA StyleOstler, J. B., & Jones, C. (2023). The Bovine Herpesvirus 1 Latency-Reactivation Cycle, a Chronic Problem in the Cattle Industry. Viruses, 15(2), 552. https://doi.org/10.3390/v15020552





