Japanese Encephalitis Enzootic and Epidemic Risks across Australia
Abstract
1. Introduction
2. Materials and Methods
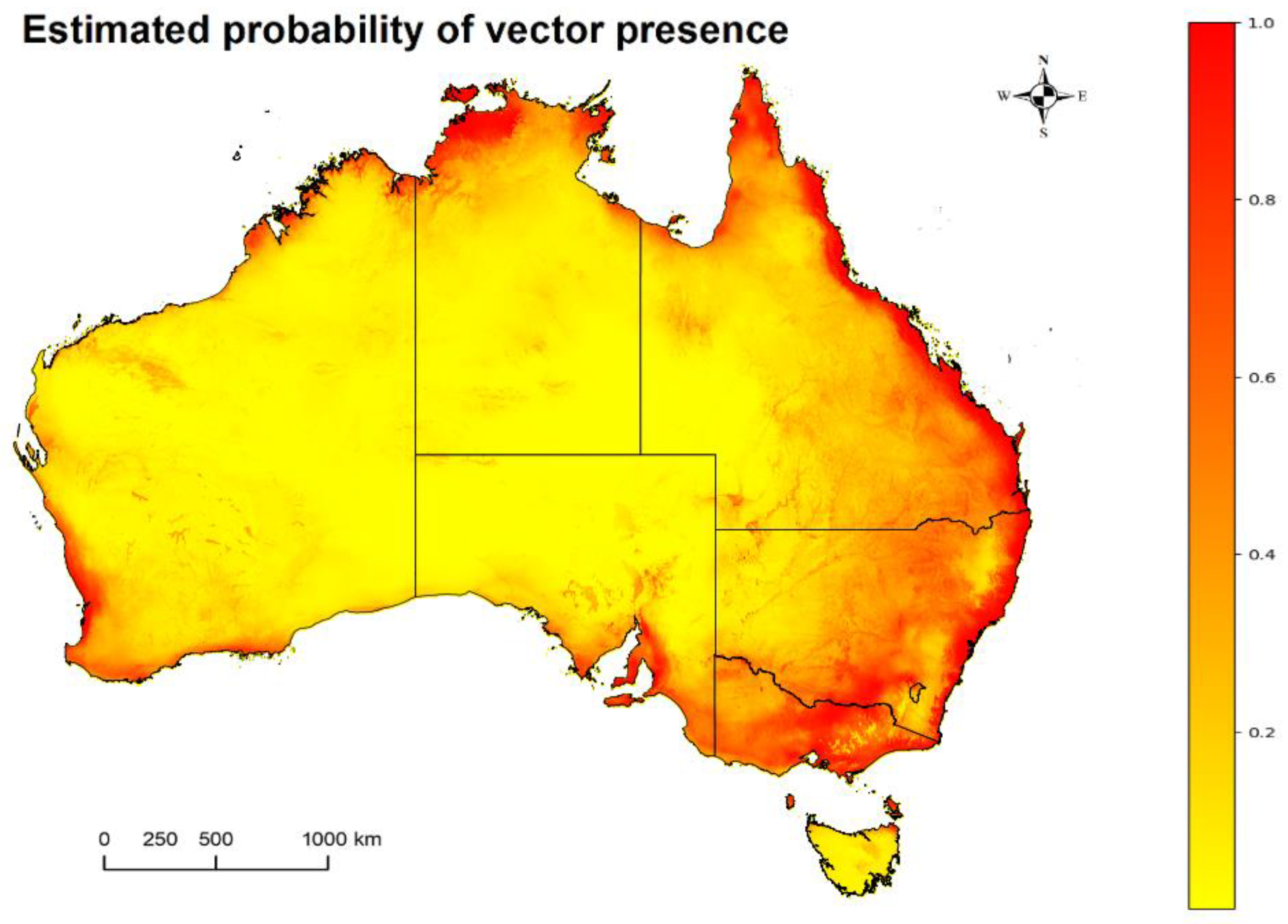
2.1. Mosquitoes: Potentially Competent JEV Vector Occurrence Estimates in Australia
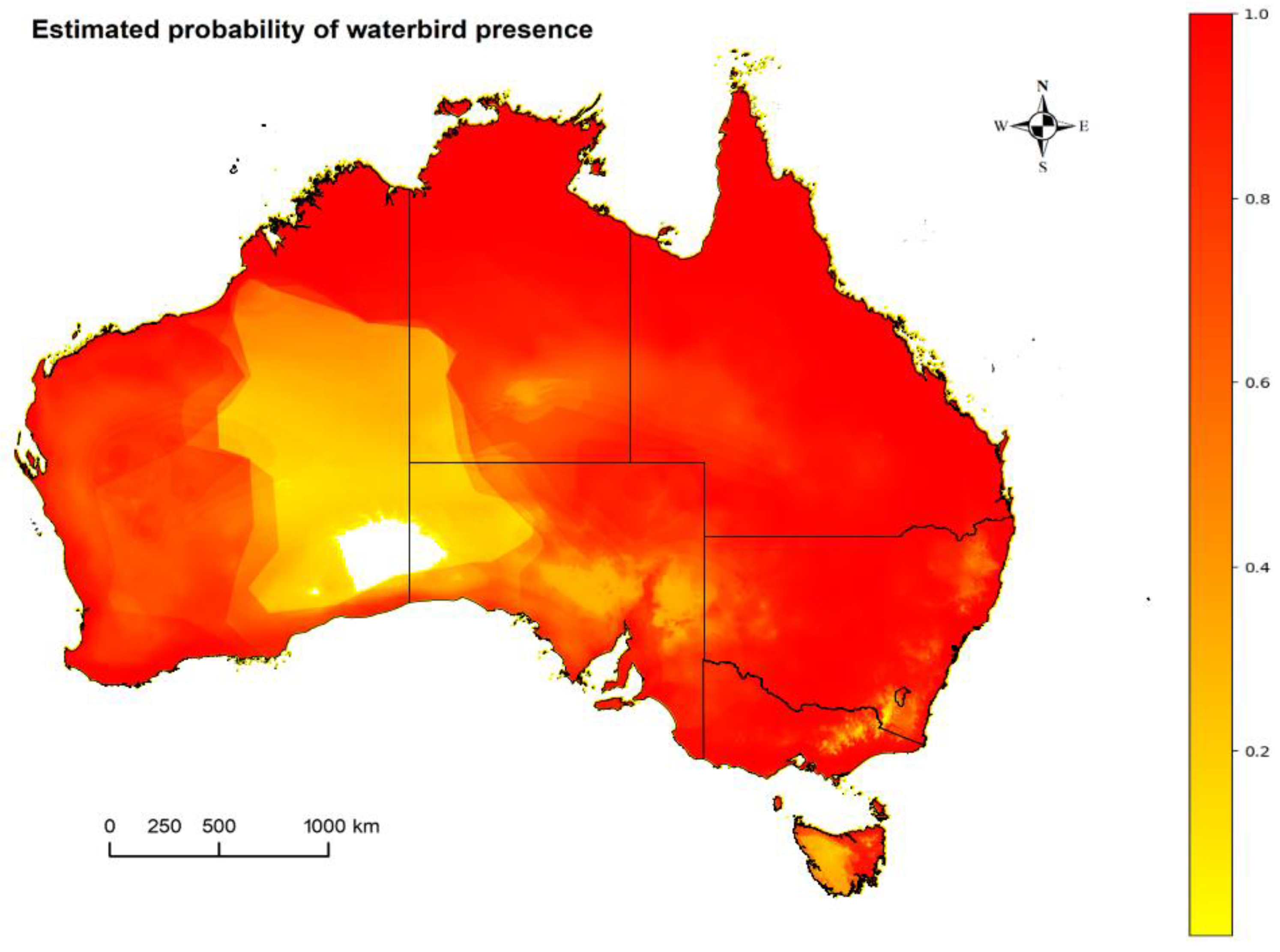
2.2. Waterbirds: Occurrence Estimates of Suspected Waterbird Reservoirs in Australia
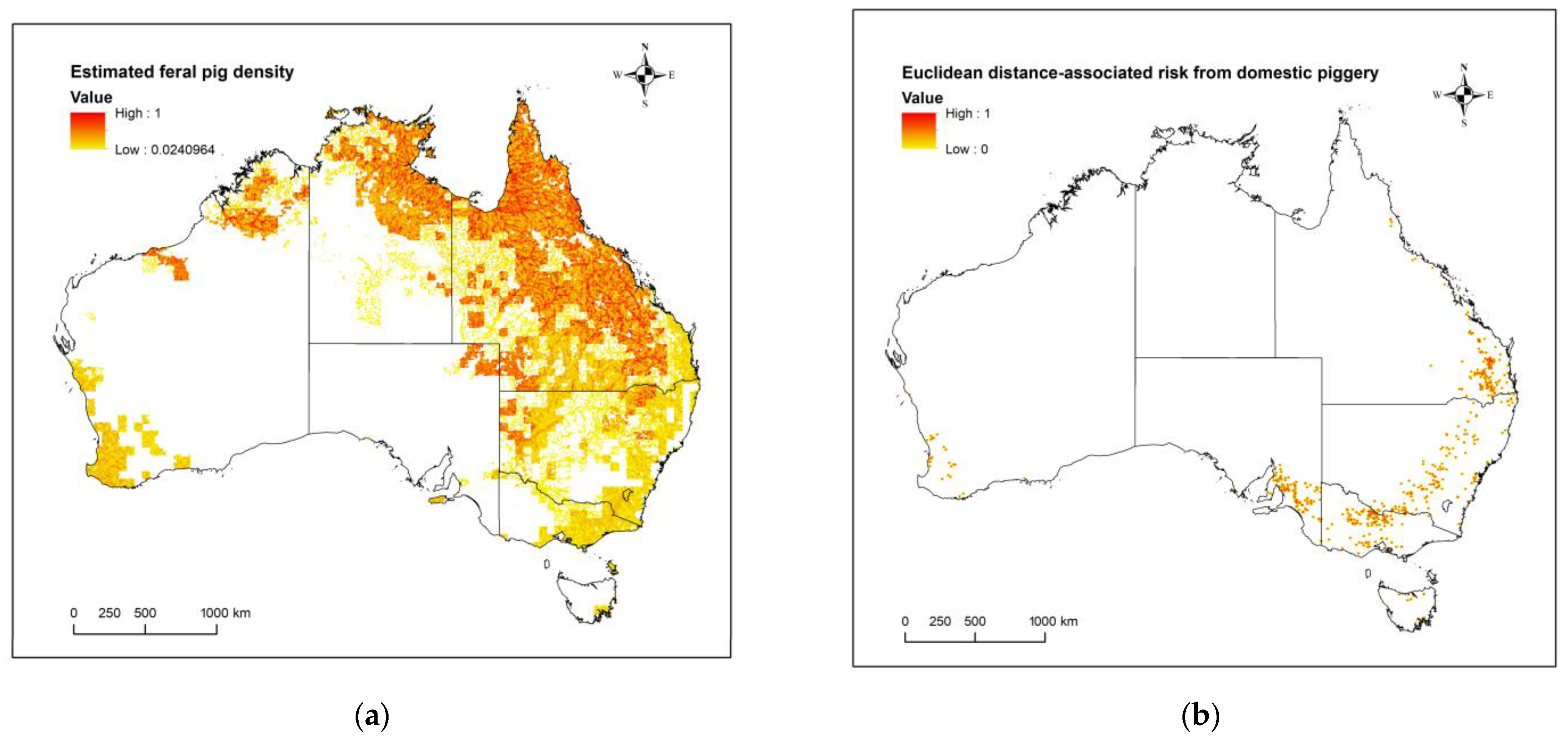
2.3. Domestic Pigs: Estimated Risk of JEV Transmission Given Distance from a Domestic Piggery
2.4. Feral Pigs: Occurrence through Population Density Estimates in Australia
2.5. Humans: Estimated Population Density in Australia
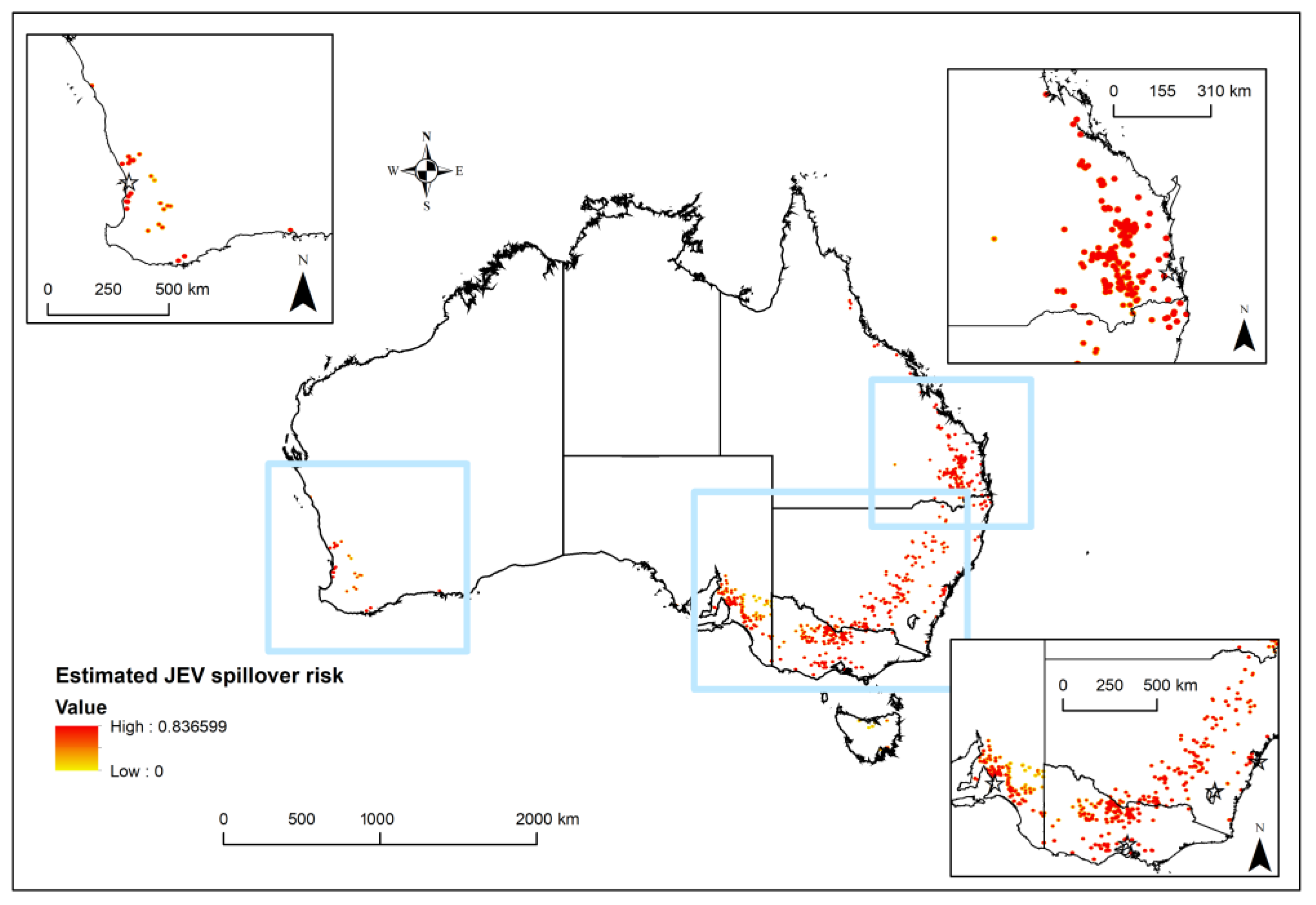
2.6. Synthesis and JEV Risk Map Combinations
2.7. Definition of Terms
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, Present, and Future of Japanese Encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Japanese Encephalitis—Fact Sheet; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 26 November 2022).
- Mackenzie, J.S.; Williams, D.T.; van den Hurk, A.F.; Smith, D.W.; Currie, B.J. Japanese encephalitis virus: The emergence of genotype IV in Australia and its potential endemicity. Viruses 2022, 14, 2480. [Google Scholar] [CrossRef]
- Australian Government Department of Agriculture, Fisheries and Forestry. Japanese Encephalitis. Available online: https://www.agriculture.gov.au/biosecurity-trade/pests-diseases-weeds/animal/japanese-encephalitis (accessed on 23 November 2022).
- Hutchinson, D.; Kunasekaran, M.; Chen, X.; Moa, A. Japanese encephalitis outbreak in South-Eastern Australia. Glob. Biosecurity 2022, 4, 159. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Skinner, E.; Ritchie, S.A.; Mackenzie, J.S. The emergence of Japanese encephalitis virus in Australia in 2022: Existing knowledge of mosquito vectors. Viruses 2022, 14, 1208. [Google Scholar] [CrossRef]
- NSW Health NG. Japanese Encephalitis (JE): NSW Health. 2022. Available online: https://www.health.nsw.gov.au/environment/pests/vector/Pages/japanese-encephalitis.aspx (accessed on 27 October 2022).
- Weaver, S.C.; Barrett, A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, A.F.; Ritchie, S.A.; Mackenzie, J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009, 54, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Williams, D.T.; Smith, D.W. Japanese encephalitis virus: The geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. Persp. Med. Virol. 2006, 16, 201–268. [Google Scholar]
- Solomon, T.; Ni, H.; Beasley, D.W.; Ekkelenkamp, M.; Cardosa, M.J.; Barrett, A.D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 2003, 5, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Schuh, A.J.; Ward, M.J.; Brown, A.L.; Barrett, A.D.T. Phylogeography of Japanese Encephalitis Virus: Genotype Is Associated with Climate. PLoS Negl. Trop. Dis. 2013, 7, e2411. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935–2017. Vector-Borne Zoonotic Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 1, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Horwood, P.F.; Duong, V.; Laurent, D.; Mey, C.; Sothy, H.; Santy, K.; Richner, B.; Heng, S.; Hem, S.; Cheval, J.; et al. Aetiology of acute meningoencephalitis in Cambodian children, 2010–2013. Emerg. Microbes Infect. 2017, 5, e35. [Google Scholar] [CrossRef]
- Friedman, D. JEV Outbreak in Australia [Webinar]. Sydney Institute for Infectious Diseases. Available online: https://www.sydney.edu.au/infectious-diseases-institute/news-and-events/distinguished-lecture-series.html (accessed on 3 June 2022).
- Quan, T.M.; Thao, T.T.N.; Duy, N.M.; Nhat, T.M.; Clapham, H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000–2015. Elife. 2020, 9, e51027. [Google Scholar] [CrossRef]
- Park, S.L.; Huang, Y.S.; Vanlandingham, D.L. Re-examining the importance of pigs in the transmission of Japanese encephalitis virus. Pathogens 2022, 11, 575. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Ståhl, K.; Chirico, J.; Boqvist, S.; Thu, H.T.; Magnusson, U. Circulation of Japanese encephalitis virus in pigs and mosquito vectors within Can Tho city, Vietnam. PLoS Negl. Trop. Dis. 2013, 4, e2153. [Google Scholar] [CrossRef]
- Sakamoto, R.; Tanimoto, T.; Takahashi, K.; Hamaki, T.; Kusumi, E.; Crump, A. Flourishing Japanese encephalitis, associated with global warming and urbanisation in Asia, demands widespread integrated vaccination programmes. Ann. Glob. Health 2019, 1, 111. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.S.; Leake, C.J. Japanese encephalitis. In The Arboviruses: Epidemiology and Ecology; CRC Press: Boca Raton, FL, USA, 2019; pp. 63–92. [Google Scholar]
- van den Hurk, A.F.; Pyke, A.T.; Mackenzie, J.S.; Hall-Mendelin, S.; Ritchie, S.A. Japanese encephalitis virus in Australia: From known known to known unknown. Trop. Med. Infect. Dis. 2019, 1, 38. [Google Scholar] [CrossRef] [PubMed]
- Endy, T.P.; Nisalak, A. Japanese encephalitis virus: Ecology and epidemiology. Curr. Top. Microbiol. Immunol. 2002, 267, 11–48. [Google Scholar]
- Ghosh, D.; Basu, A. Japanese encephalitis-a pathological and clinical perspective. PLoS Negl. Trop. Dis. 2009, 9, e437. [Google Scholar] [CrossRef]
- Cleton, N.B.; Bosco-Lauth, A.; Page, M.J.; Bowen, R.A. Age-related susceptibility to Japanese encephalitis virus in domestic ducklings and chicks. Am. J. Trop. Med. Hyg. 2014, 2, 242–246. [Google Scholar] [CrossRef]
- West, P. National Land & Water Resources Audit and Invasive Animals Cooperative Research Centre. In Assessing Invasive Animals in Australia 2008; NLWRA: Canberra, Australia, 2008. [Google Scholar]
- Hone, J. How many feral pigs in Australia? An update. Aust. J. Zool. 2019, 67, 215–220. [Google Scholar] [CrossRef]
- Scherer, W.F.; Buescher, E.L.; Mcclure, H.E. Ecologic studies of Japanese encephalitis virus in Japan. V. avian factors. Am. J. Trop. Med. Hyg. 1959, 8, 689–697. [Google Scholar] [CrossRef]
- Nitatpattana, N.; Le Flohic, G.; Thongchai, P.; Nakgoi, K.; Palaboodeewat, S.; Khin, M.; Barbazan, P.; Yoksan, S.; Gonzalez, J.P. Elevated Japanese encephalitis virus activity monitored by domestic sentinel piglets in Thailand. Vector Borne Zoonotic Dis. 2011, 4, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Ladreyt, H.; Durand, B.; Dussart, P.; Chevalier, V. How central is the domestic pig in the epidemiological cycle of Japanese encephalitis virus? A review of scientific evidence and implications for disease control. Viruses 2019, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.G.; Pattanaik, A.; Vyas, N.; Saxena, D.; Webb, C.; Sawleshwarkar, S.; Mukhopadhyay, C. A biogeographical description of the wild waterbird species associated with high-risk landscapes of Japanese encephalitis virus in India. Transbound Emerg. Dis. 2022, 5, e3015–e3023. [Google Scholar] [CrossRef] [PubMed]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 9, e2208. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.B.; Dickerman, R.W.; Marshall, I.D. Primary viraemia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Aust. J. Exp. Biol. Med. Sci. 1983, 6, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T. Control of Japanese encephalitis--within our grasp? N. Engl. J. Med. 2006, 9, 869–871. [Google Scholar] [CrossRef]
- Williams, C.R.; Webb, C.E.; Higgs, S.; van den Hurk, A.F. Japanese encephalitis virus emergence in Australia: Public health importance and implications for future surveillance. Vector Borne Zoonotic Dis. 2022, 11, 529–534. [Google Scholar] [CrossRef]
- Waller, C.; Tiemensma, M.; Currie, B.J.; Williams, D.T.; Baird, R.W.; Krause, V.L. Japanese encephalitis in Australia—A sentinel case. N. Engl. J. Med. 2022, 7, 661–662. [Google Scholar] [CrossRef]
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese encephalitis: The vectors, ecology and potential for expansion. J. Travel. Med. 2018, 25, S16–S26. [Google Scholar] [CrossRef]
- Auerswald, H.; Maquart, P.O.; Chevalier, V.; Boyer, S. Mosquito vector competence for Japanese encephalitis virus. Viruses 2021, 6, 1154. [Google Scholar] [CrossRef]
- Rosen, L. The natural history of Japanese encephalitis virus. Annu. Rev. Microbiol. 1986, 40, 395–414. [Google Scholar] [CrossRef]
- Gresser, I.; Hardy, J.L.; Hu, S.M.; Scherer, W.F. Factors influencing transmission of Japanese B encephalitis virus by a colonized strain of Culex tritaeniorhynchus Giles, from infected pigs and chicks to susceptible pigs and birds. Am. J. Trop. Med. Hyg. 1958, 4, 365–373. [Google Scholar] [CrossRef]
- Furlong, M.; Adamu, A.; Hickson, R.I.; Horwood, P.; Golchin, M.; Hoskins, A.; Russell, T. Estimating the distribution of Japanese encephalitis vectors in Australia using ecological niche modelling. Trop. Med. Infect. Dis. 2022, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.M.; Reside, A.E.; Atkinson, I.; Baird, D.; Hodgson, L.; James, C.S.; VanDerWal, J.J. Climate change and biodiversity in Australia: A systematic modelling approach to nationwide species distributions. Aust. J. Env. Mgt. 2019, 26, 112–123. [Google Scholar] [CrossRef]
- Webb, C.; Russell, R.; Doggett, S. A Guide to Mosquitoes of Australia. 2016. Available online: https://ebooks.publish.csiro.au/content/9780643104464/9780643104464 (accessed on 23 November 2022).
- Bondarenko, M.; Kerr, D.; Sorichetta, A.; Tatem, A.J. Census/Projection-Disaggregated Gridded Population Datasets, Adjusted to Match the Corresponding UNPD 2020 Estimates, for 183 Countries in 2020 Using Built-Settlement Growth Model (BSGM) Outputs; WorldPop, University of Southampton: Southampton, UK, 2020. [Google Scholar] [CrossRef]
- Australian Government Geoscience Australia. Australia’s Coastline: Adapting to Climatechange. 2011. Available online: https://www.ga.gov.au/ausgeonews/ausgeonews201103/climate.jsp (accessed on 26 October 2022).
- Lord, J.S.; Gurley, E.S.; Pulliam, J.R. Rethinking Japanese encephalitis virus transmission: A framework for implicating host and vector species. PLoS Negl. Trop. Dis. 2015, 12, e0004074. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.M.; Alkishe, A.A.; Thomas, S.M.; Wang, L.; Zhang, W. Mapping the potential distributions of etiological agent, vectors, and reservoirs of Japanese encephalitis in Asia and Australia. Acta Trop. 2018, 188, 108–117. [Google Scholar] [CrossRef]
- Johansen, C.A.; van den Hurk, A.F.; Pyke, A.T.; Zborowski, P.; Phillips, D.A.; Mackenzie, J.S.; Ritchie, S.A. Entomological investigations of an outbreak of Japanese encephalitis virus in the Torres Strait, Australia, in 1998. J. Med. Entomol. 2001, 4, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, I.; Oda, K.; Tsuchie, H.; Mahadevan, S.; Vijayamalar, B. Isolation of Japanese encephalitis virus from Culex sitiens mosquitoes in Selangor, Malaysia. J. Am. Mosq. Control. Assoc. 1994, 10, 228–229. [Google Scholar]
- Nicholson, J.; Ritchie, S.A.; van den Hurk, A.F. Aedes albopictus (Diptera: Culicidae) as a potential vector of endemic and exotic arboviruses in Australia. J. Med. Entomol. 2014, 3, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Purnell, C. The Role of Waterbirds in Australia’s 2022 Japanese Encephalitis Outbreak Unpublished—A Rapid Synthesis; Birdlife Australia: Carlton, Australia, 2002. [Google Scholar]
- Guay, P.J.; Azuolas, J.; Warner, S. Waterbird movement across the Great Dividing Range and implications for arbovirus irruption into southern Victoria. Aust. Vet. J. 2012, 5, 197–198. [Google Scholar] [CrossRef]
- McGinness, H.; Brandis, K.; Robinson, F.; Piper, M.; O’Brien, L.; Langston, A.; Hodgson, J.; Wenger, L.; Martin, J.; Bellio, M.; et al. Murray-Darling Basin Environmental Water Knowledge and Research Project: Waterbirds Theme Research Report; La Trobe University: Canberra, Australia, 2020. [Google Scholar] [CrossRef]
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.; Furuya-Kanamori, L.; Magalhaes, R.S.; Devine, G. Japanese encephalitis emergence in Australia: The potential population at risk. Clin. Infect. Dis. 2023, 76, 335–337. [Google Scholar] [CrossRef]
- Jeffries, C.L.; Walker, T. The potential use of Wolbachia-based mosquito biocontrol strategies for Japanese encephalitis. PLoS Negl. Trop. Dis. 2015, 6, e0003576. [Google Scholar] [CrossRef]
- Cappelle, J.; Duong, V.; Pring, L.; Kong, L.; Yakovleff, M.; Prasetyo, D.B.; Peng, B.; Choeung, R.; Duboz, R.; Ong, S.; et al. Intensive circulation of Japanese encephalitis virus in peri-urban sentinel pigs near Phnom Penh, Cambodia. PLoS Negl. Trop. Dis. 2016, 10, e0005149. [Google Scholar] [CrossRef]
- Ricklin, M.E.; García-Nicolás, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Nougairede, A.; Charrel, R.N.; Posthaus, H.; Oevermann, A.; Summerfield, A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016, 7, 10832. [Google Scholar] [CrossRef] [PubMed]
- ATAGI (Australian Technical Advisory Group on Immunisation). Australian Immunisation Handbook; Australian Government Department of Health: Canberra, Australia, 2018. Available online: https://immunisationhandbook.health.gov.au (accessed on 12 September 2022).
- World Health Organization Disease Outbreak News; Japanese Encephalitis—Australia. 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON365 (accessed on 2 October 2022).
- Wada, Y. Strategies for control of Japanese encephalitis in rice production systems in developing countries. In Vector-Borne Disease Control in Humans through Rice Agroecosystem Management; International Rice Research Institute: Los Banos, Philippines, 1988; pp. 153–160. [Google Scholar]
- Mulvey, P.; Duong, V.; Boyer, S.; Burgess, G.; Williams, D.T.; Dussart, P.; Horwood, P.F. The ecology and evolution of Japanese encephalitis virus. Pathogens 2021, 10, 1534. [Google Scholar] [CrossRef]
- Daniels, P.; Williams, D.; Mackenzie, J. Japanese encephalitis. In Trends in Emerging Viral Diseases of Swine; Iowa State Press: Ames, IA, USA, 2002; pp. 249–263. [Google Scholar]
- Lam, K.H.; Ellis, T.M.; Williams, D.T.; Lunt, R.A.; Daniels, P.W.; Watkins, K.L.; Riggs, C.M. Japanese encephalitis in a racing thoroughbred gelding in Hong Kong. Vet Rec. 2005, 6, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Australian Pork Limited. How many registered pig production sites are in Australia? 2022. Available online: https://australianpork.com.au/industry-facts (accessed on 13 December 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlong, M.; Adamu, A.M.; Hoskins, A.; Russell, T.L.; Gummow, B.; Golchin, M.; Hickson, R.I.; Horwood, P.F. Japanese Encephalitis Enzootic and Epidemic Risks across Australia. Viruses 2023, 15, 450. https://doi.org/10.3390/v15020450
Furlong M, Adamu AM, Hoskins A, Russell TL, Gummow B, Golchin M, Hickson RI, Horwood PF. Japanese Encephalitis Enzootic and Epidemic Risks across Australia. Viruses. 2023; 15(2):450. https://doi.org/10.3390/v15020450
Chicago/Turabian StyleFurlong, Morgan, Andrew M. Adamu, Andrew Hoskins, Tanya L. Russell, Bruce Gummow, Maryam Golchin, Roslyn I. Hickson, and Paul F. Horwood. 2023. "Japanese Encephalitis Enzootic and Epidemic Risks across Australia" Viruses 15, no. 2: 450. https://doi.org/10.3390/v15020450
APA StyleFurlong, M., Adamu, A. M., Hoskins, A., Russell, T. L., Gummow, B., Golchin, M., Hickson, R. I., & Horwood, P. F. (2023). Japanese Encephalitis Enzootic and Epidemic Risks across Australia. Viruses, 15(2), 450. https://doi.org/10.3390/v15020450






