Abstract
Human papillomavirus (HPV) is an important risk factor in a subset of head and neck squamous cell carcinomas (HNSCC), but the association with oral cavity squamous cell carcinomas (OCSCC) remains controversial. This study aimed to identify the prevalence of HPV infection in OCSCC. A systematic search on PubMed and EMBASE was performed, including articles assessing the prevalence of HPV-positive (HPV+) OCSCC published from January 2017 to December 2022. OCSCC was considered HPV+ by the detection of HPV DNA, HPV RNA, and/or p16 overexpression in the tumor mass. A meta-analysis was made determining the overall HPV+ OCSCC prevalence. We included 31 studies comprising 5007 patients from 24 countries. The study size ranged from 17 to 940 patients. The HPV+ OCSCC proportion variated widely and ranged from 0% to 37%. Tumors in the tongue were the predominant sublocation for HPV in the oral cavity. The meta-analysis revealed that the overall HPV+ OCSCC prevalence is 6% (95% CI; 3–10%), and only one study found HPV and OCSCC significantly associated. Thus, HPV may not be a necessary or a strong risk factor in OCSCC oncogenesis, and the possibility of a site misclassification of a mobile tongue with the root of the tongue cannot be excluded.
1. Introduction
Oral cavity cancer is the most frequent cancer subsite in the head and neck area with more than 377,700 cases worldwide in 2020, placing them as the 16th most common cancer overall, the 11th most prevalent cancer in men, and the 18th most frequent cancer in women [1]. In addition, human papillomavirus (HPV) is widely prevalent worldwide and is the most frequent sexually transmitted infection in the United States [2,3]. It is well established that HPV is an important risk factor in a subset of head and neck squamous cell carcinoma, particularly oropharyngeal squamous cell carcinomas (OPSCC) [4,5,6,7].
Although well-known risk factors of oral cavity cancer are the consumption of tobacco (both smoked and smokeless), alcohol, and poor oral hygiene [8], the association of HPV as a risk factor for oral cavity squamous cell carcinomas (OCSCC) remains controversial since the association was described for the first time in 1983 [9].
HPV can be classified into either a low-risk HPV or high-risk (HR) HPV regarding oncogenicity. HR-HPV genotypes currently known in oropharyngeal tumors are HPV16, 18, 26, 31, 33, 35, 45, 56, 58, 59, and 67 [10]. The predominant HR-HPV genotype in oropharyngeal tumors is HPV16, accounting for 86% of HPV+ OPSCC [10].
There are no universal HPV-testing methods available for OCSCC. The identification of HPV E6/E7 mRNA expression is considered the golden standard for measuring HPV infection by some authors, as this technique detects oncogenic transcriptionally active HPV. However, the method is expensive, technically challenging to perform, and requires fresh frozen tumor material, which is not always collected [11,12,13]. The World Health Organization (WHO) has stated that the diffuse cytoplasmic and nuclear immunoreactivity for p16 may act as a trustworthy surrogate marker for the incidence of HR-HPV in OPSCC, but the method has only been validated for OPSCC and does not apply to OCSCC [14]. Additionally, p16 does not seem to have the same role in OCSCC as in OPSCC. The overexpression of p16 has demonstrated poor performance as a prognostic marker for overall survival in OCSCC, and it has been unrecommended to use as a tool for OCSCC in study trials [15]. It has correspondingly been suggested to improve HPV detection accuracy by combining p16 immunohistochemistry and HPV DNA polymerase chain reaction analysis since this combination increases sensitivity and specificity [13,16,17].
This systematic review seeks to identify the burden of HPV infection in OCSCC globally by examining the prevalence reported in the most recently published papers.
2. Materials and Methods
2.1. Systematic Literature Search Strategy
In December 2022, a systematic search was last updated by one author (S.K.K) using the databases PubMed and EMBASE for articles regarding the prevalence of HPV+ OCSCC published between January 2017 and December 2022.
Studies including 10 or fewer patients were excluded alongside other systematic reviews and meta-analyses. Furthermore, we excluded studies if: (1) it was unclear whether the study addressed the oral cavity alone or oropharynx as well or (2) it concerned the HPV+ OCSCC prevalence in specific subpopulations stratified by gender, comorbidities, or ethnicity. An OCSCC was considered HPV+ if HPV DNA, HPV RNA, and/or p16 overexpression were detected in the tumor mass. If there was more than one way to define HPV positivity in a study, we subtracted the result in the following order: (1) double/triple positivity, (2) HPV RNA, (3) HPV DNA, and (4) p16 overexpression.
This systematic review followed the procedures of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [18]. The title and abstract of the studies were screened for eligibility while a second screening assessed the full-text. In the case of doubt regarding whether a study met the inclusion criteria, it was discussed with the author group.
The following search strategy was used when searching PubMed: (“Head and neck” or “oral cavity” or oral or mouth or lips or “buccal mucosa or tongue or “hard palate” or gingiva) AND (squamous cell carcinoma* or squamous cell neoplasm*) AND (HPV or “human papillomavirus” or “human papilloma virus” or p16) AND (prevalence or frequency or incidence). An asterisk at the end of the word means that all possible suffixes of a word are included in the search. MeSH terms (medical subject headings) were included as well: “carcinoma, squamous cell”, “neoplasm, squamous cell”, human papillomavirus, papillomaviridae. The search was restricted to the English and Danish languages.
The same keywords and Emtree (medical subject headings) were used to create four different searches in Embase, which were combined with “AND”. The search was limited to English language and articles published between 2017–2022:
- “Head and neck” or oral or “oral cavity” or mouth or lips or “buccal mucosa” or “hard palate” or gingiva or tongue;
- squamous cell carcinoma/or Squamous cell carcinoma* or squamous cell neoplasm*;
- Wart virus/or HPV or “human papillomavirus” or “human papilloma virus” or p16;
- Prevalence or incidence or frequency.
The following data were extracted from the included studies: publication year, country, female to male ratio, HPV status, anatomical sublocation, definition of HPV-positivity, mean age, and sex.
2.2. Statistical Analysis
A meta-analysis was made to determine the overall HPV+ OCSCC prevalence of the included studies. The prevalence was expressed as relative risk in the random-effects model, which was conducted due to the wide variation in the prevalence. The meta-analysis and forest plot were conducted using the software R (version 4.2.2) and the packages “meta”, “metafor”, and “forestplot”.
3. Results
The search in PubMed and Embase databases retrieved 1303 studies after duplicates were removed, shown in Figure 1. Thirty-one studies met the inclusion criteria, comprising 5007 patients with OCSCC from 24 different countries. Sixteen studies were from Asia [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], eight from Europe [35,36,37,38,39,40,41,42], two from Africa [43,44], two from South America [35,45], two from North America [46,47], and one from Asian Pacific [48]. The study size ranged from 17 patients in a study from Uganda [44] to 940 patients in the Netherlands [41]. There was no congruity in the size of the population samples regarding geographical areas.
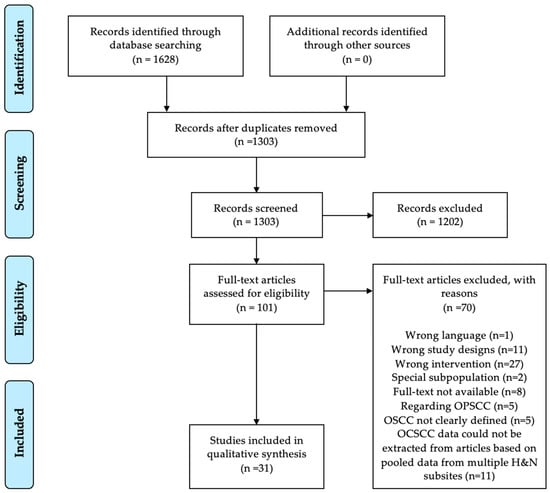
Figure 1.
Prisma Flowchart. OCSCC: oral cavity squamous cell carcinoma, OPSCC: oropharyngeal squamous cell carcinoma, OSCC: oral squamous cell carcinoma, H&N: Head and Neck.
3.1. Clinical Characteristics of HPV+ OCSCC
Five studies (n = 112) reported the mean age of patients with HPV+ OCSCC varying from 53 to 63 years. Two studies reported that HPV+ OCSCC was significantly associated with a lower mean age than HPV-negative (HPV−) OCSCC (n = 59) [38,41] while five studies found it statistically insignificant [24,27,28,30,45]. The mean age in HPV−OCSCC ranged from 57.5–64 years [37,38,41,45,49]. The overall mean age was reported in thirteen studies ranging from 45–70 years, shown in Table 1.

Table 1.
HPV-positivity in OCSCC among patients worldwide.
The female-to-male ratio varied from 1:1 in the Republic of Korea to 1:8 in Réunion Island among patients with OCSCC [23,48]. Three studies reported a significant association between patients with HPV+ OCSCCs and the male gender [37,41,49], whereas no association was reported in five studies [24,27,28,30,45].
One study (n = 26) found HPV+ OCSCC to be associated with less tobacco smoking and/or chewing [37] while six studies did not find an association [24,27,28,30,38,45].
A higher alcohol consumption and a higher number of sexual partners were demonstrated to be associated with HPV+ OCSCC in one study [38]. Two studies found an HPV association with an earlier T-stage [41,45].
Data regarding HPV+ OCSCC association with clinical characteristics could not be extracted from seventeen studies either because it was not described or the data was pooled on multiple anatomical locations in HNSCC [19,20,22,24,25,26,29,31,32,33,39,40,42,43,44,46,47].
3.2. The HPV+ OCSCC Prevalence Worldwide
The lowest prevalence of HPV+ OCSCC was 0% found in the Philippines [20], the United Kingdom (UK) [36], India [19,22], the Republic of Korea [25], and France [40]. The highest prevalence was reported to be 37% in Jordan [24]. Additionally, both low and high HPV occurrences were reported across all geographical locations. A study from India was the only study that concluded a statistically significant association of OCSCC to HPV infection by a Chi-Square Test [28], shown in Table 1.
A proportional meta-analysis was conducted, determining the total prevalence of HPV+ OCSCC to 6% (95% CI; 3–10%). There was a great heterogeneity in the prevalence as well, I2 > 75%, p < 0.01, shown in Figure 2.

Figure 2.
Meta-analysis of the HPV+ OCSCC prevalence in the included studies, representing the present HPV+ OCSCC prevalence worldwide [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. HPV+: human papillomavirus positive. OCSCC: oral cavity squamous cell carcinoma. 95% C.I: confidence interval.
The HPV status in the tumors located in different anatomical subsites of the oral cavity was specified in 16 studies (n = 284). Eight studies observed the highest proportion of HPV+ OCSCC in the tongue (n = 162) [19,24,27,28,30,35,42,45], and in three studies, the highest prevalence was seen in the floor of mouth (FoM) (n = 89) [37,38,41]. The HPV+ OCSCC proportion in the tongue varied from 0% to 100%, and among patients with tumors in the FoM, it varied from 0% to 57.1%. The specificity of the mobile tongue has been indicated as follows: * specified mobile tongue, ** specification questionable due to not following a classification system or only writing “tongue”, *** unspecified due to inclusion of overlapping lesion of tongue and/or lingual tonsil and/or tongue unspecified, shown in Table 1.
3.3. HPV Detection Methods and Definitions
Multiple different HPV detection methods were used in the included studies. Fifteen of the studies were based on double/triple positivity (n = 3743) [21,25,27,28,29,31,32,34,37,39,40,42,46,47,50], eleven based on HPV DNA (n = 1001) [20,22,24,26,30,33,36,38,43,45,49], two on HPV RNA (n = 140) [19,35], and three on p16 IHC alone (n = 123) [23,44,48], shown in Table 1. The definition of p16 IHC positivity varied among the studies. Most of the studies defined p16 overexpression as positive if staining ≥70% tumor cells were observed according to the guidelines from the College of American Pathologists [51], shown in Table 1. Both high and low HPV+ OCSCC proportions was demonstrated regardless of the detection methodology, shown in Table 1.
3.4. HPV Genotypes
Eighteen studies reported the specific HPV genotypes (n = 130) [19,21,24,25,26,27,28,29,32,34,37,39,41,42,43,45,47,49]. HPV16 was the predominant subtype in fourteen of the studies (n = 171) [21,24,25,26,28,29,32,39,41,42,43,45,47,49] and was observed in up to 100% of the HPV+ OCSCC cases in three studies (n = 17) [24,39,45]. Another common genotype was HPV18, which was the most frequent subtype in three studies (n = 28) [27,30,34] and was observed in up to 100% HPV+ OCSCC in one study (n = 8) [34]. The pooled prevalence of other HR genotypes (HPV31,33,45,52,59) presented in the enrolled studies was 6.92% (n = 9). Co-infections were reported in four studies of which HPV16 was present in most cases and was coinfected with HPV18 (n = 25), HPV31 (n = 1), and HPV39 (n = 1) [25,27,28,30].
4. Discussion
We conducted a systematic review and meta-analysis that examined the HPV+ OCSCC prevalence worldwide. Thirty-one studies were included with a total of 5007 patients. There was a wide variety in the prevalence of HPV+ OCSCC reported, with the lowest prevalence being 0% in the Philippines, the UK, India, the Republic of Korea, and France and the highest being 37% in Jordan [20,22,24,25,33,36,38,40]. Studies with a higher HPV+ proportion had greater statistical uncertainty as demonstrated in our meta-analysis, shown in Figure 2. The Jordanian paper consisted of a small study size comprising 27 patients, and they only detected HPV DNA, which should be noted. Furthermore, they found no HPV association with OCSCC or other clinical factors like tobacco, alcohol, age, or gender [24]. The studies that reported 0% HPV+ OCSCC proportion had a sample size ranging from 31 to 166 individuals and defined HPV+ as double positivity with p16+/HPV DNA or solely HPV DNA. The study from India observed the HPV+ OCSCC fraction to be 13% and was the only study to find a statistically significant association between the HPV status and OCSCC [28]. Moreover, with the HPV−OCSCC being more frequent than HPV+ OCSCC in all the studies and with our meta-analysis determining the total prevalence of HPV+ OCSCC globally being 6% (95% CI; 3–10%), it may indicate that HPV infection is not a mandatory nor a strong risk factor and does not constitute a high proportion of the OCSCC worldwide. Additionally, a study found no difference in survival outcomes between patients with HPV+ OCSCC and HPV−OCSCC when stratified on p16 overexpression status [15]. Regarding survival, our enrolled studies support that neither p16-status nor HPV-status have an impact on OCSCC patients [32,35,37,41]. Only one study reported a trend towards increased survival in HPV-positive individuals, although they did not find it statistically significant [42].
HPV+ OPSCC is more prevalent in the Western world [52,53], but in our enrolled studies there was no correlation, both high and low HPV+ OCSCC prevalence were observed regardless of the geographical area. Additionally, studies have shown HPV+ OPSCC to be more apparent among the younger patients that generally consuming less alcohol or tobacco [54,55]. Compared to HPV+ OCSCC in our study, only two enrolled studies reported such significant association with younger individuals [38,41]. Solely, one study observed HPV+ OCSCC association with less tobacco consumption [37]. A higher alcohol consumption and number of sexual partners were demonstrated to be associated with HPV+ OCSCC in one study as well [38]. Interestingly, the same study demonstrated a significant HPV+ OCSCC association to an earlier T-stage and concluded that it may indicate that both alcohol intake and oral HPV infection act synergistically, explaining earlier tumor onset. Overall, this could emphasize that the HPV+ OCSCC is most frequently seen in elderly patients, and the association with less alcohol and tobacco consumption is weak in contrast to the HPV+ OPSCC. Presumably, the oncogenicity of HPV-infection is of less magnitude in the genesis of OCSCC compared to OPSCC. This is supported by the decrease in incidences of OCSCC (i.e., in the USA while HPV-related OPSCC incidents have been increasing) [56].
We found that HPV16 was the predominant genotype and HPV18 was the next common genotype in the oral cavity. Other HPV HR-subtypes (HPV 31,33,45,52 and 59) were less frequent, but as a shortcoming, some studies only examined the presents of HPV16 and/or HPV 18. The 9-valent HPV vaccine (Gardasil9) may in theory reduce the possible HPV-caused carcinogenesis in the oral cavity since it covers all these subtypes except for HPV 59, which was only present in one case [49].
The anatomical subsites of HPV+ OCSCC was specified in 14 of the studies. Discrepancies were observed regarding the most prevalent sublocation. Ten studies did not investigate the subsites of the tumors, which complicates the interpretation of the results and is a limitation for determining the true representation of HPV+ OCSCC sublocations [23,29,31,37,39,43,44,46,47,48]. However, most of the included studies reported that HPV+ OCSCC were more apparent in the tongue followed by the FoM. This raises an interesting issue as to whether some of the tumors in the tongue might have arisen from the base of the tongue, which is classified as a part of the oropharynx instead of the mobile tongue, which is considered a subsite of the oral cavity.
Hence, a shortcoming can be attributed to the different definitions of the oral cavity among the studies regarding the included subsites. The Surveillance, Epidemiology, and End Results (SEER) database and Systematized Nomenclature of Medicine—Clinical Terms (SNOMED-CT) do not distinguish between the base of the tongue and the mobile tongue when describing the oral sites but labels them both as lingual or tongue, which can cause some anatomical site misclassification and contribute to the confounding of the reported HPV+ OCSCC proportion [57,58]. Furthermore, SNOMED-CT consider the palate as a part of the oral cavity without distinguishing between the hard palate and the soft palate, with the latter being a part of the oropharynx [58]. Our study demonstrates some outlying studies that report a higher prevalence of HPV+ OCSCC than the pooled prevalence, and a high proportion of the tumors are found in the tongue [19,24,28,30,38]. In the paper with the highest reported HPV+ OCSCC proportion, we observed that all HPV+ cases were seen in the tongue, and 50% (10/20) of all the tongue tumors were HPV+, shown in Table 1. Nonetheless, they distinguished between the base of tongue and the mobile part, but considering the ICD-10 codes, overlapping lesions might have been involved (C.02.8 “overlapping lesion of tongue”) as they do not report the exact included ICD-10 codes [59]. Another example to notice is that the only study that found a significant association between HPV and OCSCC included the soft palate as a part of the oral cavity, as shown in Table 1. Moreover, we excluded some relevant studies due to the unclear definition of the term “oral”, which might solely refer to the oral cavity or the combination of the oral cavity and oropharynx. On the contrary, these mistakes should easily be avoided as the anatomical sites are clearly defined [59,60]. A previous study has investigated the prevalence of HPV in palatine tonsillar squamous cell carcinoma, subdivided, according to the certainty of tonsillar tumor origin into specified tonsillar squamous cell carcinoma (STSCC) and non-specified tonsillar squamous cell carcinoma (NSTSCC). The study observed the proportion of HPV+/p16+ to be 72% for STSCC and 21% for NSTSCC [61]. Hence, it would be interesting to explore if there was a big difference in the prevalence of HPV+ tumor according to the certainty of mobile tongue origin as well. Eventually, the HPV+ OCSCC fraction globally might be smaller than the 6% determined in this study.
The inconsistency of HPV detection methods among the studies was remarkable and a limitation to this review. PCR, ISH, hybrid capture 2 high-risk HPV (HC2-HPV DNA), and p16 IHC were among the different methods applied in the studies. We prioritized results from the methods that have defined HPV presence by double positivity since studies based on HPV DNA and p16 combined are more reliable, as the method has demonstrated a high sensitivity and specificity when considering HPV16 E6/E7 mRNA detection as the golden standard [13,16,17]. Many of the enrolled studies only detected HPV DNA with PCR, which is not the equivalent to the virus being transcriptionally active and could lead to false-positive test results, making this method suboptimal [17,62]. A few studies were based on p16 IHC positivity, and they all reported a higher HPV+ OCSCC prevalence than the pooled result determined by our meta-analysis. The overexpression of p16 might not reveal the true result, as it is only identified as valid for OPSCC, where it furthermore yields false-positive results in up to 20% when compared to the detection of HPV DNA [50,62]. It has also been recommended not to use p16 IHC as a tool for OCSCC in study trials since it has shown poor performance as a prognostic marker for overall survival in OCSCC [15].
To compare the studies and determine the HPV+ OCSCC fraction worldwide, it is important to find a homogenous HPV detection method that is both highly sensitive and specific for biologically active HPV and considered beneficial in the cost-effective context.
Moreover, there is no consistency in the definition of p16-positivity. Most of the studies defined p16 IHC overexpression as positive if staining ≥70% of the tumor cells was observed. Other studies set the limit of the staining for p16 to be positive at >75%, >50%, and >10%, and one study used a 5-tiered point system to determine p16+. The wide variety of p16-positive definition can also result in a wrong interpretation of the HPV+ OCSCC proportion.
Lastly, a meta-analysis was conducted, revealing significant heterogeneity I2 > 75%, p < 0.01, and the global burden of HPV+ OCSCC to 6% (95% CI; 3–10%). (Figure 2). We did not stratify for the various detection methods because some of the detection methods were too few for a valid meta-analysis, which is a limitation that should be noted. We excluded from our review studies and meta-analyses that assessed the prevalence of HPV in the head and neck area if data from the oral cavity could not be extracted due to pooled data from multiple anatomical locations. Hence, it entails some missing data that could be used in our study.
To the best of our knowledge, with over 5000 patients and 31 studies, this is the largest systematic review and meta-analysis conducted evaluating the association of HPV infection in solely OCSCC worldwide within the last five years.
5. Conclusions
In conclusion, there was a significant heterogeneity in the HPV+ OCSCC prevalence worldwide, varying from 0% to 37%. Studies with higher HPV+ fraction had greater statistical uncertainty. The most prevalent HPV genotype was HPV 16, and the second most prevalent was HPV 18. Tumors in the tongue were the predominant sublocation for HPV in the oral cavity. The meta-analysis revealed the pooled HPV+ OCSCC prevalence worldwide to be 6% (95% CI; 3–10%) from the included studies. HPV−OCSCC were more frequent than HPV+ OCSCC in all the studies and only one study found HPV significantly associated with OCSCC. Thus, taking the heterogeneity and limitations into account, HPV may not be a necessary factor nor a high-impact risk factor regarding OCSCC development, and the possibility of site misclassification of the mobile tongue with the root of the tongue cannot be excluded.
Author Contributions
Conceptualization, S.K.K., K.K.J., C.G. and C.v.B.; validation, All authors; formal analysis, S.K.K. and M.P.G.; investigation, S.K.K.; writing—original draft preparation, S.K.K.; writing—review and editing, All authors; supervision, C.v.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Cancer Research Fund International Page. Available online: https://www.wcrf.org/cancer-trends/mouth-and-oral-cancer-statistics/ (accessed on 12 December 2022).
- Centers for Disease Control and Prevention Page. Available online: https://www.cdc.gov/std/general/default.htm (accessed on 12 December 2022).
- World Health Organization Page. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 12 December 2022).
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. JNCI J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Castellsagué, X.; Pawlita, M.; Lissowska, J.; Kee, F.; Balaram, P.; Rajkumar, T.; Sridhar, H.; Rose, B.; Pintos, J.; et al. Human papillomavirus and oral cancer: The international agency for research on cancer multicenter study. J. Natl. Cancer Inst. 2003, 95, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M.; Daling, J.R.; Doody, D.R.; Wipf, G.C.; Carter, J.J.; Madeleine, M.M.; Mao, E.J.; Fitzgibbons, E.D.; Huang, S.; Beckmann, A.M.; et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J. Natl. Cancer Inst. 1998, 90, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Smeets, S.J.; Braakhuis, B.J.M.; Abbas, S.; Snijders, P.J.F.; Ylstra, B.; Van De Wiel, M.A.; Meijer, G.A.; Leemans, C.R.; Brakenhoff, R.H. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene 2006, 25, 2558–2564. [Google Scholar] [CrossRef]
- Irani, S. New Insights into Oral Cancer-Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, K.J.; Pyrhönen, S.; Syrjänen, S.M.; Lamberg, M.A. Immunohistochemical demonstration of human papilloma virus (HPV) antigens in oral squamous cell lesions. Br. J. Oral. Surg. 1983, 21, 147–153. [Google Scholar] [CrossRef]
- Zamani, M.; Gronhoj, C.; Jensen, D.H.; Carlander, A.F.; Agander, T.; Kiss, K.; Olsen, C.; Baandrup, L.; Nielsen, F.C.; Andersen, E.; et al. The current epidemic of HPV-associated oropharyngeal cancer: An 18-year Danish population-based study with 2,169 patients. Eur. J. Cancer 2020, 134, 52–59. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Schache, A.G.; Liloglou, T.; Risk, J.M.; Filia, A.; Jones, T.M.; Sheard, J.; Woolgar, J.A.; Helliwell, T.R.; Triantafyllou, A.; Robinson, M.; et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: Sensitivity, specificity, and prognostic discrimination. Clin. Cancer Res. 2011, 17, 6262–6271. [Google Scholar] [CrossRef]
- Smeets, S.J.; Hesselink, A.T.; Speel, E.J.; Haesevoets, A.; Snijders, P.J.; Pawlita, M.; Meijer, C.J.; Braakhuis, B.J.; Leemans, C.R.; Brakenhoff, R.H. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int. J. Cancer 2007, 121, 2465–2472. [Google Scholar] [CrossRef]
- Katabi, N.; Lewis, J.S. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What Is New in the 2017 WHO Blue Book for Tumors and Tumor-Like Lesions of the Neck and Lymph Nodes. Head Neck Pathol. 2017, 11, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Jakobsen, K.K.; Jensen, J.S.; Wessel, I.; Christensen, A.; Specht, L.; Lelkaitis, G.; Mirian, C.; Buchwald, C.v.; Grønhøj, C. Impact of p16-overexpression on overall and progression-free survival outcomes in oral cavity squamous cell carcinomas: A semi-national, population-based study. Oral. Oncol. 2020, 111, 105031. [Google Scholar] [CrossRef]
- Grønhøj, C.; Jensen, D.H.; Dehlendorff, C.; Marklund, L.; Wagner, S.; Mehanna, H.; Munck-Wikland, E.; Ramqvist, T.; Näsman, A.; Wittekindt, C.; et al. Development and external validation of nomograms in oropharyngeal cancer patients with known HPV-DNA status: A European Multicentre Study (OroGrams). Br. J. Cancer 2018, 118, 1672–1681. [Google Scholar] [CrossRef]
- Hauck, F.; Oliveira-Silva, M.; Dreyer, J.H.; Perrusi, V.J.; Arcuri, R.A.; Hassan, R.; Bonvicino, C.R.; Barros, M.H.; Niedobitek, G. Prevalence of HPV infection in head and neck carcinomas shows geographical variability: A comparative study from Brazil and Germany. Virchows Arch. 2015, 466, 685–693. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Palve, V.; Bagwan, J.; Krishnan, N.M.; Pareek, M.; Chandola, U.; Suresh, A.; Siddappa, G.; James, B.L.; Kekatpure, V.; Kuriakose, M.A.; et al. Detection of High-Risk Human Papillomavirus in Oral Cavity Squamous Cell Carcinoma Using Multiple Analytes and Their Role in Patient Survival. J. Glob. Oncol. 2018, 4, 1–33. [Google Scholar] [CrossRef]
- Albano, P.M.; Holzinger, D.; Salvador, C.; Orosa, J., 3rd; Racelis, S.; Leaño, M.; Sanchez, D., Jr.; Angeles, L.M.; Halec, G.; Schmitt, M.; et al. Low prevalence of human papillomavirus in head and neck squamous cell carcinoma in the northwest region of the Philippines. PLoS ONE 2017, 12, e0172240. [Google Scholar] [CrossRef] [PubMed]
- Alsbeih, G.; Al-Harbi, N.; Bin Judia, S.; Al-Qahtani, W.; Khoja, H.; El-Sebaie, M.; Tulbah, A. Prevalence of Human Papillomavirus (HPV) Infection and the Association with Survival in Saudi Patients with Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 820. [Google Scholar] [CrossRef]
- Dalakoti, P.; Ramaswamy, B.; Bhandarkar, A.M.; Nayak, D.R.; Sabeena, S.; Arunkumar, G. Prevalence of HPV in Oral Squamous Cell Carcinoma in South West India. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 657–664. [Google Scholar] [CrossRef]
- Jun, H.W.; Ji, Y.B.; Song, C.M.; Myung, J.K.; Park, H.J.; Tae, K. Positive Rate of Human Papillomavirus and Its Trend in Head and Neck Cancer in South Korea. Front. Surg. 2021, 8, 833048. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, A.I.; Himsawi, N.; Abu-Raideh, J.; Salameh, M.; Abdullah, N.; Khasawneh, R.; Saleh, T. Prevalence of Human Papillomavirus Associated with Head and Neck Squamous Cell Carcinoma in Jordanian Patients. Open Microbiol. J. 2020, 14, 57–64. [Google Scholar] [CrossRef]
- Kim, Y.; Joo, Y.H.; Kim, M.S.; Lee, Y.S. Prevalence of high-risk human papillomavirus and its genotype distribution in head and neck squamous cell carcinomas. J. Pathol. Transl. Med. 2020, 54, 411–418. [Google Scholar] [CrossRef]
- Köksal, M.O.; Yalçın, B.K.; Keskin, F.; Çiftçi, S.; Yağcı, I.; Hasçiçek, S.Ö.; Başaran, B.; Değer, K.; Ağaçfidan, A.; Quaas, A.; et al. Genotype Distribution and Prevalence of Human Papillomavirus in Head and Neck Cancer Samples from Istanbul, Turkey. Pathogens 2021, 10, 1533. [Google Scholar] [CrossRef]
- Komolmalai, N.; Pongsiriwet, S.; Lertprasertsuke, N.; Lekwanavijit, S.; Kintarak, S.; Phattarataratip, E.; Subarnbhesaj, A.; Dhanuthai, K.; Chaisuparat, R.; Iamaroon, A. Human Papillomavirus 16 and 18 Infection in Oral Cancer in Thailand: A Multicenter Study. Asian Pac. J. Cancer Prev. 2020, 21, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Verma, H.; Tanveer, N.; Sudheer, A.K.; Kakkar, A.; Tanwar, P. Demographic Profile of p16 Immunopositive and HPV DNA PCR Positive Oral Squamous Cell Carcinoma in a Large Cohort of Indian Patients. Asian Pac. J. Cancer Prev. 2022, 23, 529–536. [Google Scholar] [CrossRef]
- Nopmaneepaisarn, T.; Tangjaturonrasme, N.; Rawangban, W.; Vinayanuwattikun, C.; Keelawat, S.; Bychkov, A. Low prevalence of p16-positive HPV-related head-neck cancers in Thailand: Tertiary referral center experience. BMC Cancer 2019, 19, 1050. [Google Scholar] [CrossRef]
- Purwanto, D.J.; Soedarsono, N.; Reuwpassa, J.O.; Adisasmita, A.C.; Ramli, M.; Djuwita, R. The prevalence of oral high-risk HPV infection in Indonesian oral squamous cell carcinoma patients. Oral Dis. 2020, 26, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.H.; Khan, A.I.; Sadat, A.; Chowdhury, A.H.; Jinnah, S.A.; Gopalan, V.; Lam, A.K.; Clarke, D.T.W.; McMillan, N.A.J.; Johnson, N.W. Prevalence and types of high-risk human papillomaviruses in head and neck cancers from Bangladesh. BMC Cancer 2017, 17, 792. [Google Scholar] [CrossRef]
- Tokuzen, N.; Nakashiro, K.I.; Tojo, S.; Goda, H.; Kuribayashi, N.; Uchida, D. Human papillomavirus-16 infection and p16 expression in oral squamous cell carcinoma. Oncol. Lett. 2021, 22, 528. [Google Scholar] [CrossRef]
- Rajesh, D.; Azeem Mohiyuddin, S.M.; Moideen Kutty, A.V.; Balakrishna, S. Prevalence of human papillomavirus in oral squamous cell carcinoma: A rural teaching hospital-based cross-sectional study. Indian J. Cancer 2017, 54, 498–501. [Google Scholar] [CrossRef]
- Rungraungrayabkul, D.; Panpradit, N.; Lapthanasupkul, P.; Kitkumthorn, N.; Klanrit, P.; Subarnbhesaj, A.; Sresumatchai, V.; Klongnoi, B.; Khovidhunkit, S.o.P. Detection of Human Papillomavirus and p16INK4a Expression in Thai Patients with Oral Squamous Cell Carcinoma. Head Neck Pathol. 2022, 16, 444–452. [Google Scholar] [CrossRef]
- Abreu, P.M.; Valle, I.B.; Damasceno, T.C.D.; Có, A.C.G.; Pansini, P.F.; Podestá, J.R.V.; Souza, E.D.; Rocha, R.M.; Curado, M.P.; Mehanna, H.; et al. Human Papillomavirus E6/E7 mRNA detection by in situ hybridization in oral cavity squamous cell carcinoma. Arch. Oral Biol. 2020, 116, 104746. [Google Scholar] [CrossRef]
- Al-Dabbagh, R.; Al-Hazmi, N.; Alhazzazi, T.; Barrett, A.W.; Speight, P. Human papillomavirus and head and neck squamous cell carcinoma in a UK population: Is there an association? Indian J. Cancer 2022, 59, 65–72. [Google Scholar] [CrossRef]
- Alsharif, U.; Hofmann, M.; Gebhard, M.; Tharun, L.; Rades, D.; Sieg, P.; Hakim, S.G. Double positivity for hpv dna/p16ink4a does not influence survival of patients with oral squamous cell carcinoma. Anticancer. Res. 2021, 41, 5557–5568. [Google Scholar] [CrossRef]
- Dalla Torre, D.; Burtscher, D.; Soelder, E.; Offermanns, V.; Rasse, M.; Puelacher, W. Human papillomavirus prevalence in a Mid-European oral squamous cell cancer population: A cohort study. Oral. Dis. 2018, 24, 948–956. [Google Scholar] [CrossRef]
- Janecka-Widła, A.; Mucha-Małecka, A.; Majchrzyk, K.; Halaszka, K.; Przewoźnik, M.; Słonina, D.; Biesaga, B. Active HPV infection and its influence on survival in head and neck squamous-cell cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Molimard, C.; L’Huillier, V.; Overs, A.; Soret, C.; Algros, M.-P.; Mougin, C.; Guenat, D.; Mauvais, O.; Prétet, J.-L. Human papillomavirus DNA and p16 expression in head and neck squamous cell carcinoma in young French patients. J. Int. Med. Res. 2021, 49, 03000605211022534. [Google Scholar] [CrossRef] [PubMed]
- Nauta, I.H.; Heideman, D.A.M.; Brink, A.; van der Steen, B.; Bloemena, E.; Koljenović, S.; Baatenburg de Jong, R.J.; Leemans, C.R.; Brakenhoff, R.H. The unveiled reality of human papillomavirus as risk factor for oral cavity squamous cell carcinoma. Int. J. Cancer 2021, 149, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Vidal Loustau, A.C.; Dulguerov, N.; Curvoisier, D.; McKee, T.; Lombardi, T. Low prevalence of HPV-induced oral squamous cell carcinoma in Geneva, Switzerland. Oral Dis. 2019, 25, 1283–1290. [Google Scholar] [CrossRef]
- Aboagye, E.; Agyemang-Yeboah, F.; Duduyemi, B.M.; Obirikorang, C. Human Papillomavirus Detection in Head and Neck Squamous Cell Carcinomas at a Tertiary Hospital in Sub-Saharan Africa. ScientificWorldJournal 2019, 2019, 2561530. [Google Scholar] [CrossRef] [PubMed]
- Kabagenyi, F.; Otiti, J.; Namwagala, J.; Kamulegeya, A.; Kalungi, S. A descriptive study of human papilloma virus in upper aero-digestive squamous cell carcinoma at Uganda cancer institute assessed by P16 immunohistochemistry. Cancers Head Neck 2020, 5, 10. [Google Scholar] [CrossRef]
- de Abreu, P.M.; Có, A.C.G.; Azevedo, P.L.; do Valle, I.B.; de Oliveira, K.G.; Gouvea, S.A.; Cordeiro-Silva, M.F.; Louro, I.D.; de Podestá, J.R.V.; Lenzi, J.; et al. Frequency of HPV in oral cavity squamous cell carcinoma. BMC Cancer 2018, 18, 324. [Google Scholar] [CrossRef]
- D’Souza, G.; Westra, W.H.; Wang, S.J.; Van Zante, A.; Wentz, A.; Kluz, N.; Rettig, E.; Ryan, W.R.; Ha, P.K.; Kang, H.; et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017, 3, 169–177. [Google Scholar] [CrossRef]
- Méndez-Matías, G.; Velázquez-Velázquez, C.; Castro-Oropeza, R.; Mantilla-Morales, A.; Ocampo-Sandoval, D.; Burgos-González, A.; Heredia-Gutiérrez, C.; Alvarado-Cabrero, I.; Sánchez-Sandoval, R.; Barco-Bazán, A.; et al. Prevalence of HPV in Mexican Patients with Head and Neck Squamous Carcinoma and Identification of Potential Prognostic Biomarkers. Cancers 2021, 13, 5602. [Google Scholar] [CrossRef] [PubMed]
- Delagranda, A.; Leterme, G.; Chirpaz, E.; Ferdynus, C.; Fernandez, C.; Rubin, F. Epidemiological features of cancers of the oral cavity, oropharynx, hypopharynx and larynx cancer in Réunion Island. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 175–181. [Google Scholar] [CrossRef]
- Bulane, A.; Goedhals, D.; Seedat, R.Y.; Goedhals, J.; Burt, F. Human papillomavirus DNA in head and neck squamous cell carcinomas in the Free State, South Africa. J. Med. Virol. 2020, 92, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nauta, I.H.; Rietbergen, M.M.; van Bokhoven, A.; Bloemena, E.; Lissenberg-Witte, B.I.; Heideman, D.A.M.; Baatenburg de Jong, R.J.; Brakenhoff, R.H.; Leemans, C.R. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in the Netherlands and the importance of additional HPV DNA testing. Ann. Oncol. 2018, 29, 1273–1279. [Google Scholar] [CrossRef]
- Lewis, J.S.; Beadle, B.; Bishop, J.A.; Chernock, R.D.; Colasacco, C.; Lacchetti, C.; Moncur, J.T.; Rocco, J.W.; Schwartz, M.R.; Seethala, R.R.; et al. Human papillomavirus testing in head and neck carcinomas guideline from the college of American pathologists. Arch. Pathol. Lab. Med. 2018, 142, 559–597. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Rosenberg, P.S.; Bray, F.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550–4559. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.I.; Westra, W.H. Molecular pathology of head and neck cancer: Implications for diagnosis, prognosis, and treatment. Annu. Rev. Pathol. 2009, 4, 49–70. [Google Scholar] [CrossRef]
- Syrjanen, S. The role of human papillomavirus infection in head and neck cancers. Ann. Oncol. 2010, 21 (Suppl. S7), vii243–vii245. [Google Scholar] [CrossRef]
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Nishimoto, I.N.; Califano, J.A.; Kowalski, L.P. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int. J. Cancer 2005, 114, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER). Cancer Statistics Review, National Cancer Institute. Available online: https://seer.cancer.gov/archive/csr/1975_2018/browse_csr.php?sectionSEL=1&pageSEL=sect_01_table.01 (accessed on 21 December 2022).
- SNOMED International Page—Formerly International Health Terminology Standards Development Organisation. Available online: https://browser.ihtsdotools.org/?perspective=full&conceptId1=314808005&edition=MAIN/2022-11-30&release=&languages=en (accessed on 19 December 2022).
- International Classification of Diseases 10th Revision (ICD-10)-WHO. Available online: https://icd.who.int/browse10/2019/en#/C00-C14 (accessed on 20 December 2022).
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—An update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Garnaes, E.; Frederiksen, K.; Kiss, K.; Andersen, L.; Therkildsen, M.H.; Franzmann, M.B.; Specht, L.; Andersen, E.; Norrild, B.; Kjaer, S.K.; et al. Double positivity for HPV DNA/p16 in tonsillar and base of tongue cancer improves prognostication: Insights from a large population-based study. Int. J. Cancer 2016, 139, 2598–2605. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Pawlita, M.; Holzinger, D. From HPV-positive towards HPV-driven oropharyngeal squamous cell carcinomas. Cancer Treat. Rev. 2016, 42, 24–29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).