Follow-Up of PRRSv-Vaccinated Piglets Born from PRRSv-Vaccinated, ELISA-Seropositive and ELISA-Seronegative Sows
Abstract
1. Introduction
2. Materials and Methods
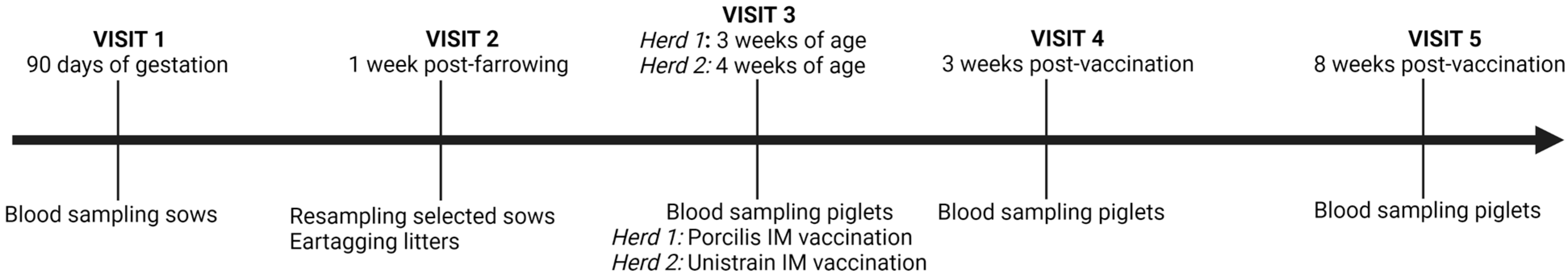
2.1. Study Design
2.2. Blood Sampling
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Virus Neutralization Assay (VN)
2.5. RNA Extraction and Real-Time Polymerase Chain Reaction (qPCR)
2.6. Statistical Analysis
3. Results
3.1. Sow Selection
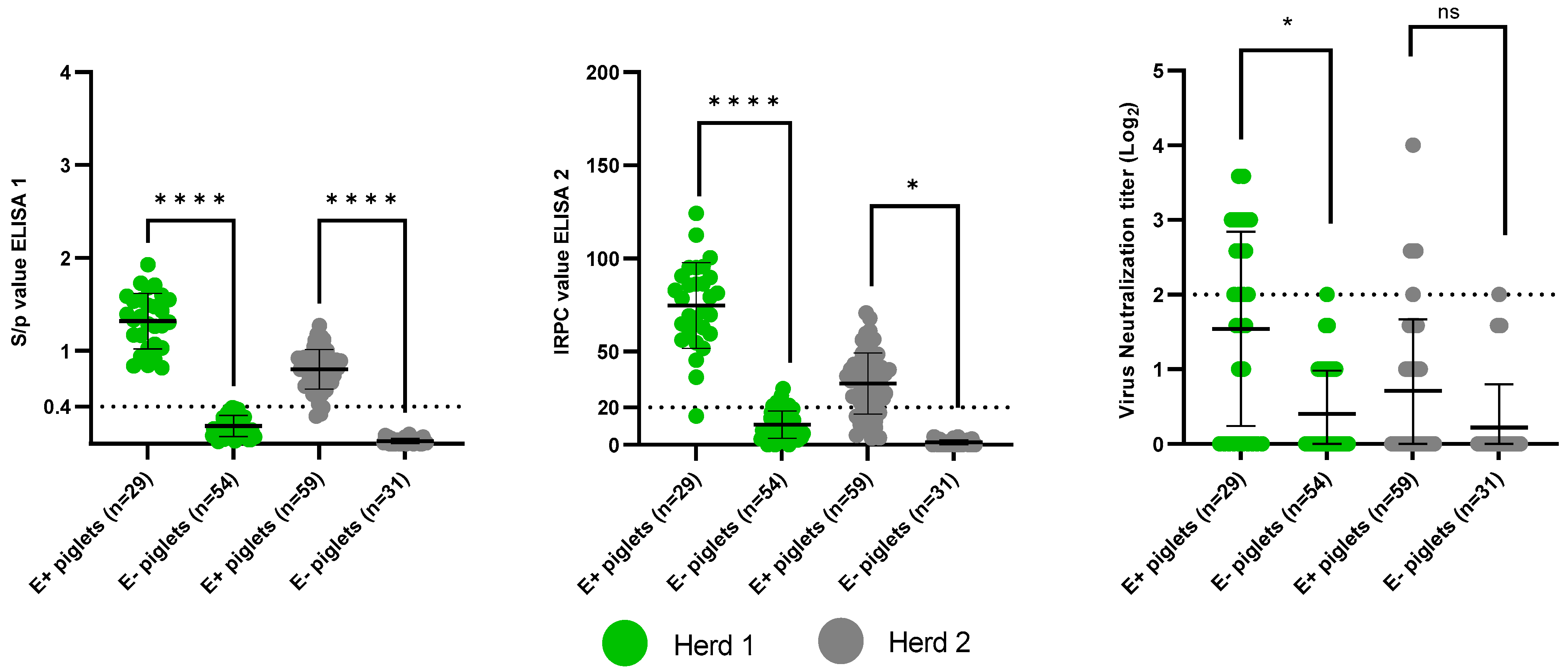
3.2. Presence of Maternally-Derived Antibodies
3.2.1. Presence of PRRSv-Specific MDAs
3.2.2. Presence of PCV2-Specific MDAs: Control for Colostrum Intake
3.3. Antibody Responses Post-Vaccination
3.3.1. Antibody Responses 3 Weeks Post-Vaccination
3.3.2. Antibody Responses 8 Weeks Post-Vaccination
3.3.3. Correlation between MDAs and the Absence of an Antibody Response Post-Vaccination
3.4. Vaccine Viremia Post-Vaccination
3.4.1. Vaccine Viremia 3 Weeks Post-Vaccination
3.4.2. Vaccine Viremia 8 Weeks Post-Vaccination
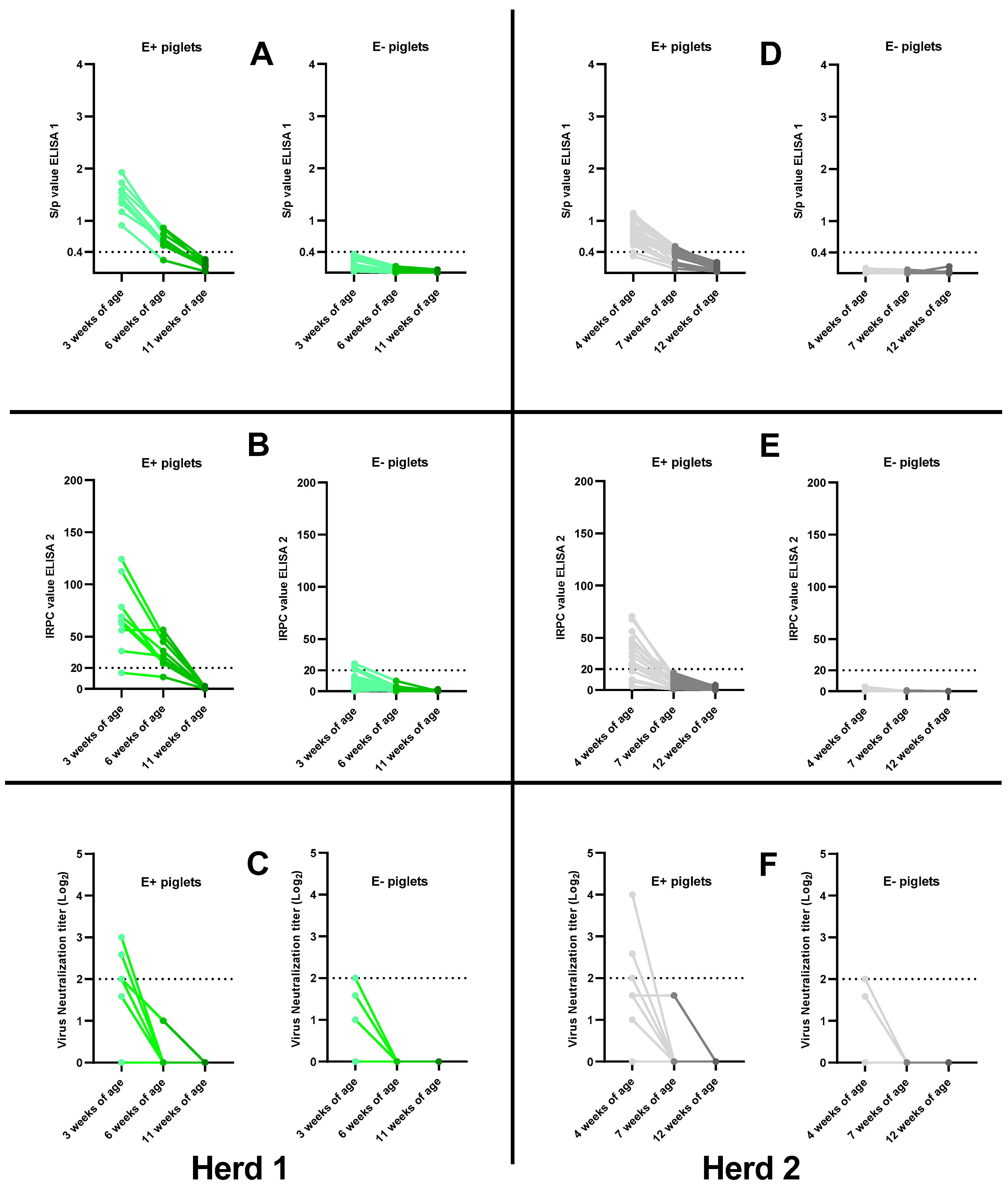
3.5. Absence of Field PRRSv Circulation: Waning of PRRSv-Specific MDAs in Control Piglets
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392. [Google Scholar]
- Nathues, H.; Alarcon, P.; Rushton, J.; Jolie, R.; Fiebig, K.; Jimenez, M.; Geurts, V.; Nathues, C. Cost of porcine reproductive and respiratory syndrome virus at individual farm level—An economic disease model. Prev. Vet. Med. 2017, 142, 16–29. [Google Scholar]
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. ICTV Virus Taxonomy Profile: Arteriviridae 2021. J. Gen. Virol. 2021, 102, 001632. [Google Scholar]
- Zimmerman, J.J.; Dee, S.A.; Holtkamp, D.J.; Murtaugh, M.P.; Stadejek, T.; Stevenson, G.W.; Torremorell, M.; Yang, H.; Zhang, J. Porcine Reproductive and Respiratory Syndrome Viruses (Porcine Arteriviruses) in Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 685–708. [Google Scholar]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Kimman, T.G.; Cornelissen, L.A.; Moormann, R.J.; Rebel, J.M.; Stockhofe-Zurwieden, N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine 2009, 27, 3704–3718. [Google Scholar] [PubMed]
- Zhou, L.; Ge, X.; Yang, H. Porcine Reproductive and Respiratory Syndrome Modified Live Virus Vaccine: A “Leaky” Vaccine with Depatable Efficacy and Safety. Vaccines 2021, 9, 362. [Google Scholar] [PubMed]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.J.; Zhou, E.M. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef] [PubMed]
- Eclercy, J.; Renson, P.; Lebret, A.; Hirchaud, E.; Normand, V.; Andraud, M.; Paboeuf, F.; Blanchard, Y.; Rose, N.; Bourry, O. A Field Recombinant Strain Derived from Two Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV-1) Modified Live Vaccines Shows Increased Viremia and Transmission in SPF Pigs. Viruses 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, X.; Zhai, J.; Wei, C.; Dai, A.; Yang, X.; Luo, M. Recombination in JXA1-R vaccine and NADC30-like strain of porcine reproductive and respiratory syndrome viruses. Vet. Microbiol. 2017, 204, 110–120. [Google Scholar] [PubMed]
- Li, B.; Fang, L.; Xu, Z.; Liu, S.; Gao, J.; Jiang, Y.; Chen, H.; Xiao, S. Recombination in vaccine and circulating strains of porcine reproductive and respiratory syndrome viruses. Emerg. Infect. Dis. 2009, 15, 2032–2035. [Google Scholar]
- Wenhui, L.; Zhongyan, W.; Guanqun, Z.; Zhili, L.; JingYun, M.; Qingmei, X.; Baoli, S.; Yingzuo, B. Complete genome sequence of a novel variant porcine reproductive and respiratory syndrome virus (PRRSV) strain: Evidence for recombination between vaccine and wild-type PRRSV strains. J. Virol. 2012, 86, 9543. [Google Scholar]
- Kristensen, C.S.; Christiansen, M.G.; Pedersen, K.; Larsen, L.E. Production losses five months after outbreak with a recombinant of two PRRSV vaccine strains in 13 Danish sow herds. Porcine Health Manag. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Mathijs, E.; Tignon, M.; Vandersmissen, T.; Cay, A.B. WGS- versus ORF5-Based Typing of PRRSV: A Belgian Case Study. Viruses 2021, 13, 2419. [Google Scholar] [CrossRef] [PubMed]
- Fiers, J.; Tignon, M.; Cay, A.B.; Simons, X.; Maes, D. Porcine Reproductive and Respiratory Syndrome virus (PRRSv): A Cross-Sectional Study on ELISA Seronegative, Multivaccinated Sows. Viruses 2022, 14, 1944. [Google Scholar] [CrossRef]
- Fablet, C.; Renson, P.; Eono, F.; Mahé, S.; Eveno, E.; Le Dimna, M.; Normand, V.; Lebret, A.; Rose, N.; Bourry, O. Maternally derived antibodies (MDAs) impair piglets’ humoral and cellular immune responses to vaccination against porcine reproductive and respiratory syndrome (PRRS). Vet. Microbiol. 2016, 192, 175–180. [Google Scholar] [PubMed]
- Renson, P.; Fablet, C.; Andraud, M.; Normand, V.; Lebret, A.; Paboeuf, F.; Rose, N.; Bourry, O. Maternally-derived neutralizing antibodies reduce vaccine efficacy against porcine reproductive and respiratory syndrome virus infection. Vaccine 2019, 37, 4318–4324. [Google Scholar] [PubMed]
- Sattler, T.; Pikalo, J.; Wodak, E.; Schmoll, F. Performance of ELISAs for detection of antibodies against porcine respiratory and reproductive syndrome virus in serum of pigs after PRRSV type 2 live vaccination and challenge. Porcine Health Manag. 2015, 1, 19. [Google Scholar] [PubMed]
- Yoon, I.J.; Joo, H.S.; Goyal, S.M.; Molitor, T.W. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J. Vet. Diagn. Investig. 1994, 6, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Dee, S.; Halbur, P.; Keffaber, K.; Lautner, B.; McCaw, M.; Rodibaugh, M.; Sanford, E.; Yeske, P. Laboratory diagnosis of porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Swine Health Prod. 1996, 4, 33–35. [Google Scholar]
- Martinez-Lobo, F.J.; Carracosa de Lome, L.; Diez-Fuertes, F.; Segalés, J.; Garcia-Artiga, C.; Simarro, I.; Castro, J.M.; Prieto, C. Safety of Porcine Reproductive and Respiratory Syndrome Modified Live Virus (MLV) vaccine strains in a young pig infection model. Vet. Res. 2013, 44, 115. [Google Scholar] [PubMed]
- Mahe, S.; Renson, P.; Andraud, M.; Le Dimna, M.; Rose, N.; Paboeuf, F.; Bourry, O. Evaluation of PRRS MLV viremia and transmission for a better prevention of recombination between vaccine strains. In Proceedings of the European Symposium of Porcine Health Management, Budapest, Hungary, 11–13 May 2022. [Google Scholar]


| E+ Piglets | E− Piglets | |||
|---|---|---|---|---|
| Vaccinated | Control | Vaccinated | Control | |
| Herd 1 | 20 | 9 | 36 | 18 |
| Herd 2 | 39 | 20 | 20 | 11 |
| Sow Number (Parity) | S/p ELISA 1 (90d Gestation) | IRPC ELISA 2 (90d Gestation) | S/p ELISA 1 (1 wpf) | IRPC ELISA 2 (1 wpf) | Log2 VN Titer (1 wpf) | PRRSv Serostatus |
|---|---|---|---|---|---|---|
| Herd 1 | ||||||
| Sow 1 (7) | 0.36 | 6.62 | 0.19 | 5.84 | 0.00 | Seronegative |
| Sow 2 (4) | 0.39 | 28.04 | 0.30 | 15.56 | 2.00 | Seronegative |
| Sow 3 (7) | 0.79 | 38.88 | 0.44 | 17.45 | 2.00 | Seronegative |
| Sow 4 (5) | 0.65 | 20.98 | 0.63 | 20.10 | 0.00 | Seronegative |
| Sow 5 (1) | 0.27 | 18.07 | 0.15 | 19.86 | 0.00 | Seronegative |
| Sow 6 (6) | 2.20 | 128.93 | 1.96 | 100.85 | 4.58 | Seropositive |
| Sow 7 (5) | 2.32 | 134.10 | 2.00 | 123.99 | 4.58 | Seropositive |
| Sow 8 (1) | 1.82 | 113.93 | 1.08 | 94.21 | 0.00 | Seropositive |
| Herd 2 | ||||||
| Sow 1 (5) | 0.14 | 6.41 | 0.14 | 5.96 | 2.00 | Seronegative |
| Sow 2 (3) | 0.43 | 17.67 | 0.37 | 13.12 | 1.58 | Seronegative |
| Sow 3 (3) | 0.29 | 12.06 | 0.27 | 7.50 | 2.58 | Seronegative |
| Sow 4 (4) | 1.93 | 117.40 | 1.47 | 65.04 | 1.58 | Seropositive |
| Sow 5 (3) | 1.75 | 109.76 | 1.71 | 89.25 | 2.00 | Seropositive |
| Sow 6 (3) | 1.30 | 46.18 | 1.41 | 54.30 | 2.58 | Seropositive |
| Sow 7 (1) | 0.59 | 47.30 | 2.01 | 120.24 | 4.58 | Seropositive |
| Sow 8 (1) | 2.05 | 105.60 | 1.87 | 85.11 | 1.58 | Seropositive |
| Sow Number (Parity) | S/p ELISA 1 (1 wpf) | IRPC ELISA 2 (1 wpf) | Log2 VN Titer (1 wpf) | S/p ELISA 1 Piglets | IRPC ELISA 2 Piglets | Log2 VN Titer Piglets |
|---|---|---|---|---|---|---|
| Herd 1 | ||||||
| Sow 1 (7) | 0.19 | 5.84 | 0 | 0.13 ± 0.02 | 1.81 ± 1.36 | 0.70 ± 0.61 |
| Sow 2 (4) | 0.30 | 15.56 | 2 | 0.13 ± 0.03 | 12.67 ± 5.60 | 0.91 ± 0.30 |
| Sow 3 (7) | 0.44 | 17.45 | 2 | 0.28 ± 0.08 | 12.63 ± 9.73 | 0.20 ± 0.50 |
| Sow 4 (5) | 0.63 | 20.10 | 0 | 0.32 ± 0.04 | 13.95 ± 4.62 | 0.33 ± 0.73 |
| Sow 5 (1) | 0.15 | 19.86 | 0 | 0.06 ± 0.03 | 9.66 ± 5.68 | 0.00 ± 0.00 |
| Sow 6 (6) | 1.96 | 100.85 | 4.58 | 1.48 ± 0.21 | 73.33 ± 14.68 | 2.49 ± 0.77 |
| Sow 7 (5) | 2.00 | 123.99 | 4.58 | 1.46 ± 0.26 | 85.24 ± 30.89 | 2.08 ± 1.05 |
| Sow 8 (1) | 1.08 | 94.21 | 0 | 1.04 ± 0.19 | 66.62 ± 19.93 | 0.10 ± 0.32 |
| Herd 2 | ||||||
| Sow 1 (5) | 0.14 | 5.96 | 2 | 0.01 ± 0.01 | 0.39 ± 0.52 | 0.00 ± 0.00 |
| Sow 2 (3) | 0.37 | 13.12 | 1.58 | 0.03 ± 0.04 | 1.07 ± 1.17 | 0.63 ± 0.87 |
| Sow 3 (3) | 0.27 | 7.50 | 2.58 | 0.04 ± 0.03 | 1.65 ± 1.20 | 0.00 ± 0.00 |
| Sow 4 (4) | 1.47 | 65.04 | 1.58 | 0.93 ± 0.1 | 37.27 ± 6.46 | 0.13 ± 0.35 |
| Sow 5 (3) | 1.71 | 89.25 | 2 | 0.78 ± 0.11 | 38.84 ± 13.60 | 0.00 ± 0.00 |
| Sow 6 (3) | 1.41 | 54.30 | 2.58 | 0.54 ± 0.12 | 10.50 ± 4.40 | 0.38 ± 0.52 |
| Sow 7 (1) | 2.01 | 120.24 | 4.58 | 0.89 ± 0.34 | 41.89 ± 16.39 | 1.99 ± 1.00 |
| Sow 8 (1) | 1.87 | 85.11 | 1.58 | 0.87 ± 0.21 | 35.75 ± 16.15 | 0.68 ± 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiers, J.; Tignon, M.; Maes, D.; Cay, A.-B. Follow-Up of PRRSv-Vaccinated Piglets Born from PRRSv-Vaccinated, ELISA-Seropositive and ELISA-Seronegative Sows. Viruses 2023, 15, 479. https://doi.org/10.3390/v15020479
Fiers J, Tignon M, Maes D, Cay A-B. Follow-Up of PRRSv-Vaccinated Piglets Born from PRRSv-Vaccinated, ELISA-Seropositive and ELISA-Seronegative Sows. Viruses. 2023; 15(2):479. https://doi.org/10.3390/v15020479
Chicago/Turabian StyleFiers, Jorian, Marylène Tignon, Dominiek Maes, and Ann-Brigitte Cay. 2023. "Follow-Up of PRRSv-Vaccinated Piglets Born from PRRSv-Vaccinated, ELISA-Seropositive and ELISA-Seronegative Sows" Viruses 15, no. 2: 479. https://doi.org/10.3390/v15020479
APA StyleFiers, J., Tignon, M., Maes, D., & Cay, A.-B. (2023). Follow-Up of PRRSv-Vaccinated Piglets Born from PRRSv-Vaccinated, ELISA-Seropositive and ELISA-Seronegative Sows. Viruses, 15(2), 479. https://doi.org/10.3390/v15020479







