Correlates of Healthy Aging in Geriatric HIV (CHANGE HIV)—CTN 314
Abstract
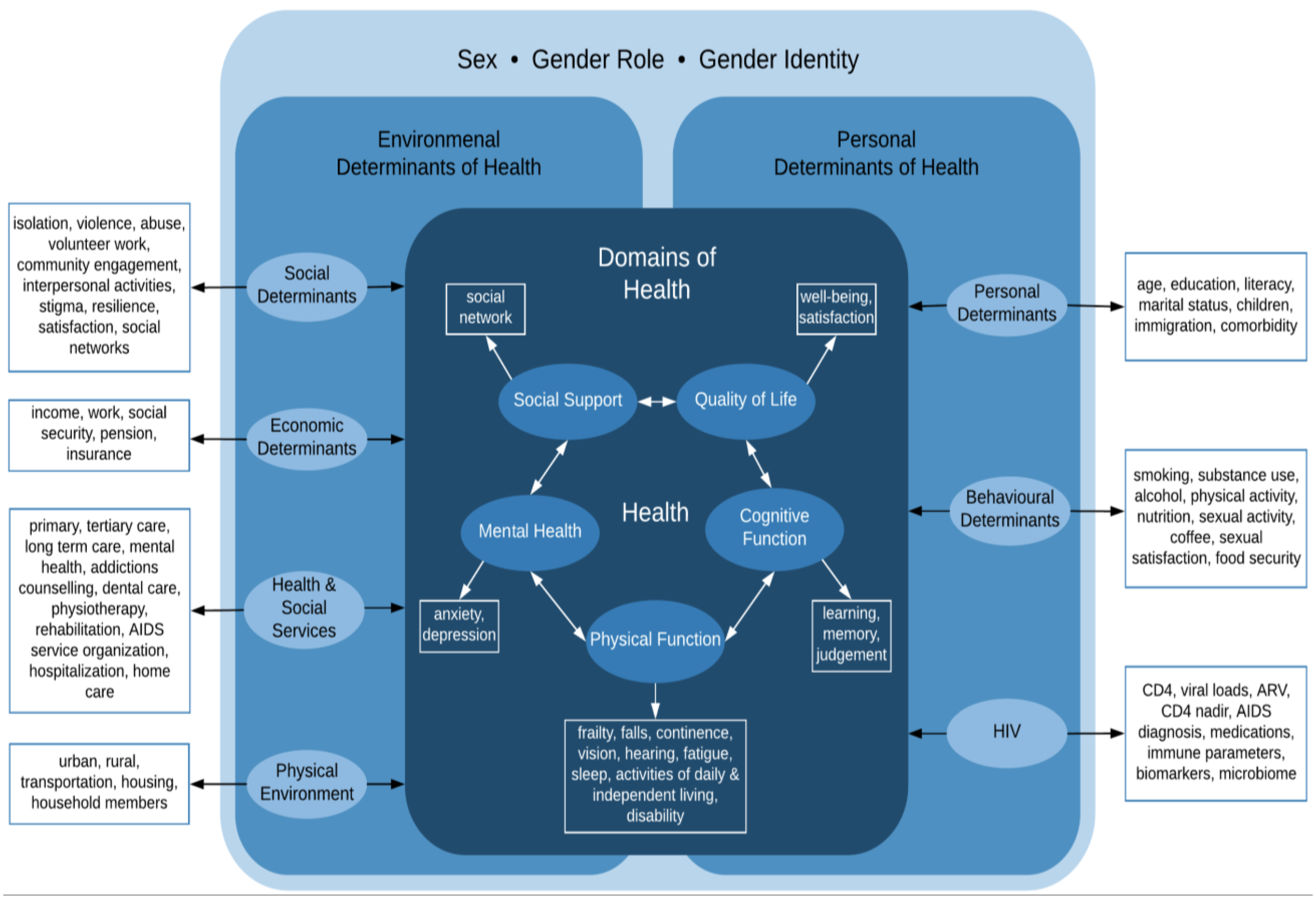
:1. Introduction
2. Methods
2.1. Study Administration
2.2. Source Population, Eligibility, and Recruitment
2.3. Data Collection
2.4. Data Management
3. Results
3.1. Enrolment, Retention and Mortality Rate
3.2. Participant Demographics
3.3. Participant Medical Background
3.4. Participant Frailty, Cognitive Function, Loneliness, and Resilience
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trickey, A.; May, M.T.; Vehreschild, J.-J.; Obel, N.; Gill, M.J.; Crane, H.M.; Boesecke, C.; Patterson, S.; Grabar, S.; Cazanave, C.; et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Estimates of HIV Incidence, Prevalence and Canada’s Progress on Meeting the 90–90–90 HIV Targets. 2020. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/estimates-hiv-incidence-prevalence-canada-meeting-90-90-90-targets-2020.html (accessed on 10 December 2022).
- Public Health Agency of Canada. HIV in Canada: 2021 Surveillance Highlights. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/hiv-2021-surveillance-highlights.html (accessed on 10 December 2022).
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the Gap: Increases in Life Expectancy among Treated HIV-Positive Individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; De Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Brothers, T.D.; Zona, S.; Stentarelli, C.; Carli, F.; Malagoli, A.; Santoro, A.; Menozzi, M.; Mussi, C.; Mussini, C.; et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. Aids 2015, 29, 1633–1641. [Google Scholar] [CrossRef]
- O’Brien, K.K.; Solomon, P.; Bergin, C.; O’Dea, S.; Stratford, P.; Iku, N.; Bayoumi, A.M. Reliability and validity of a new HIV-specific questionnaire with adults living with HIV in Canada and Ireland: The HIV Disability Questionnaire (HDQ). Health Qual. Life Outcomes 2015, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Bruce, B.; Fries, J.F. The Stanford Health Assessment Questionnaire: Dimensions and Practical Applications. Health Qual. Life Outcomes 2003, 1, 20. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Tassiopoulos, K.; Abdo, M.; Wu, K.; Koletar, S.L.; Palella, F.J.; Kalayjian, R.; Taiwo, B.; Erlandson, K.M. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. Aids 2017, 31, 2287–2294. [Google Scholar] [CrossRef]
- Elsawy, B.; Higgins, K.E. The geriatric assessment. Am. Fam. Physician 2011, 83, 48–56. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K. Prospective evaluation of visual acuity assessment: A comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Am. J. Ophthalmol. 2009, 107, 311–324. [Google Scholar]
- Ventry, I.M.; Weinstein, B.E. The hearing handicap inventory for the elderly: A new tool. Ear Hear 1982, 3, 128–134. [Google Scholar] [CrossRef]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988, 14, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Collet, L.; Cottraux, J. The shortened Beck depression inventory (13 items). Study of the concurrent validity with the Hamilton scale and Widlocher’s retardation scale. Encephale 1986, 12, 77–79. [Google Scholar]
- Gana, K.; Bailly, N.; Broc, G.; Cazauvieilh, C.; Boudouda, N.E. The Geriatric Depression Scale: Does it measure depressive mood, depressive affect, or both? Int. J. Geriatr. Psychiatry 2016, 32, 1150–1157. [Google Scholar] [CrossRef]
- Himmelfarb, S.; Murrell, S.A. Reliability and Validity of Five Mental Health Scales in Older Persons. J. Gerontol. 1983, 38, 333–339. [Google Scholar] [CrossRef]
- Mohebbi, M.; Nguyen, V.; McNeil, J.J.; Woods, R.L.; Nelson, M.R.; Shah, R.C.; Storey, E.; Murray, A.M.; Reid, C.M.; Kirpach, B.; et al. Psychometric properties of a short form of the Center for Epidemiologic Studies Depression (CES-D-10) scale for screening depressive symptoms in healthy community dwelling older adults. Gen. Hosp. Psychiatry 2018, 51, 118–125. [Google Scholar] [CrossRef]
- Lacy, M.; Kaemmerer, T.; Czipri, S. Standardized mini-mental state examination scores and verbal memory performance at a memory center: Implications for cognitive screening. Am. J. Alzheimers. Dis. Other Demen. 2015, 30, 145–152. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Henrich, G.H.P. Questions on Life Satisfaction (FLZ M): A short questionnaire for assessing subjective quality of life. Eur. J. Psychol. Assess. 2000, 16, 150–159. [Google Scholar] [CrossRef]
- Wolfs, C.A.; Dirksen, C.D.; Kessels, A.; Willems, D.C.; Verhey, F.R.; Severens, J.L. Performance of the EQ-5D and the EQ-5D+C in elderly patients with cognitive impairments. Health Qual. Life Outcomes 2007, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Schnall, R.; Liu, J.; Cho, H.; Hirshfield, S.; Siegel, K.; Olender, S. A Health-Related Quality-of-Life Measure for Use in Patients with HIV: A Validation Study. AIDS Patient Care STDs 2017, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Koenig, H.G.; Westlund, R.E.; George, L.K.; Hughes, D.C.; Blazer, D.G.; Hybels, C. Abbreviating the Duke Social Support Index for Use in Chronically Ill Elderly Individuals. Psychosomatics 1993, 34, 61–69. [Google Scholar] [CrossRef]
- Sherbourne, C.D.; Stewart, A.L. The MOS social support survey. Soc. Sci. Med. 1991, 32, 705–714. [Google Scholar] [CrossRef]
- Ausín, B.; Muñoz, M.; Martin, T.; Pérez-Santos, E.; Castellanos, M. Confirmatory factor analysis of the Revised UCLA Loneliness Scale (UCLA LS-R) in individuals over 65. Aging Ment. Health 2018, 23, 345–351. [Google Scholar] [CrossRef]
- Jaspers, L.; Schoufour, J.D.; Erler, N.S.; Darweesh, S.K.; Portegies, M.L.; Sedaghat, S.; Lahousse, L.; Brusselle, G.G.; Stricker, B.H.; Tiemeier, H.; et al. Development of a Healthy Aging Score in the Population-Based Rotterdam Study: Evaluating Age and Sex Differences. J. Am. Med. Dir. Assoc. 2017, 18, 276.e1–276.e7. [Google Scholar] [CrossRef]
- Sebastiani, G.; Rollet-Kurhajec, K.C.; Pexos, C.; Gilmore, N.; Klein, M. Incidence and Predictors of Hepatic Steatosis and Fibrosis by Serum Biomarkers in a Large Cohort of Human Immunodeficiency Virus Mono-Infected Patients. Open Forum Infect. Dis. 2015, 2, ofv015. [Google Scholar] [CrossRef]
- Rourke, S.B.; Gardner, S.; Burchell, A.N.; Raboud, J.; Rueda, S.; Bayoumi, A.M.; Loutfy, M.; Cooper, C.; Smieja, M.; Taylor, D.; et al. Cohort Profile: The Ontario HIV Treatment Network Cohort Study (OCS). Leuk. Res. 2012, 42, 402–411. [Google Scholar] [CrossRef]
- Loutfy, M.; Greene, S.; Kennedy, V.L.; Lewis, J.; Thomas-Pavanel, J.; Conway, T.; de Pokomandy, A.; O’Brien, N.; Carter, A.; Tharao, W.; et al. Establishing the Canadian HIV Women’s Sexual and Reproductive Health Cohort Study (CHIWOS): Operationalizing Community-based Research in a Large National Quantitative Study. BMC Med. Res. Methodol. 2016, 16, 101. [Google Scholar] [CrossRef]
- Štulhofer, A.; Buško, V.; Brouillard, P. Development and Bicultural Validation of the New Sexual Satisfaction Scale. J. Sex Res. 2009, 47, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.E.; Lin, L.; Weinfurt, K.P. Sexual function and satisfaction among heterosexual and sexual minority U.S. adults: A cross-sectional survey. PLoS ONE 2017, 12, e0174981. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.B.; Saeed, S.; Yang, H.; Cohen, J.; Conway, B.; Cooper, C.; Côté, P.; Cox, J.; Gill, J.; Haase, D.; et al. Cohort Profile: The Canadian HIV-Hepatitis C Co-infection Cohort Study. Leuk. Res. 2009, 39, 1162–1169. [Google Scholar] [CrossRef]
- Hetling, A.; Hoge, G.L.; Postmus, J.L. What is economic self-sufficiency? Validating a measurement scale for policy, practice, and research. J. Poverty 2016, 20, 214–235. [Google Scholar] [CrossRef]
- Eakman, A.M.; Carlson, M.E.; Clark, F.A. The Meaningful Activity Participation Assessment: A Measure of Engagement in Personally Valued Activities. Int. J. Aging Hum. Dev. 2010, 70, 299–317. [Google Scholar] [CrossRef]
- Wagnild, G. A Review of the Resilience Scale. J. Nurs. Meas. 2009, 17, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, S.C.; Simbayi, L.C.; Cloete, A.; Mthembu, P.P.; Mkhonta, R.N.; Ginindza, T. Measuring AIDS stigmas in people living with HIV/AIDS: The Internalized AIDS-Related Stigma Scale. AIDS Care 2008, 21, 87–93. [Google Scholar] [CrossRef]
- Cyranowski, J.M.; Zill, N.; Bode, R.; Butt, Z.; Kelly, M.A.R.; Pilkonis, P.A.; Salsman, J.M.; Cella, D. Assessing social support, companionship, and distress: National Institute of Health (NIH) Toolbox Adult Social Relationship Scales. Health Psychol. 2013, 32, 293–301. [Google Scholar] [CrossRef]
- Carver, L.F.; Vafaei, A.; Guerra, R.; Freire, A.; Phillips, S.P. Gender Differences: Examination of the 12-Item Bem Sex Role Inventory (BSRI-12) in an Older Brazilian Population. PLoS ONE 2013, 8, e76356. [Google Scholar] [CrossRef]
- Stern, B.B.; Barak, B.; Gould, S.J. Sexual identity scale: A new self-assessment measure. Sex Roles 1987, 17, 503–519. [Google Scholar] [CrossRef]
- Arslantaş, H.; Adana, F.; Ergin, F.A.; Kayar, D.; Acar, G. Loneliness in Elderly People, Associated Factors and Its Correlation with Quality of Life: A Field Study from Western Turkey. Iran. J. Public Health 2015, 44, 43–50. [Google Scholar]
- Davidson, J.R.T. Connor-Davidson Resilience Scale (CDRISC) Manual. Unpublished. 19 August 2018. Available online: www.cdrisc.com (accessed on 10 December 2022).
- Greene, M.; Hessol, N.A.; Perissinotto, C.; Zepf, R.; Parrott, A.H.; Foreman, C.; Whirry, R.; Gandhi, M.; John, M. Loneliness in Older Adults Living with HIV. AIDS Behav. 2017, 22, 1475–1484. [Google Scholar] [CrossRef]
- Blanco-Reina, E.; Aguilar-Cano, L.; García-Merino, M.R.; Ocaña-Riola, R.; Valdellós, J.; Bellido-Estévez, I.; Ariza-Zafra, G. Assessing Prevalence and Factors Related to Frailty in Community-Dwelling Older Adults: A Multinomial Logistic Analysis. J. Clin. Med. 2021, 10, 3576. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef] [PubMed]
- Kooij, K.W.; Wit, F.W.; Schouten, J.; van der Valk, M.; Godfried, M.H.; Stolte, I.G.; Prins, M.; Falutz, J.; Reiss, P. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. Aids 2016, 30, 241–250. [Google Scholar] [CrossRef]
- Doctor, J.; Winston, A.; Vera, J.; Post, F.A.; Boffito, M.; Mallon, P.; Anderson, J.; Durkina, M.; Williams, J.; Johnson, M.; et al. Anticholinergic medications associated with falls and frailty in people with HIV. In Proceedings of the 2022 CROI, Virtual, 12–16 and 22–24 February 2022. Abstract 35. [Google Scholar]
- Justice, A.C.; Dombrowski, E.; Conigliaro, J.; Fultz, S.L.; Gibson, D.; Madenwald, T.; Goulet, J.; Simberkoff, M.; Butt, A.A.; Rimland, D.; et al. Veterans Aging Cohort Study (VACS): Overview and description. Med. Care 2006, 44, S13–S24. [Google Scholar] [CrossRef]
- Karpiak, S.E.; Shippy, R.A.; Cantor, M.H. Research on Older Adults with HIV; AIDS Community Research Initiative of America: New York, NY, USA, 2006. [Google Scholar]
- Schouten, J.; Wit, F.W.; Stolte, I.G.; Kootstra, N.A.; van der Valk, M.; Geerlings, S.E.; Prins, M.; Reiss, P. Cross-sectional Comparison of the Prevalence of Age-Associated Comorbidities and Their Risk Factors Between HIV-Infected and Uninfected Individuals: The AGEhIV Cohort Study. Clin. Infect. Dis. 2014, 59, 1787–1797. [Google Scholar] [CrossRef]
- Sabine, C.; Winston, A. A Prospective, Observational Study to Examine the Effects of Ageing on the ’Pharmacokinetic and Clinical Observations in People Over Fifty’ (POPPY). 13 January 2017. Available online: ClinicalTrials.gov (accessed on 10 December 2022).
| Participant Characteristics | Men | Women |
|---|---|---|
| (n = 323) | (n = 30) | |
| Age at enrolment (years) | ||
| 65–69 | 49% | 57% |
| 70–74 | 35% | 33% |
| 75–79 | 13% | 7% |
| ≥80 | 3% | 3% |
| Race/ethnicity | ||
| White | 79% | 53% |
| Black | 9% | 37% |
| Indigenous | 0.3% | 0% |
| Asian | 3% | 7% |
| Hispanic | 3% | 0% |
| Other | 6% | 3% |
| Canadian born | 66% | 40% |
| Highest level of education completed | ||
| Elementary school | 5% | 23% |
| Secondary school diploma or equivalent | 19% | 27% |
| Apprenticeship or trades certificate | 3% | 3% |
| College or other non-university certificate | 18% | 20% |
| University certificate or diploma | 9% | 7% |
| Bachelor’s degree | 24% | 10% |
| Master’s degree | 17% | 7% |
| PhD | 4% | 3% |
| Retired | 74% | 73% |
| Gross Annual Household Income (CAD) | ||
| Under CAD 20,000 | 17% | 31% |
| CAD 20,000–49,999 | 35% | 34% |
| CAD 50,000–99,999 | 28% | 31% |
| CAD ≥100,000 | 20% | 3% |
| Marital status | ||
| Single | 42% | 37% |
| Divorced or separated | 11% | 27% |
| Widowed | 8% | 30% |
| Steady partner | 7% | 0% |
| Common law partner | 16% | 3% |
| Married | 16% | 3% |
| Living arrangement | ||
| Alone | 53% | 73% |
| With spouse/partner | 38% | 10% |
| With roommate | 7% | 0% |
| With friends | 1% | 3% |
| With family | 2% | 10% |
| Other | 1% | 3% |
| HIV exposure category | ||
| Same sex only | 83% | 10% |
| Heterosexual only | 11% | 73% |
| Injection drug use only | 1% | 3% |
| Sex and injection drug use | 2% | 0% |
| Blood products | 3% | 13% |
| Duration of HIV infection at enrolment (years) | ||
| <10 | 5% | 13% |
| 10–19 | 19% | 27% |
| 20–29 | 38% | 43% |
| ≥30 | 37% | 17% |
| CD4 nadir (cells/mm3) | ||
| <100 | 26% | 21% |
| 100–199 | 24% | 42% |
| 200–299 | 21% | 25% |
| 300–399 | 9% | 8% |
| 400–499 | 9% | 4% |
| ≥500 | 11% | 0% |
| Comorbidities and Risk Factors | Men (n = 323) | Women (n = 30) |
|---|---|---|
| Dyslipidemia | 52% | 50% |
| Hypertension | 42% | 40% |
| Cancer | 32% | 13% |
| Diabetes | 24% | 23% |
| Arthritis | 21% | 30% |
| Coronary Artery Disease | 17% | 13% |
| Peripheral Neuropathy | 16% | 23% |
| Liver Disease | 16% | 17% |
| Osteoporosis | 15% | 20% |
| Depression and/or Anxiety | 15% | 20% |
| Chronic Obstructive Lung Disease and/or Asthma | 14% | 14% |
| Chronic Kidney Disease | 11% | 7% |
| Substance Use Disorder | 8% | 3% |
| Human Papillomavirus-related Disease | 8% | 13% |
| Stroke | 5% | 3% |
| Body Mass Index | ||
| <18.5 (Underweight range) | 2% | 4% |
| 18.5 to <25 (Healthy Weight Range) | 41% | 32% |
| 25 to <30 (Overweight Range) | 40% | 32% |
| ≥30 (Obesity Range) | 17% | 32% |
| Hepatitis B Core Antibody Positive | 31% | 19% |
| Hepatitis C | ||
| Antibody positive | 10% | 11% |
| RNA positive | 5% | 11% |
| Tobacco Smoking History | ||
| Current | 13% | 3% |
| Past | 50% | 33% |
| Never | 37% | 63% |
| Alcohol Use | ||
| None | 23% | 23% |
| Few Times Per Week or Less | 58% | 70% |
| Daily | 19% | 7% |
| Measure | Men | Women |
|---|---|---|
| Fried Frailty Phenotype | (n = 290) | (n = 28) |
| Non-frail | 19% | 36% |
| Pre-frail | 65% | 57% |
| Frail | 16% | 7% |
| MMSE | (n = 295) | (n = 27) |
| 30–26 Normal | 94% | 74% |
| 25–20 Mild | 5% | 26% |
| <20 Moderate-severe | 1% | 0% |
| UCLA loneliness scale | (n = 255) | (n = 27) |
| Low degree of loneliness | 67% | 67% |
| Moderate degree of loneliness | 19% | 15% |
| Moderately high degree of loneliness | 7% | 18% |
| High degree of loneliness | 6% | 0% |
| Connor-Davidson Resilience Scale | (n = 255) | (n = 27) |
| Median score (IQR) | 32 (27, 36) | 32 (27, 36) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhabokritsky, A.; Clarke, R.; Rosenes, R.; Smith, G.; Loutfy, M.; Andany, N.; Falutz, J.; Klein, M.; Harris, M.; Guillemi, S.; et al. Correlates of Healthy Aging in Geriatric HIV (CHANGE HIV)—CTN 314. Viruses 2023, 15, 517. https://doi.org/10.3390/v15020517
Zhabokritsky A, Clarke R, Rosenes R, Smith G, Loutfy M, Andany N, Falutz J, Klein M, Harris M, Guillemi S, et al. Correlates of Healthy Aging in Geriatric HIV (CHANGE HIV)—CTN 314. Viruses. 2023; 15(2):517. https://doi.org/10.3390/v15020517
Chicago/Turabian StyleZhabokritsky, Alice, Rosemarie Clarke, Ron Rosenes, Graham Smith, Mona Loutfy, Nisha Andany, Julian Falutz, Marina Klein, Marianne Harris, Silvia Guillemi, and et al. 2023. "Correlates of Healthy Aging in Geriatric HIV (CHANGE HIV)—CTN 314" Viruses 15, no. 2: 517. https://doi.org/10.3390/v15020517




