Risk Factors of Glecaprevir/Pibrentasvir-Induced Liver Injury and Efficacy of Ursodeoxycholic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment and Follow-Up
2.3. Assessment of GP-Induced Liver Injury
2.4. Assessment of Efficacy of Add-On UDCA for GP-Induced Liver Injury
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. SVR and GP-Induced Liver Injury
3.3. Risk Factors Contributing to GP-Induced Liver Injury
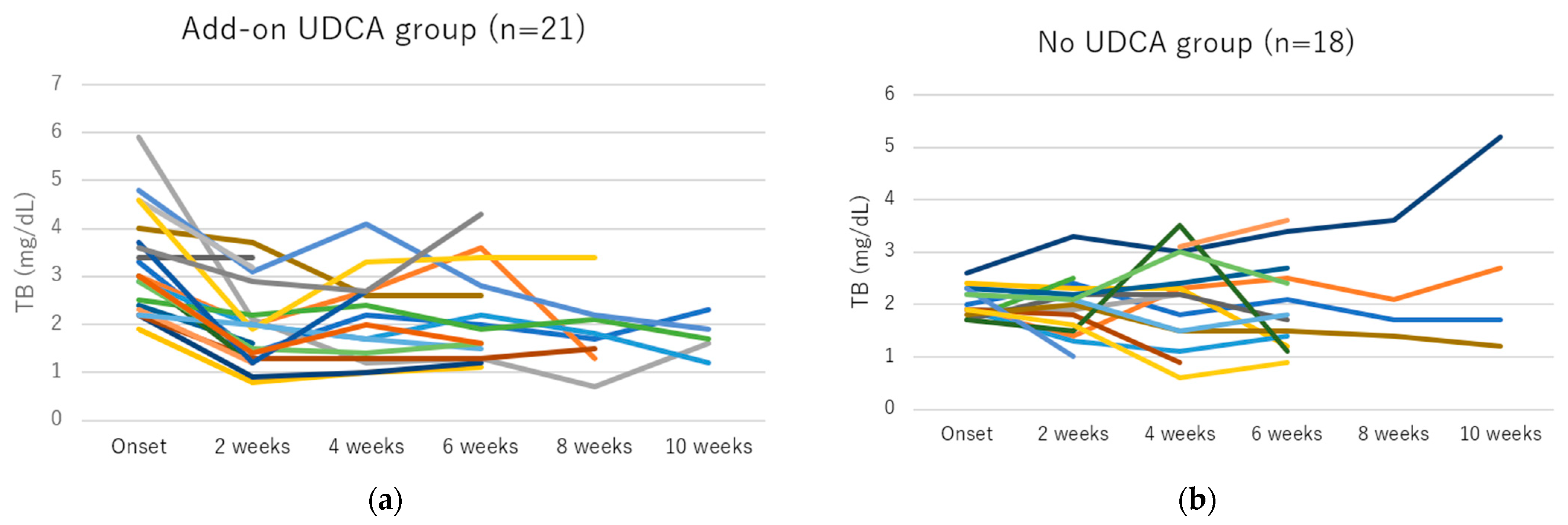
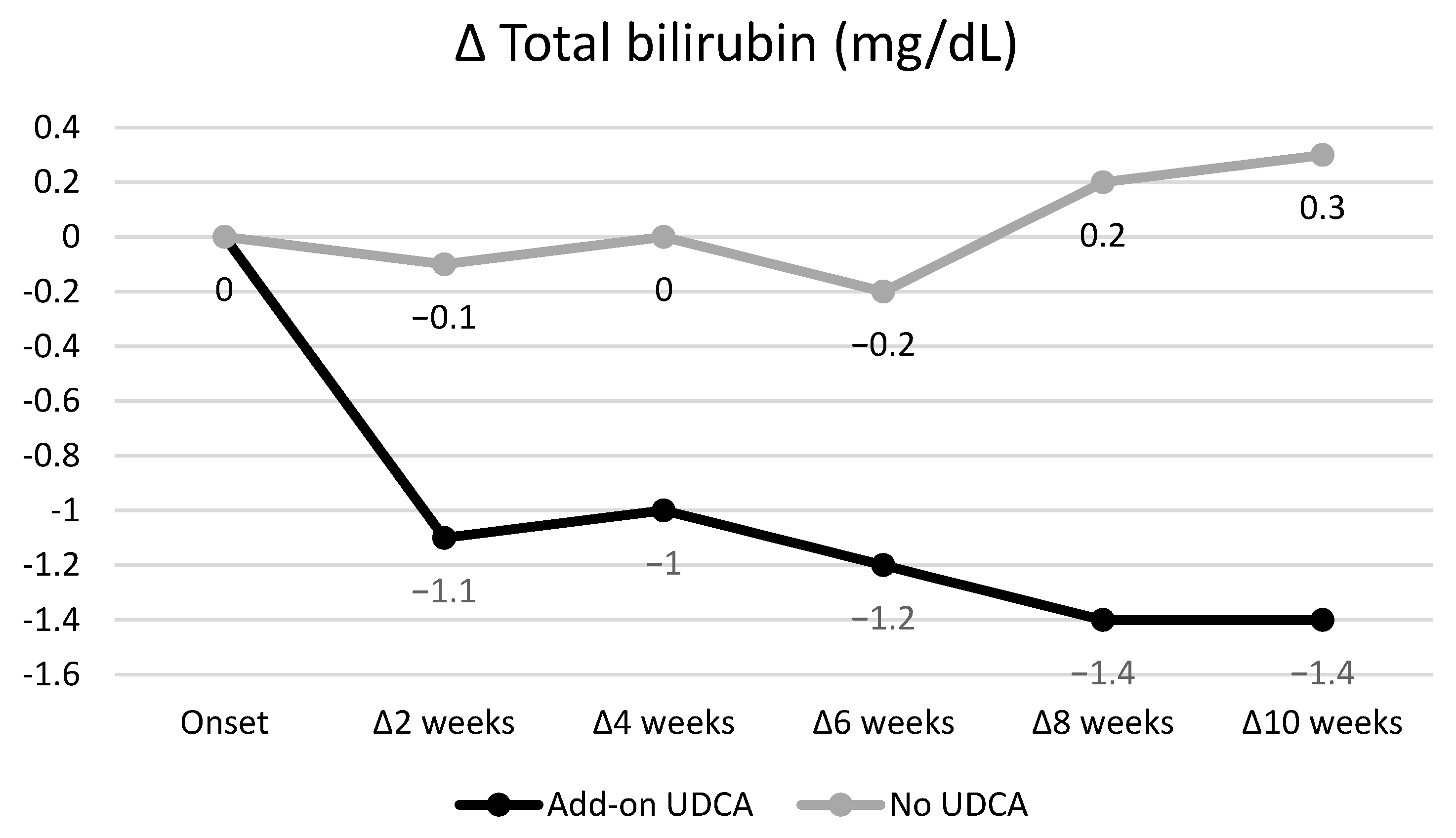
3.4. Efficacy of Add-On UDCA for GP-Induced Liver Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chayama, K.; Suzuki, F.; Karino, Y.; Kawakami, Y.; Sato, K.; Atarashi, T.; Naganuma, A.; Watanabe, T.; Eguchi, Y.; Yoshiji, H.; et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis. J. Gastroenterol. 2018, 53, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Chayama, K.; Suzuki, F.; Sato, K.; Atarashi, T.; Watanabe, T.; Atsukawa, M.; Naganuma, A.; Notsumata, K.; Osaki, Y.; et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection. Hepatol. (Baltim. Md.) 2018, 67, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Morgan, T.R. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatol. (Baltim. Md.) 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan society of hepatology guidelines for the management of hepatitis C virus infection: 2019 update. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2020, 50, 791–816. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H.; European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series (☆). J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Carrión, J.A.; Curry, M.; Turnes, J.; Cornberg, M.; Negro, F.; Brown, A.; Persico, M.; Wick, N.; Porcalla, A.; et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: A meta-analysis. J. Hepatol. 2020, 72, 1112–1121. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, S.M.; Kang, G.; Kim, H.J.; Jun, C.H.; Choi, S.K. A case report of glecaprevir/pibrentasvir-induced severe hyperbilirubinemia in a patient with compensated liver cirrhosis. Medicine 2019, 98, e17343. [Google Scholar] [CrossRef]
- Hammami, M.B.; Aboushaar, R.; Alsabbagh, E. Glecaprevir/pibrentasvir-associated acute liver injury in non-cirrhotic, chronic HCV infection without HBV co-infection. BMJ Case Rep. 2019, 12, e226622. [Google Scholar] [CrossRef]
- Hara, T.; Ohara, T.; Taniguchi, M.; Sakai, H.; Oka, K.; Iwai, N.; Tsuji, T.; Okuda, T.; Nagata, A.; Komaki, T.; et al. Severe liver injury associated with glecaprevir plus pibrentasvir therapy in a patient with treatment-naïve hepatitis C virus infection. Intern. Med. 2021, 60, 2437–2443. [Google Scholar] [CrossRef]
- Jain, A.; Mumtaz, K. Acute liver injury due to glecaprevir/pibrentasvir in a patient with chronic hepatitis C virus infection without cirrhosis. Avicenna J. Med. 2022, 12, 154–156. [Google Scholar] [CrossRef]
- Garcia-Cortes, M.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug induced liver injury: An update. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef] [PubMed]
- Robles-Díaz, M.; Nezic, L.; Vujic-Aleksic, V.; Björnsson, E.S. Role of ursodeoxycholic acid in treating and preventing idiosyncratic drug-induced liver injury. A systematic review. Front. Pharmacol. 2021, 12, 744488. [Google Scholar] [CrossRef] [PubMed]
- Taki, S.; Tamai, H.; Ida, Y.; Shingaki, N.; Kawashima, A.; Shimizu, R.; Moribata, K.; Maekita, T.; Iguchi, M.; Kato, J.; et al. The real-world safety and efficacy of daclatasvir and asunaprevir for elderly patients. Gut Liver 2018, 12, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Leise, M.D.; Poterucha, J.J.; Talwalkar, J.A. Drug-induced liver injury. Mayo Clin. Proc. 2014, 89, 95–106. [Google Scholar] [CrossRef]
- Naganuma, A.; Chayama, K.; Notsumata, K.; Gane, E.; Foster, G.R.; Wyles, D.; Kwo, P.; Crown, E.; Bhagat, A.; Mensa, F.J.; et al. Integrated analysis of 8-week glecaprevir/pibrentasvir in Japanese and overseas patients without cirrhosis and with hepatitis C virus genotype 1 or 2 infection. J. Gastroenterol. 2019, 54, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, G.; Alami, N.N.; Xie, W.; Heo, J.; Xie, Q.; Zhang, M.; Kim, Y.J.; Lim, S.G.; Fredrick, L.M.; et al. Glecaprevir-pibrentasvir to treat chronic hepatitis C virus infection in Asia: Two multicentre, phase 3 studies- a randomised, double-blind study (VOYAGE-1) and an open-label, single-arm study (VOYAGE-2). Lancet Gastroenterol. Hepatol. 2020, 5, 839–849. [Google Scholar] [CrossRef]
- Brown, R.S., Jr.; Buti, M.; Rodrigues, L.; Chulanov, V.; Chuang, W.L.; Aguilar, H.; Horváth, G.; Zuckerman, E.; Carrion, B.R.; Rodriguez-Perez, F.; et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J. Hepatol. 2020, 72, 441–449. [Google Scholar] [CrossRef]
- Heo, J.; Kim, Y.J.; Lee, J.W.; Kim, J.H.; Lim, Y.S.; Han, K.H.; Jeong, S.H.; Cho, M.; Yoon, K.T.; Bae, S.H.; et al. Efficacy and Safety of Glecaprevir/Pibrentasvir in Korean Patients with Chronic Hepatitis C: A Pooled Analysis of Five Phase II/III Trials. Gut Liver 2021, 15, 895–903. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Deng, H.; Zhang, X.; Zhang, K.; Li, N.; Han, Q.; Lv, Y.; Liu, Z. Efficacy and safety of glecaprevir/pibrentasvir for chronic hepatitis C virus genotypes 1-6 infection: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2019, 54, 780–789. [Google Scholar] [CrossRef]
- Liu, X.; Hu, P. Efficacy and safety of glecaprevir/pibrentasvir in patients with chronic HCV infection. J. Clin. Transl. Hepatol. 2021, 9, 125–132. [Google Scholar] [CrossRef]
- Hung, H.Y.; Hung, W.L.; Shih, C.L.; Chen, C.Y. Drug-induced liver injury by glecaprevir/pibrentasvir treatment for chronic hepatitis C infection: A systematic review and meta-analysis. Ann. Med. 2022, 54, 108–120. [Google Scholar] [CrossRef]
- Tamori, A.; Inoue, K.; Kagawa, T.; Takaguchi, K.; Nouso, K.; Iwasaki, Y.; Minami, M.; Hai, H.; Enomoto, M.; Kawada, N. Intention-to-treat assessment of glecaprevir + pibrentasvir combination therapy for patients with chronic hepatitis C in the real world. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2019, 49, 1365–1373. [Google Scholar] [CrossRef]
- Ogawa, E.; Furusyo, N.; Nakamuta, M.; Nomura, H.; Satoh, T.; Takahashi, K.; Koyanagi, T.; Kajiwara, E.; Dohmen, K.; Kawano, A.; et al. Glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C genotype 1 or 2 infection: Results from a multicenter, real-world cohort study. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2019, 49, 617–626. [Google Scholar] [CrossRef]
- Watanabe, S.; Morimoto, N.; Miura, K.; Murohisa, T.; Tahara, T.; Sato, T.; Tano, S.; Fukaya, Y.; Kurata, H.; Okamura, Y.; et al. Efficacy and safety of glecaprevir and pibrentasvir combination therapy in old-aged patients with chronic hepatitis C virus infection. J. Rural. Med. JRM 2020, 15, 139–145. [Google Scholar] [CrossRef]
- Nozaki, A.; Atsukawa, M.; Kondo, C.; Toyoda, H.; Chuma, M.; Nakamuta, M.; Uojima, H.; Takaguchi, K.; Ikeda, H.; Watanabe, T.; et al. The effectiveness and safety of glecaprevir/pibrentasvir in chronic hepatitis C patients with refractory factors in the real world: A comprehensive analysis of a prospective multicenter study. Hepatol. Int. 2020, 14, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Komaki, Y.; Ozono, Y.; Nakamura, K.; Iwakiri, H.; Hasuike, S.; Sueta, M.; Miike, T.; Yamamoto, S.; Uto, H.; Kusumoto, K.; et al. Efficacy and safety of glecaprevir and pibrentasvir in Japanese patients with hepatitis C virus infection aged 75 years or older. BMC Gastroenterol. 2022, 22, 210. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, A.; Yoshida, Y.; Murakami, A.; Hoshino, T.; Sawara, K.; Numao, H.; Takikawa, Y. Safety and efficacy of glecaprevir and pibrentasvir in north Tohoku Japanese patients with genotype 1/2 hepatitis C virus infection. Health Sci. Rep. 2022, 5, e458. [Google Scholar] [CrossRef] [PubMed]
- Kosloski, M.P.; Wang, H.; Pugatch, D.; Mensa, F.J.; Gane, E.; Lawitz, E.; Marbury, T.C.; Preston, R.A.; Kort, J.; Liu, W. Pharmacokinetics and safety of glecaprevir and pibrentasvir in HCV-negative subjects with hepatic impairment. Eur. J. Clin. Pharmacol. 2019, 75, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Sotaniemi, E.A.; Arranto, A.J.; Pelkonen, O.; Pasanen, M. Age and cytochrome P450-linked drug metabolism in humans: An analysis of 226 subjects with equal histopathologic conditions. Clin. Pharmacol. Ther. 1997, 61, 331–339. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakura, H.; Tateishi, T.; Watanabe, M.; Nakaya, S.; Kumai, T.; Kobayashi, S. Ursodeoxycholic acid prevents hepatic cytochrome P450 isozyme reduction in rats with deoxycholic acid-induced liver injury. J. Hepatol. 1999, 31, 263–270. [Google Scholar] [CrossRef] [PubMed]
| N = 236 | |
|---|---|
| Age, years (range) | 70 (14–94) |
| ≥75 years | 85 (36%) |
| Sex, male/female | 132/104 |
| Height, cm (range) | 161.0 (133.2–180) |
| Weight, kg (range) | 58 (29.6–140.5) |
| Body mass index, kg/m2 (range) | 22.8 (15.6–58.5) |
| Cirrhosis | 78 (33%) |
| Genotype (1/2/3/4/5/6/unknown) | 132/101/1/0/0/1/1 |
| HCV-RNA (logIU/mL) | 6.3 (2.1–7.6) |
| History of HCC treatment | 28 (12%) |
| History of IFN-based therapy | 24 (10%) |
| History of DAA treatment | 13 (6%) |
| White blood cells (/mm3) | 5180 (1200–11,700) |
| Hemoglobin (g/dL) | 13.7 (7.8–17.3) |
| Platelets (×104/mm3) | 17.9 (5.1–96.7) |
| AST (U/L) | 39 (13–432) |
| ALT (U/L) | 36 (8–558) |
| ALP (U/L) | 87 (35–303) |
| γ-GT (U/L) | 37 (7–748) |
| PT (%) | 97 (12–141) |
| Alb (g/dL) | 4.1 (2.3–5.2) |
| TB (mg/dL) | 0.8 (0.3–2.6) |
| ALBI grade (1/2/3) | 154/80/2 |
| N = 236 | |
|---|---|
| Occurrence of liver dysfunction | 146 (62%) |
| Liver injury grade | |
| AST elevation (Grade 1/2/≥3) | 7 (6/1/0) |
| ALT elevation (Grade 1/2/≥3) | 14 (13/1/0) |
| ALP elevation (Grade 1/2/≥3) | 60 (60/0/0) |
| γ-GT elevation (Grade 1/2/≥3) | 4 (2/1/1) |
| TB elevation (Grade 1/2/≥3) | 118 (75/34/9) |
| Time to liver injury | |
| AST elevation after 2/4/6/8/10/12 weeks | 2/2/2/0/0/1 |
| ALT elevation after 2/4/6/8/10/12 weeks | 8/1/2/1/1/1 |
| ALP elevation after 2/4/6/8/10/12 weeks | 31/14/6/7/1/1 |
| γ-GT elevation after 2/4/6/8/10/12 weeks | 2/1/0/0/1/0 |
| TB elevation after 2/4/6/8/10/12 weeks | 94/12/7/5/0/0 |
| Management of liver injury | |
| Treatment discontinuation due to hyperbilirubinemia | 1 |
| Dose reduction due to hyperbilirubinemia | 1 |
| Add-on UDCA for hyperbilirubinemia | 21 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Factors | p | OR | 95% CI | p | OR | 95% CI |
| Age (per 1-year increase) | <0.001 | 1.056 | 1.033–1.079 | 0.005 | 1.044 | 1.013–1.076 |
| Sex (female) | 0.209 | 1.408 | 0.826–2.402 | |||
| Height (per 1-cm increase) | 0.005 | 0.960 | 0.932–0.987 | 0.614 | 1.028 | 0.923–1.145 |
| Weight (per 1-kg increase) | 0.002 | 0.971 | 0.953–0.990 | 0.351 | 0.939 | 0.822–1.072 |
| BMI (per 1-kg/m2 increase) | 0.040 | 0.939 | 0.885–0.997 | 0.341 | 1.179 | 0.840–1.655 |
| Cirrhosis | 0.103 | 1.614 | 0.908–2.870 | |||
| History of HCC treatment | 0.133 | 1.992 | 0.810–4.896 | |||
| History of IFN-based therapy | 0.343 | 1.563 | 0.621–3.930 | |||
| History of DAA treatment | 0.085 | 0.363 | 0.115–1.148 | |||
| HCV-RNA (per 1-logIU/mL increase) | 0.528 | 1.090 | 0.834–1.424 | |||
| White blood cells (per 1/mm3 increase) | 0.043 | 1.000 | 1.000–1.000 | 0.829 | 1.000 | 1.000–1.000 |
| Hemoglobin (per 1-g/dL increase) | 0.139 | 0.893 | 0.768–1.037 | |||
| Platelets (per 1 × 104/mm3 increase) | 0.008 | 0.946 | 0.908–0.986 | 0.623 | 0.989 | 0.946–1.034 |
| AST (per 1-IU/L increase) | 0.175 | 0.996 | 0.990–1.002 | |||
| ALT (per 1-IU/L increase) | 0.039 | 0.995 | 0.991–1.000 | 0.659 | 0.999 | 0.992–1.005 |
| ALP (per 1-IU/L increase) | 0.914 | 1.000 | 0.993–1.006 | |||
| γ-GT (per 1-IU/L increase) | 0.016 | 0.996 | 0.993–0.999 | 0.093 | 0.997 | 0.993–1.001 |
| PT (per 1% increase) | 0.022 | 0.979 | 0.962–0.997 | 0.305 | 0.989 | 0.969–1.010 |
| Alb (per 1-g/dL increase) | 0.013 | 0.467 | 0.256–0.852 | 0.676 | 1.302 | 0.377–4.492 |
| TB (per 1-mg/dL increase) | <0.001 | 10.668 | 3.640–31.267 | <0.001 | 14.700 | 3.941–54.822 |
| ALBI grade (≥2) | 0.001 | 2.836 | 1.552–5.184 | 0.482 | 1.501 | 0.484–4.653 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Factors | p | OR | 95% CI | p | OR | 95% CI |
| Age (per 1-year increase) | 0.009 | 1.038 | 1.010–1.068 | 0.614 | 1.010 | 0.974–1.047 |
| Sex (female) | 0.747 | 0.896 | 0.459–1.749 | |||
| Height (per 1-cm increase) | 0.066 | 0.969 | 0.937–1.002 | |||
| Weight (per 1-kg increase) | 0.024 | 0.970 | 0.944–0.996 | 0.575 | 0.984 | 0.931–1.040 |
| BMI (per 1-kg/m2 increase) | 0.045 | 0.911 | 0.831–0.998 | 0.683 | 0.963 | 0.803–1.154 |
| Cirrhosis | 0.006 | 2.563 | 1.306–5.028 | 0.195 | 1.771 | 0.746–4.205 |
| History of HCC treatment | 0.136 | 1.977 | 0.806–4.847 | |||
| History of IFN-based therapy | 0.448 | 0.614 | 0.175–2.161 | |||
| History of DAA treatment | 0.332 | 0.359 | 0.045–2.839 | |||
| HCV-RNA (per 1-logIU/mL increase) | 0.106 | 0.773 | 0.566–1.056 | |||
| White blood cells (per 1/mm3 increase /mm3) | 0.064 | 1.000 | 1.000–1.000 | |||
| Hemoglobin (per 1-g/dL increase) | 0.080 | 0.852 | 0.712–1.019 | |||
| Platelets (per 1 × 104/mm3 increase) | 0.026 | 0.938 | 0.886–0.992 | 0.554 | 0.980 | 0.920–1.045 |
| AST (per 1-IU/L increase) | 0.789 | 0.999 | 0.991–1.007 | |||
| ALT (per 1-IU/L increase) | 0.312 | 1.004 | 0.996–1.011 | |||
| ALP (per 1-IU/L increase) | 0.914 | 1.000 | 0.993–1.006 | |||
| γ-GT (per 1-IU/L increase) | 0.335 | 0.998 | 0.993–1.002 | |||
| PT (per 1% increase) | 0.019 | 0.976 | 0.957–0.996 | 0.157 | 0.985 | 0.963–1.006 |
| Alb (per 1-g/dL increase) | 0.023 | 0.451 | 0.227–0.896 | 0.958 | 1.033 | 0.313–3.411 |
| TB (per 1-mg/dL increase) | 0.001 | 5.024 | 1.932–13.069 | 0.004 | 5.132 | 1.679–15.683 |
| ALBI grade (≥2) | 0.001 | 2.834 | 1.494–5.377 | 0.651 | 1.320 | 0.396–4.392 |
| Add-On UDCA Group (n = 21) | No UDCA Group (n = 18) | |||
|---|---|---|---|---|
| Total Bilirubin (mg/dL) | p | Total Bilirubin (mg/dL) | p | |
| At onset | 3.0 (1.9–5.9) | 2.0 (1.6–3.5) | ||
| After 2 weeks | 1.9 (0.8–3.7) | <0.001 | 2.0 (1.0–3.3) | 0.690 |
| After 4 weeks | 2.1 (1.0–4.1) | <0.001 | 2.2 (0.6–3.5) | 0.950 |
| After 6 weeks | 1.6 (1.1–4.3) | <0.001 | 1.8 (0.8–3.6) | 0.363 |
| After 8 weeks | 1.8 (0.7–3.4) | 0.012 | 1.9 (1.4–3.6) | 0.500 |
| After 10 weeks | 1.7 (1.0–3.5) | 0.018 | 1.9 (1.2–5.2) | 0.465 |
| Δ Total Bilirubin | Add-On UDCA Group (n = 21) | No UDCA Group (n = 18) | p |
|---|---|---|---|
| After 2 weeks | −1.1 (−3.8–0.0) | −0.1 (−1.3–0.8) | <0.001 |
| After 4 weeks | −1.0 (−4.7–−1.0) | 0.0 (−1.3–1.8) | <0.001 |
| After 6 weeks | −1.2 (−4.6–0.7) | −0.2 (−1.5–0.8) | 0.004 |
| After 8 weeks | −1.4 (−5.2–−0.4) | 0.2 (−0.4–1.8) | 0.002 |
| After 10 weeks | −1.4 (−4.3–−0.5) | 0.3 (−0.6–2.6) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamai, H.; Okamura, J. Risk Factors of Glecaprevir/Pibrentasvir-Induced Liver Injury and Efficacy of Ursodeoxycholic Acid. Viruses 2023, 15, 489. https://doi.org/10.3390/v15020489
Tamai H, Okamura J. Risk Factors of Glecaprevir/Pibrentasvir-Induced Liver Injury and Efficacy of Ursodeoxycholic Acid. Viruses. 2023; 15(2):489. https://doi.org/10.3390/v15020489
Chicago/Turabian StyleTamai, Hideyuki, and Jumpei Okamura. 2023. "Risk Factors of Glecaprevir/Pibrentasvir-Induced Liver Injury and Efficacy of Ursodeoxycholic Acid" Viruses 15, no. 2: 489. https://doi.org/10.3390/v15020489
APA StyleTamai, H., & Okamura, J. (2023). Risk Factors of Glecaprevir/Pibrentasvir-Induced Liver Injury and Efficacy of Ursodeoxycholic Acid. Viruses, 15(2), 489. https://doi.org/10.3390/v15020489






