Obesity-Associated Hepatic Steatosis, Somatotropic Axis Impairment, and Ferritin Levels Are Strong Predictors of COVID-19 Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Measurements
2.4. CT Imaging of the Liver
2.5. Statistical Analysis
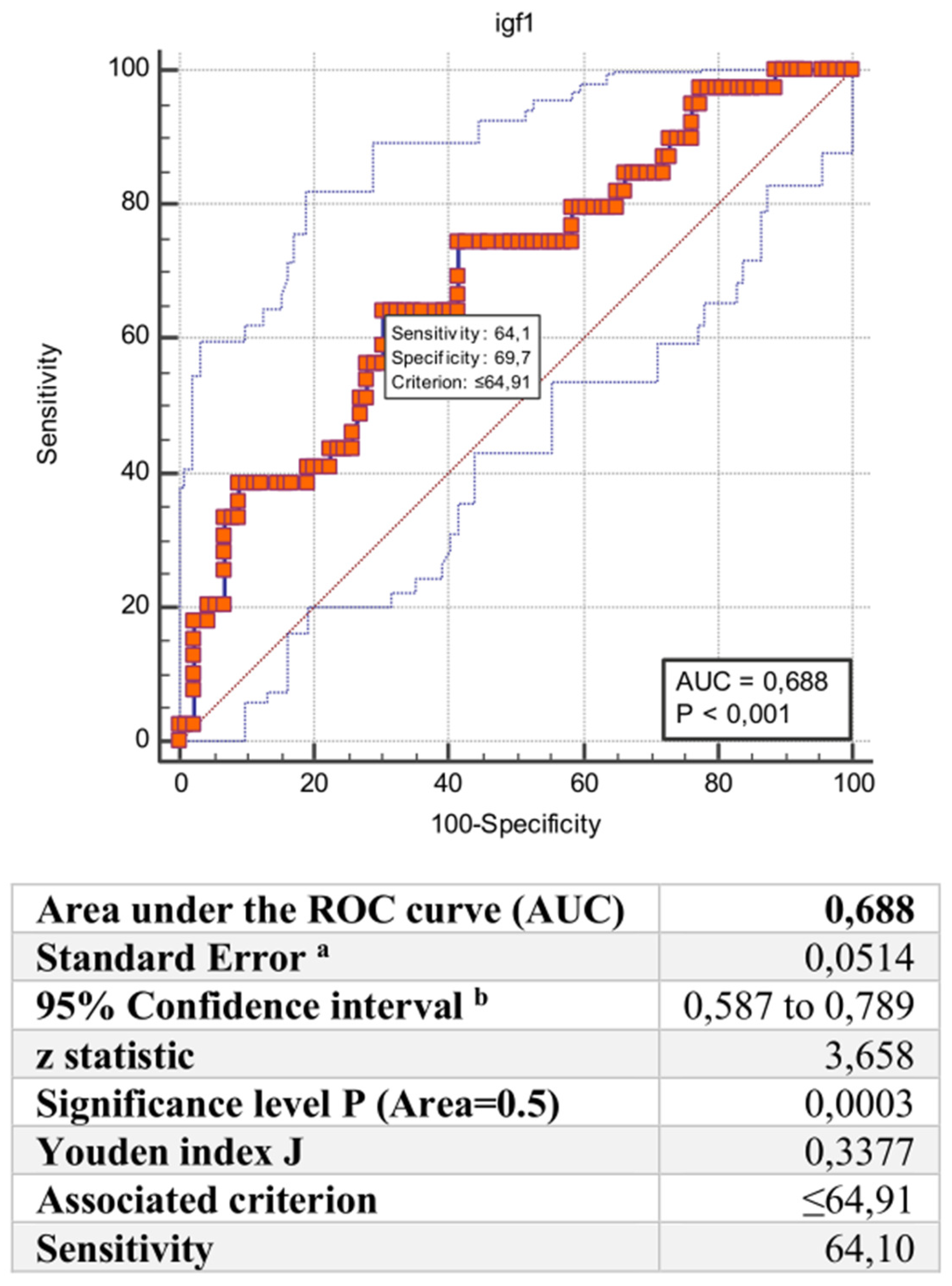
3. Results
Predicors of COVID-19 Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Singh, R.; Kaur, J.; Pandey, S.; Sharma, V.; Thakur, L.; Sati, S.; Mani, S.; Asthana, S.; Sharma, T.K.; et al. Wuhan to World: The COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2021, 11, 596201. [Google Scholar] [CrossRef] [PubMed]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic Factors for Severity and Mortality in Patients Infected with COVID-19: A Systematic Review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef] [PubMed]
- Gandini, O.; Criniti, A.; Ballesio, L.; Giglio, S.; Galardo, G.; Gianni, W.; Santoro, L.; Angeloni, A.; Lubrano, C. Serum Ferritin Is an Independent Risk Factor for Acute Respiratory Distress Syndrome in COVID-19. J. Infect. 2020, 81, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Gandini, O.; Criniti, A.; Gagliardi, M.C.; Ballesio, L.; Giglio, S.; Balena, A.; Caputi, A.; Angeloni, A.; Lubrano, C. Sex-Disaggregated Data Confirm Serum Ferritin as an Independent Predictor of Disease Severity Both in Male and Female COVID-19 Patients. J. Infect. 2021, 82, 414–451. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of Angiotensin-Converting Enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Colonnello, E.; Criniti, A.; Lorusso, E.; Curreli, M.; Santulli, M.; Angeloni, A.; Gnessi, L.; Gandini, O.; Lubrano, C. Thyroid Hormones and Platelet Activation in COVID-19 Patients. J. Endocrinol. Investig. 2022, 46, 261–269. [Google Scholar] [CrossRef]
- Somasundaram, N.P.; Ranathunga, I.; Ratnasamy, V.; Wijewickrama, P.S.A.; Dissanayake, H.A.; Yogendranathan, N.; Gamage, K.K.K.; de Silva, N.L.; Sumanatilleke, M.; Katulanda, P.; et al. The Impact of SARS-CoV-2 Virus Infection on the Endocrine System. J. Endocr. Soc. 2020, 4, bvaa082. [Google Scholar] [CrossRef]
- Masi, D.; Risi, R.; Gnessi, L.; Watanabe, M.; Mariani, S.; Lubrano, C. Letter to the Editor: “Our Response to COVID-19 as Endocrinologists and Diabetologists”. J. Clin. Endocrinol. Metab. 2020, 105, e2665–e2666. [Google Scholar] [CrossRef]
- Ilias, I.; Diamantopoulos, A.; Botoula, E.; Athanasiou, N.; Zacharis, A.; Tsipilis, S.; Jahaj, E.; Vassiliou, A.G.; Vassiliadi, D.A.; Kotanidou, A.; et al. COVID-19 and Growth Hormone/Insulin-Like Growth Factor 1: Study in Critically and Non-Critically Ill Patients. Front. Endocrinol. 2021, 12, 644055. [Google Scholar] [CrossRef]
- Dhindsa, S.; Zhang, N.; McPhaul, M.J.; Wu, Z.; Ghoshal, A.K.; Erlich, E.C.; Mani, K.; Randolph, G.J.; Edwards, J.R.; Mudd, P.A.; et al. Association of Circulating Sex Hormones with Inflammation and Disease Severity in Patients With COVID-19. JAMA Netw. Open 2021, 4, e2111398. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Huang, G.; Thuluvath, P.J.; Satapathy, S.K. Elevated Liver Biochemistries in Hospitalized Chinese Patients with Severe COVID-19: Systematic Review and Meta-analysis. Hepatology 2021, 73, 1521–1530. [Google Scholar] [CrossRef]
- Luo, M. SARS-CoV-2 Infection and Liver Involvement. Hepatol. Int. 2022, 16, 755–774. [Google Scholar] [CrossRef]
- Lu, J.Y.; Anand, H.; Frager, S.Z.; Hou, W.; Duong, T.Q. Longitudinal Progression of Clinical Variables Associated with Graded Liver Injury in COVID-19 Patients. Hepatol. Int. 2021, 15, 1018–1026. [Google Scholar] [CrossRef]
- Adams, L.A.; Feldstein, A.; Lindor, K.D.; Angulo, P. Nonalcoholic Fatty Liver Disease among Patients with Hypothalamic and Pituitary Dysfunction. Hepatology 2004, 39, 909–914. [Google Scholar] [CrossRef]
- Koehler, E.; Swain, J.; Sanderson, S.; Krishnan, A.; Watt, K.; Charlton, M. Growth Hormone, Dehydroepiandrosterone and Adiponectin Levels in Non-Alcoholic Steatohepatitis: An Endocrine Signature for Advanced Fibrosis in Obese Patients. Liver Int. 2012, 32, 279–286. [Google Scholar] [CrossRef]
- Lubrano, C.; Tenuta, M.; Costantini, D.; Specchia, P.; Barbaro, G.; Basciani, S.; Mariani, S.; Pontecorvi, A.; Lenzi, A.; Gnessi, L. Severe Growth Hormone Deficiency and Empty Sella in Obesity: A Cross-Sectional Study. Endocrine 2015, 49, 503–511. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Fatty Liver Index Associates with Relative Sarcopenia and GH/ IGF- 1 Status in Obese Subjects. PLoS ONE 2016, 11, e0145811. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Onodera, T.; Fukuda, S.; Kita, S.; Fukuhara, A.; Shimomura, I. SARS-CoV-2 Infection Impairs the Insulin/IGF Signaling Pathway in the Lung, Liver, Adipose Tissue, and Pancreatic Cells via IRF1. Metabolism 2022, 133, 155236. [Google Scholar] [CrossRef]
- Brevini, T.; Maes, M.; Webb, G.J.; John, B.V.; Fuchs, C.D.; Buescher, G.; Wang, L.; Griffiths, C.; Brown, M.L.; Scott, W.E.; et al. FXR Inhibition May Protect from SARS-CoV-2 Infection by Reducing ACE2. Nature 2022. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, C.; Han, L.; Xu, T.; Saeed, K.; Han, J.; Liu, J.; Klaassen, C.D.; Gonzalez, F.J.; Lu, Y.; et al. Suppressed Farnesoid X Receptor by Iron Overload in Mice and Humans Potentiates Iron-induced Hepatotoxicity. Hepatology 2022, 76, 387–403. [Google Scholar] [CrossRef]
- Šeda, O.; Cahová, M.; Míková, I.; Šedová, L.; Daňková, H.; Heczková, M.; Brátová, M.; Ďásková, N.; Erhartová, D.; Čapek, V.; et al. Hepatic Gene Expression Profiles Differentiate Steatotic and Non-Steatotic Grafts in Liver Transplant Recipients. Front. Endocrinol. 2019, 10, 270. [Google Scholar] [CrossRef]
- Bickler, S.W.; Cauvi, D.M.; Fisch, K.M.; Prieto, J.M.; Sykes, A.G.; Thangarajah, H.; Lazar, D.A.; Ignacio, R.C.; Gerstmann, D.R.; Ryan, A.F.; et al. Extremes of Age Are Associated with Differences in the Expression of Selected Pattern Recognition Receptor Genes and ACE2, the Receptor for SARS-CoV-2: Implications for the Epidemiology of COVID-19 Disease. BMC Med. Genom. 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, C.; Masi, D.; Risi, R.; Balena, A.; Watanabe, M.; Mariani, S.; Gnessi, L. Is Growth Hormone Insufficiency the Missing Link Between Obesity, Male Gender, Age, and COVID-19 Severity? Obesity 2020, 28, 2038–2039. [Google Scholar] [CrossRef] [PubMed]
- Frara, S.; Loli, P.; Allora, A.; Santini, C.; di Filippo, L.; Mortini, P.; Fleseriu, M.; Giustina, A. COVID-19 and Hypopituitarism. Rev. Endocr. Metab. Disord. 2022, 23, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral Fat Shows the Strongest Association with the Need of Intensive Care in Patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef]
- Risi, R.; Masieri, S.; Poggiogalle, E.; Watanabe, M.; Caputi, A.; Tozzi, R.; Gangitano, E.; Masi, D.; Mariani, S.; Gnessi, L.; et al. Nickel Sensitivity Is Associated with GH-IGF-1 Axis Impairment and Pituitary Abnormalities on MRI in Overweight and Obese Subjects. Int. J. Mol. Sci. 2020, 21, 9733. [Google Scholar] [CrossRef]
- Briand, F.; Sencio, V.; Robil, C.; Heumel, S.; Deruyter, L.; Machelart, A.; Barthelemy, J.; Bogard, G.; Hoffmann, E.; Infanti, F.; et al. Diet-Induced Obesity and NASH Impair Disease Recovery in SARS-CoV-2-Infected Golden Hamsters. Viruses 2022, 14, 2067. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.; Arya, R.; Anand, U.; Priyadarshi, R.N. Association of COVID-19 with Hepatic Metabolic Dysfunction. World J. Virol. 2022, 11, 237–251. [Google Scholar] [CrossRef]
- Lubrano, C.; Genovesi, G.; Specchia, P.; Costantini, D.; Mariani, S.; Petrangeli, E.; Lenzi, A.; Gnessi, L. Obesity and Metabolic Comorbidities: Environmental Diseases? Oxidative Med. Cell. Longev. 2013, 2013, 640673. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Corey, K.E.; Haines, M.S.; Chicote, M.L.; Kimball, A.; Colling, C.; Simon, T.G.; Long, M.T.; Husseini, J.; Bredella, M.A.; et al. The GH/IGF-1 Axis Is Associated with Intrahepatic Lipid Content and Hepatocellular Damage in Overweight/Obesity. J. Clin. Endocrinol. Metab. 2022, 107, e3624–e3632. [Google Scholar] [CrossRef]
- Elkarow, M.H.; Hamdy, A. A Suggested Role of Human Growth Hormone in Control of the COVID-19 Pandemic. Front. Endocrinol. 2020, 11, 569633. [Google Scholar] [CrossRef]
- Devin, J.K.; Blevins, L.S.; Verity, D.K.; Chen, Q.; Bloodworth, J.R.; Covington, J.; Vaughan, D.E. Markedly Impaired Fibrinolytic Balance Contributes to Cardiovascular Risk in Adults with Growth Hormone Deficiency. J. Clin. Endocrinol. Metab. 2007, 92, 3633–3639. [Google Scholar] [CrossRef]
- Bachler, M.; Bösch, J.; Stürzel, D.P.; Hell, T.; Giebl, A.; Ströhle, M.; Klein, S.J.; Schäfer, V.; Lehner, G.F.; Joannidis, M.; et al. Impaired Fibrinolysis in Critically Ill COVID-19 Patients. Br. J. Anaesth. 2021, 126, 590–598. [Google Scholar] [CrossRef]
- Kelesidis, T.; Mantzoros, C.S. Cross-Talk between SARS-CoV-2 Infection and the Insulin/IGF Signaling Pathway: Implications for Metabolic Diseases in COVID-19 and for Post-Acute Sequelae of SARS-CoV-2 Infection. Metab. Clin. Exp. 2022, 134. [Google Scholar] [CrossRef]
- Sunada, N.; Honda, H.; Nakano, Y.; Yamamoto, K.; Tokumasu, K.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; Otsuka, Y.; Obika, M.; et al. Hormonal Trends in Patients Suffering from Long COVID Symptoms. Endocr. J. 2022, 69, 1173–1181. [Google Scholar] [CrossRef]
- WHO. Clinical Management of COVID-19; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Marino, L.; Suppa, M.; Rosa, A.; Servello, A.; Coppola, A.; Palladino, M.; Mazzocchitti, A.M.; Bresciani, E.; Petramala, L.; Bertazzoni, G.; et al. Time to Hospitalisation, CT Pulmonary Involvement and In-hospital Death in COVID-19 Patients in an Emergency Medicine Unit. Int. J. Clin. Pract. 2021, 75, e14426. [Google Scholar] [CrossRef]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT Score in COVID-19 Patients: Correlation with Disease Severity and Short-Term Prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef]
- Chanson, P.; Arnoux, A.; Mavromati, M.; Brailly-Tabard, S.; Massart, C.; Young, J.; Piketty, M.-L.; Souberbielle, J.-C.; for the VARIETE Investigators. Reference Values for IGF-I Serum Concentrations: Comparison of Six Immunoassays. J. Clin. Endocrinol. Metab. 2016, 101, 3450–3458. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic Steatosis Index: A Simple Screening Tool Reflecting Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An Inexpensive and Accurate Marker of Fibrosis in HCV Infection. Comparison with Liver Biopsy and Fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Chung, J.; Park, H.-S.; Kim, Y.-J.; Yu, M.-H.; Park, S.; Jung, S.-I. Association of Hepatic Steatosis Index with Nonalcoholic Fatty Liver Disease Diagnosed by Non-Enhanced CT in a Screening Population. Diagnostics 2021, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar]
- Ferritin as a Predictive Biomarker of Severity in COVID-19. Ann. Biol. Clin. 2022, 80, 363–368. [CrossRef]
- Foulkes, A.S.; Selvaggi, C.; Shinnick, D.; Lumish, H.; Kim, E.; Cao, T.; Thaweethai, T.; Qian, J.; Lu, F.; Yan, J.; et al. Understanding the Link Between Obesity and Severe COVID-19 Outcomes: Causal Mediation by Systemic Inflammatory Response. J. Clin. Endocrinol. Metab. 2022, 107, e698–e707. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central Obesity, Smoking Habit, and Hypertension Are Associated with Lower Antibody Titres in Response to COVID-19 MRNA Vaccine. Diabetes Metab. Res. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Hattori, N.; Saito, T.; Yagyu, T.; Jiang, B.-H.; Kitagawa, K.; Inagaki, C. GH, GH Receptor, GH Secretagogue Receptor, and Ghrelin Expression in Human T Cells, B Cells, and Neutrophils. J. Clin. Endocrinol. Metab. 2001, 86, 4284–4291. [Google Scholar] [CrossRef]
- Hazrati, E.; Gholami, M.; Farahani, R.H.; Ghorban, K.; Ghayomzadeh, M.; Rouzbahani, N.H. The Effect of IGF-1 Plasma Concentration on COVID-19 Severity. Microb. Pathog. 2022, 164, 105416. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Yuan, S.; Zhou, X.; Wang, L.; Sun, J.; Yu, L.; Zhu, J.; Zhang, H.; Yang, N.; et al. Genetically Predicted High IGF-1 Levels Showed Protective Effects on COVID-19 Susceptibility and Hospitalization: A Mendelian Randomisation Study with Data from 60 Studies across 25 Countries. eLife 2022, 11, e79720. [Google Scholar] [CrossRef]
- Feizollahi, P.; Matin, S.; Roghani, S.A.; Mostafaei, S.; Safarzadeh, E.; Taghadosi, M. Evaluation Serum Levels of Insulin Growth Factor-1 (IGF-1) and Its Association with Clinical Parameters in Severe COVID-19. Inflammopharmacology 2022, 30, 199–205. [Google Scholar] [CrossRef]
- Aguirre, G.A.; De Ita, J.R.; de la Garza, R.G.; Castilla-Cortazar, I. Insulin-like Growth Factor-1 Deficiency and Metabolic Syndrome. J. Transl. Med. 2016, 14, 3. [Google Scholar] [CrossRef]
- Gangitano, E.; Barbaro, G.; Susi, M.; Rossetti, R.; Spoltore, M.E.; Masi, D.; Tozzi, R.; Mariani, S.; Gnessi, L.; Lubrano, C. Growth Hormone Secretory Capacity Is Associated with Cardiac Morphology and Function in Overweight and Obese Patients: A Controlled, Cross-Sectional Study. Cells 2022, 11, 2420. [Google Scholar] [CrossRef]
- Fornari, R.; Marocco, C.; Francomano, D.; Fittipaldi, S.; Lubrano, C.; Bimonte, V.M.; Donini, L.M.; Nicolai, E.; Aversa, A.; Lenzi, A.; et al. Insulin Growth Factor-1 Correlates with Higher Bone Mineral Density and Lower Inflammation Status in Obese Adult Subjects. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2018, 23, 375–381. [Google Scholar] [CrossRef]
- Masi, D.; Risi, R.; Biagi, F.; Vasquez Barahona, D.; Watanabe, M.; Zilich, R.; Gabrielli, G.; Santin, P.; Mariani, S.; Lubrano, C.; et al. Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome. Nutrients 2022, 14, 373. [Google Scholar] [CrossRef]
- Bucci, T.; Galardo, G.; Gandini, O.; Vicario, T.; Paganelli, C.; Cerretti, S.; Bucci, C.; Pugliese, F.; Pastori, D.; the Research on Medical Patients Admitted to the Emergency Department (ROMA-ED) Study Group; et al. Fibrosis-4 (FIB-4) Index and Mortality in COVID-19 Patients Admitted to the Emergency Department. Intern. Emerg. Med. 2022, 17, 1777–1784. [Google Scholar] [CrossRef]
- Gandini, O.; Lubrano, C. Use of Rapid Ferritin Test to Predict Clinical Deterioration in at Home COVID-19 Patients. J. Infect. 2021, 82, e11–e13. [Google Scholar] [CrossRef]
- Sampaziotis, F.; Brevini, T. A Liver Drug Reduces SARS-CoV-2 Entry into Cells. Nature 2022, 22. [Google Scholar] [CrossRef]
| 143 Patients (68 Females–75 Males) | ||||
|---|---|---|---|---|
| Variables | Mean | S.D. | Minimum | Maximum |
| Age (years) | 60.63 | 17.05 | 20.00 | 92.00 |
| Liver attenuation (HU) | 50.72 | 9.46 | 25.00 | 72.00 |
| BMI (Kg/m2) | 27.00 | 3.64 | 20.06 | 38.30 |
| Ferritin (µg/L) | 847.13 | 799.15 | 12.00 | 4000.00 |
| CRP (mg/dL) | 7.16 | 9.14 | 0.09 | 45.40 |
| DD (µg/dL) | 1242.37 | 1216.64 | 169.00 | 4610.00 |
| Fibrinogen (mg/dL) | 498.65 | 87.25 | 241.00 | 829.00 |
| Platelet × 109/L | 214.12 | 89.56 | 40.00 | 656.00 |
| LDH (UI/L) | 324.41 | 137.51 | 132.00 | 874.00 |
| Leukocytes × 103/µL | 7.09 | 3.74 | 2.00 | 22.23 |
| Neutrophils × 103/µL | 5.44 | 3.47 | 1.12 | 19.38 |
| Lymphocytes × 103/µL | 1.08 | 0.69 | 0.19 | 5.44 |
| Monocytes × 103/µL | 0.43 | 0.59 | 0.05 | 6.10 |
| Glycemia (mg/dL) | 123.79 | 49.53 | 72.00 | 397.00 |
| HbA1c (%) | 5.76 | 0.98 | 4.50 | 9.00 |
| Creatinine (mg/dL) | 0.97 | 0.53 | 0.40 | 5.00 |
| Na (mM/L) | 137.47 | 5.17 | 117.00 | 154.00 |
| K (mM/L) | 4.43 | 3.75 | 2.91 | 38.00 |
| Ca (mg/dL) | 8.70 | 0.74 | 7.40 | 10.90 |
| AST (mU/mL) | 36.81 | 29.46 | 3.86 | 259.00 |
| ALT (mU/mL) | 43.49 | 122.57 | 7.00 | 1379.00 |
| GGT (U/L) | 46.48 | 70.37 | 0.38 | 529.00 |
| CPK (U/L) | 154.03 | 174.11 | 20.00 | 779.00 |
| GH (ng/mL) | 0.92 | 1.06 | 0.05 | 6.52 |
| IGF-1 (ng/dL) | 97.15 | 63.40 | 20.90 | 426.50 |
| zSDS–IGF-1 | –2.62 | 1.64 | –5.30 | 2.37 |
| P/F ratio | 310.46 | 115.14 | 58.00 | 590.00 |
| HSI | 36.48 | 6.87 | 27.15 | 69.99 |
| FIB–4 | 2.31 | 1.86 | 0.06 | 12.62 |
| Number of comorbidities | 1.01 | 1.24 | 0.00 | 7.00 |
| Group 0 | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| Variables | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F | P |
| Number (F/M) | 17 (10/7) | 26 (9/17) | 37 (22/15) | 63 (27/36) | 1.63 | 0.19 |
| Age (years) | 49.82 (18.75) | 61.80 (15.23) | 60.84 (17.78) | 62.83 (16.21) | 2.75 | 0.05 |
| Liver attenuation (HU) | 58.69 (6.02) | 51.61 (10.43) | 51.42 (6.24) | 46.86 (10.03) | 8.04 | 0.00 |
| BMI (Kg/m2) | 24.37 (3.52) | 25.67 (3.30) | 26.77 (2.49) | 28.47 (3.78) | 5.27 | 0.00 |
| Ferritin (µg/L) | 234.12 (184.92) | 478.32 (383.11) | 733.99 (677.99) | 1203.92 (897.70 | 11.91 | 0.00 |
| CRP (mg/dL) | 1.57 (3.49) | 3.89 (3.60) | 7.74 (9.56) | 9.59 (10.50) | 4.95 | 0.00 |
| DD (µg/dL) | 468.29 (269.95) | 1186.84 (1148.91) | 1392.64 (1208.16) | 1399.61 (1358.70) | 2.91 | 0.04 |
| Fibrinogen (mg/dL) | 429.12 (82.49) | 475.84 (77.52) | 493.17 (89.19) | 531.29 (77.53) | 7.96 | 0.00 |
| Platelet × 103/µL | 207.88 (62.60) | 193.96 (70.70) | 221.54 (76.67) | 220.85 (109.32) | 0.64 | 0.59 |
| LDH (UI/L) | 211.31 (69.71) | 292.41 (109.41) | 308.34 (118.60) | 376.96 (145.58) | 8.08 | 0.00 |
| Leukocytes × 109/L | 6.30 (3.25) | 6.86 (3.44) | 6.75 (2.94) | 7.70 (4.33) | 0.93 | 0.43 |
| Neutrophils × 109/L | 4.51 (3.20) | 4.92 (2.78) | 5.01 (2.69) | 6.23 (4.07) | 1.86 | 0.14 |
| Lymphocytes × 109/L | 1.23 (0.68) | 1.13 (0.61) | 1.20 (0.93) | 0.95 (0.52) | 1.40 | 0.25 |
| Monocytes × 109/L | 0.35 (0.15) | 0.55 (0.68) | 0.53 (0.96) | 0.36 (0.19) | 1.15 | 0.33 |
| Glycemia (mg/dL) | 132.50 (35.77) | 105.88 (18.26) | 117.53 (33.06) | 130.66 (64.82) | 1.38 | 0.25 |
| Glycated Hb % | 5.3 (0.27) | 5.53 (0.32) | 5.71 (0.44) | 5.85 (1.25) | 0.20 | 0.89 |
| Creatinine (mg/dL) | 0.89 (0.56) | 1.14 (0.98) | 0.85 (0.329 | 0.99 (0.41) | 1.36 | 0.26 |
| Na (mM/L) | 137.07 (2.99) | 138.71 (5.94) | 138.59 (3.36) | 136.68 (6.15) | 1.16 | 0.33 |
| K (mM/L) | 5.33 (5.20) | 3.91 (0.57) | 5.12 (6.34) | 3.94 (0.55) | 1.00 | 0.40 |
| Ca (mg/dL) | 8.60 (1.19) | 8.00 (0.42) | 8.93 (0.44) | 8.68 (0.77) | 0.91 | 0.45 |
| AST (mU/mL) | 26.94 (9.39) | 29.55 (10.43) | 31.26 (19.69) | 44.76 (38.96) | 3.01 | 0.03 |
| ALT (mU/mL) | 26.76 (23.62) | 29.65 (16.31) | 31.33 (18.32) | 60.17 (181.05) | 0.65 | 0.59 |
| GGT (U/L) | 20.40 (9.23) | 38.44 (17.31) | 61.74 (121.49) | 47.90 (46.13) | 0.78 | 0.51 |
| CPK (U/L) | 68.33 (20.80) | 110.38 (60.15) | 134.96 (135.87) | 180.62 (203.27) | 1.42 | 0.24 |
| GH (ng/mL) | 2.27 (5.46) | 1.39 (1.53) | 0.99 (1.23) | 0.68 (0.68) | 2.36 | 0.07 |
| IGF-1 (ng/dL) | 141.51 (61.62) | 123.74 (89.17) | 92.97 (49.44) | 76.68 (48.52) | 6.85 | 0.00 |
| zSDS–IGF-1 | –0.51 (2.04) | –0.48 (3.19) | –2.01 (1.51) | –2.43 (1.47) | 5.89 | 0.00 |
| P/F ratio | 420.56 (54.29) | 371.11 (71.57) | 329.97 (95.59) | 239.85 (109.83) | 18.97 | 0.00 |
| HSI | 33.79 (2.93) | 33.80 (3.12) | 37.56 (5.27) | 37.55 (8.66) | 1.45 | 0.24 |
| FIB–4 | 1.40 (0.58) | 2.09 (1.10) | 1.88 (1.34) | 2.35 (1.46) | 2.63 | 0.05 |
| Number of comorbidities | 0.50 (0.51) | 0.61 (0.87) | 1.03 (1.03) | 1.30 (1.60) | 1.94 | 0.13 |
| Mean | S.D. | Mean | S.D. | p | |
|---|---|---|---|---|---|
| 0 | 0 | 1 | 1 | ||
| Number (F/M) | 83 (43/40) | 59 (25/34) | |||
| Age (years) | 58,140 | 17,395 | 63,980 | 16,188 | 0.044 |
| Liver attenuation (HU) | 51,830 | 10,080 | 48,160 | 8470 | 0.030 |
| BMI (Kg/m2) | 26,010 | 3198 | 28,710 | 3754 | 0.000 |
| Ferritin (µg/L) | 559,330 | 554,794 | 1229,120 | 911,059 | 0.000 |
| CRP (mg/dL) | 5150 | 7283 | 10,000 | 10,720 | 0.001 |
| DD (µg/dL) | 1096,410 | 1094,369 | 1451,490 | 1368,123 | 0.097 |
| Fibrinogen (mg/dL) | 481,000 | 83,847 | 524,410 | 87,028 | 0.004 |
| Platelet × 109/L | 209,710 | 73,770 | 221,890 | 109,861 | 0.436 |
| LDH (UI/L) | 290,150 | 116,370 | 372,810 | 148,903 | 0.000 |
| Leucocytes × 109/L | 6780 | 3260 | 7630 | 4301 | 0.183 |
| Neutrophils × 109/L | 4950 | 2875 | 6210 | 4100 | 0.034 |
| Lymphocytes × 109/L | 1190 | 0763 | 0920 | 0521 | 0.018 |
| Monocytes × 109/L | 0490 | 0741 | 0350 | 0194 | 0.149 |
| Glycemia (mg/dL) | 116,420 | 31,295 | 134,730 | 66,943 | 0.049 |
| Glycated Hemoglobin (%) | 5480 | 510 | 6080 | 1270 | 0.068 |
| Creatinine (mg/dL) | 0930 | 0604 | 1020 | 0419 | 0.357 |
| Na (mM/L) | 138,250 | 3331 | 136,480 | 6820 | 0.073 |
| K (mM/L) | 4780 | 4970 | 3970 | 0560 | 0.262 |
| AST (mU/mL) | 30,600 | 15,948 | 44,940 | 40,339 | 0.005 |
| ALT (mU/mL) | 29,570 | 18,736 | 63,280 | 189,207 | 0.127 |
| Ca (mg/dL) | 8760 | 0696 | 8610 | 0812 | 0.582 |
| GGT (U/L) | 45,230 | 83,849 | 48,430 | 46,435 | 0.855 |
| CPK (U/L) | 112,860 | 104,999 | 190,860 | 211,875 | 0.036 |
| GH (ng/mL) | 1400 | 2774 | 0,640 | 0709 | 0.060 |
| IGF-1 (ng/dL) | 109,680 | 69,411 | 79,760 | 49,500 | 0.008 |
| zSDS–IGF-1 | −1380 | 2243 | −2330 | 1522 | 0.028 |
| P/F ratio | 352,680 | 98,920 | 243,950 | 108,527 | 0.000 |
| HSI | 35,090 | 4458 | 38,570 | 9104 | 0.037 |
| FIB–4 | 1980 | 1431 | 2860 | 2383 | 0.039 |
| Number of comorbidities | 0760 | 0893 | 1480 | 1671 | 0.007 |
| R = 0.56452776 R2 = 0.31869159 Adjusted R2 = 0.29179784 F (3.76) = 11,850 p | ||||||
|---|---|---|---|---|---|---|
| N = 143 | b* | S.E. of b* | b | S.E. of b | t (76) | p-Value |
| Intercept | 3.25839 | 0.61712 | 5.27995 | 0.00000 | ||
| Ferritin, µg/L | 0.36698 | 0.09620 | 0.00029 | 0.00008 | 3.81465 | 0.00028 |
| Liver attenuation, HU | −0.363571 | 0.10228 | −0.038050 | 0.01071 | −3.55451 | 0.00066 |
| zSDS–IGF-1 | −0.214087 | 0.10080 | −0.143060 | 0.06736 | −2.12378 | 0.03694 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, D.; Gangitano, E.; Criniti, A.; Ballesio, L.; Anzuini, A.; Marino, L.; Gnessi, L.; Angeloni, A.; Gandini, O.; Lubrano, C., on behalf of the Sapienza University MOrbility and Treatment Options in Obesity (SUMOTO) Study Group. Obesity-Associated Hepatic Steatosis, Somatotropic Axis Impairment, and Ferritin Levels Are Strong Predictors of COVID-19 Severity. Viruses 2023, 15, 488. https://doi.org/10.3390/v15020488
Masi D, Gangitano E, Criniti A, Ballesio L, Anzuini A, Marino L, Gnessi L, Angeloni A, Gandini O, Lubrano C on behalf of the Sapienza University MOrbility and Treatment Options in Obesity (SUMOTO) Study Group. Obesity-Associated Hepatic Steatosis, Somatotropic Axis Impairment, and Ferritin Levels Are Strong Predictors of COVID-19 Severity. Viruses. 2023; 15(2):488. https://doi.org/10.3390/v15020488
Chicago/Turabian StyleMasi, Davide, Elena Gangitano, Anna Criniti, Laura Ballesio, Antonella Anzuini, Luca Marino, Lucio Gnessi, Antonio Angeloni, Orietta Gandini, and Carla Lubrano on behalf of the Sapienza University MOrbility and Treatment Options in Obesity (SUMOTO) Study Group. 2023. "Obesity-Associated Hepatic Steatosis, Somatotropic Axis Impairment, and Ferritin Levels Are Strong Predictors of COVID-19 Severity" Viruses 15, no. 2: 488. https://doi.org/10.3390/v15020488
APA StyleMasi, D., Gangitano, E., Criniti, A., Ballesio, L., Anzuini, A., Marino, L., Gnessi, L., Angeloni, A., Gandini, O., & Lubrano, C., on behalf of the Sapienza University MOrbility and Treatment Options in Obesity (SUMOTO) Study Group. (2023). Obesity-Associated Hepatic Steatosis, Somatotropic Axis Impairment, and Ferritin Levels Are Strong Predictors of COVID-19 Severity. Viruses, 15(2), 488. https://doi.org/10.3390/v15020488












