The Frequency and Function of NKG2C+CD57+ Adaptive NK Cells in Cytomagalovirus Co-Infected People Living with HIV Decline with Duration of Antiretroviral Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. CMV Testing
2.4. Staining PBMC for Adaptive NK Cells
2.5. AD NK Cell Activation (ADNKA) Assay
2.6. Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Study Population Characteristics
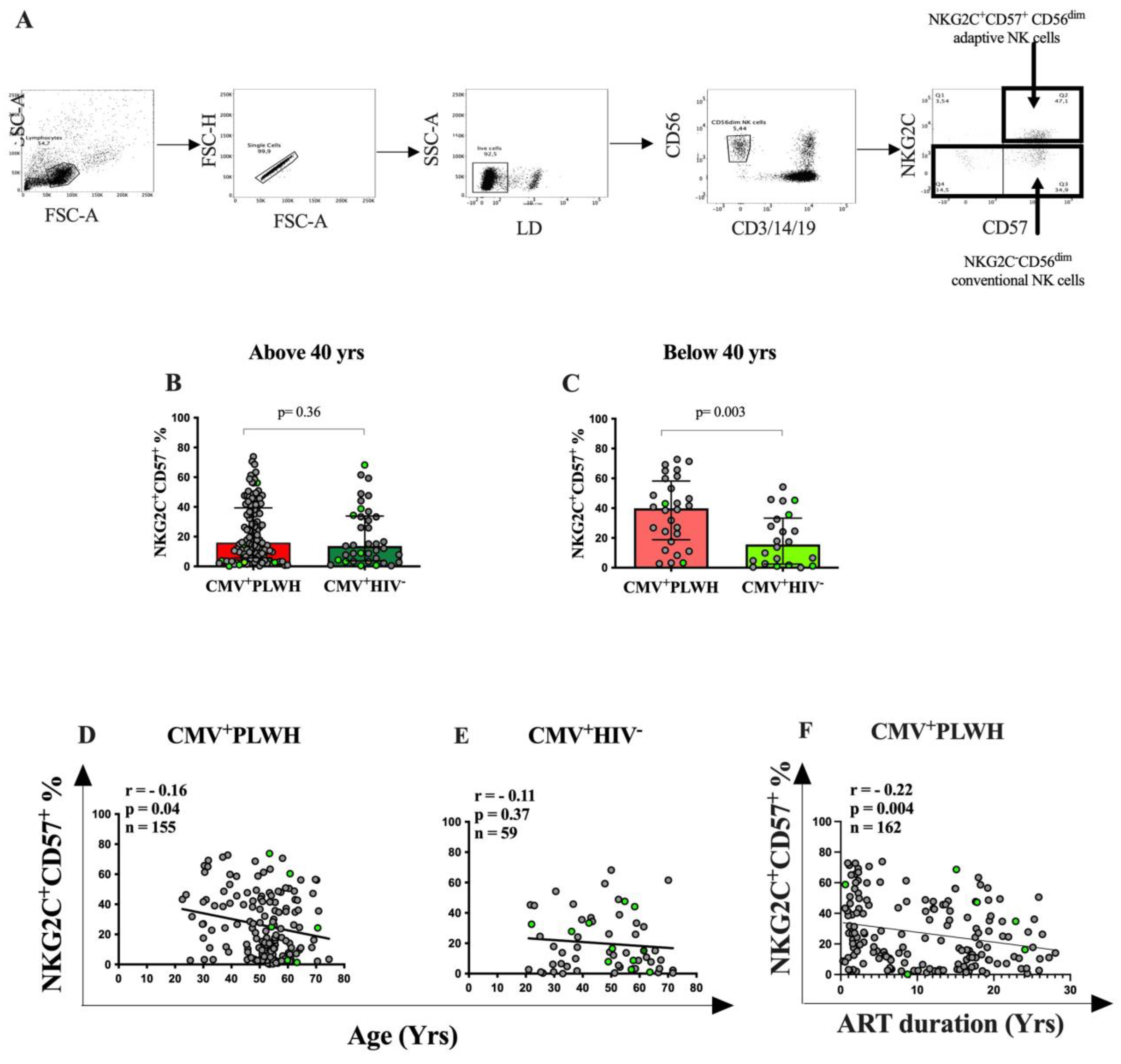
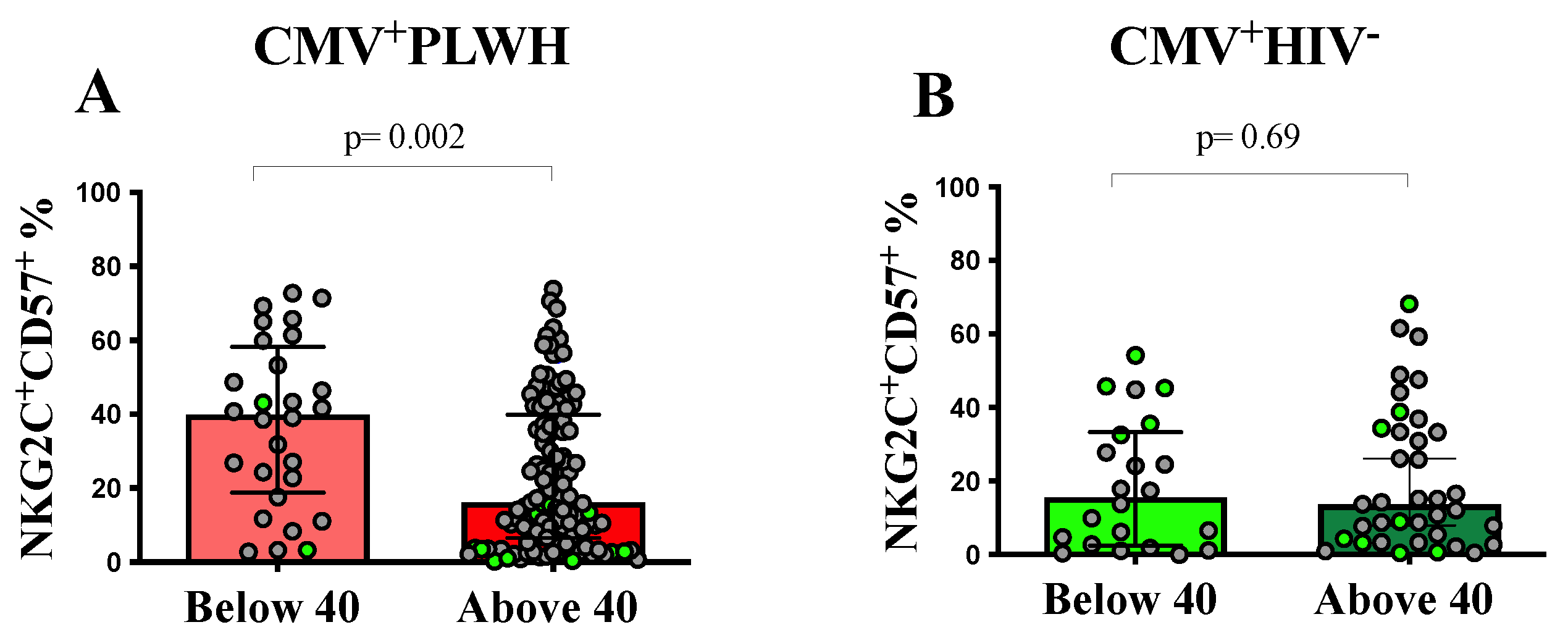
3.2. The Frequency of NKG2C+CD57+CD56dim Adaptive NK Cells Is Dependent on the Duration of ART
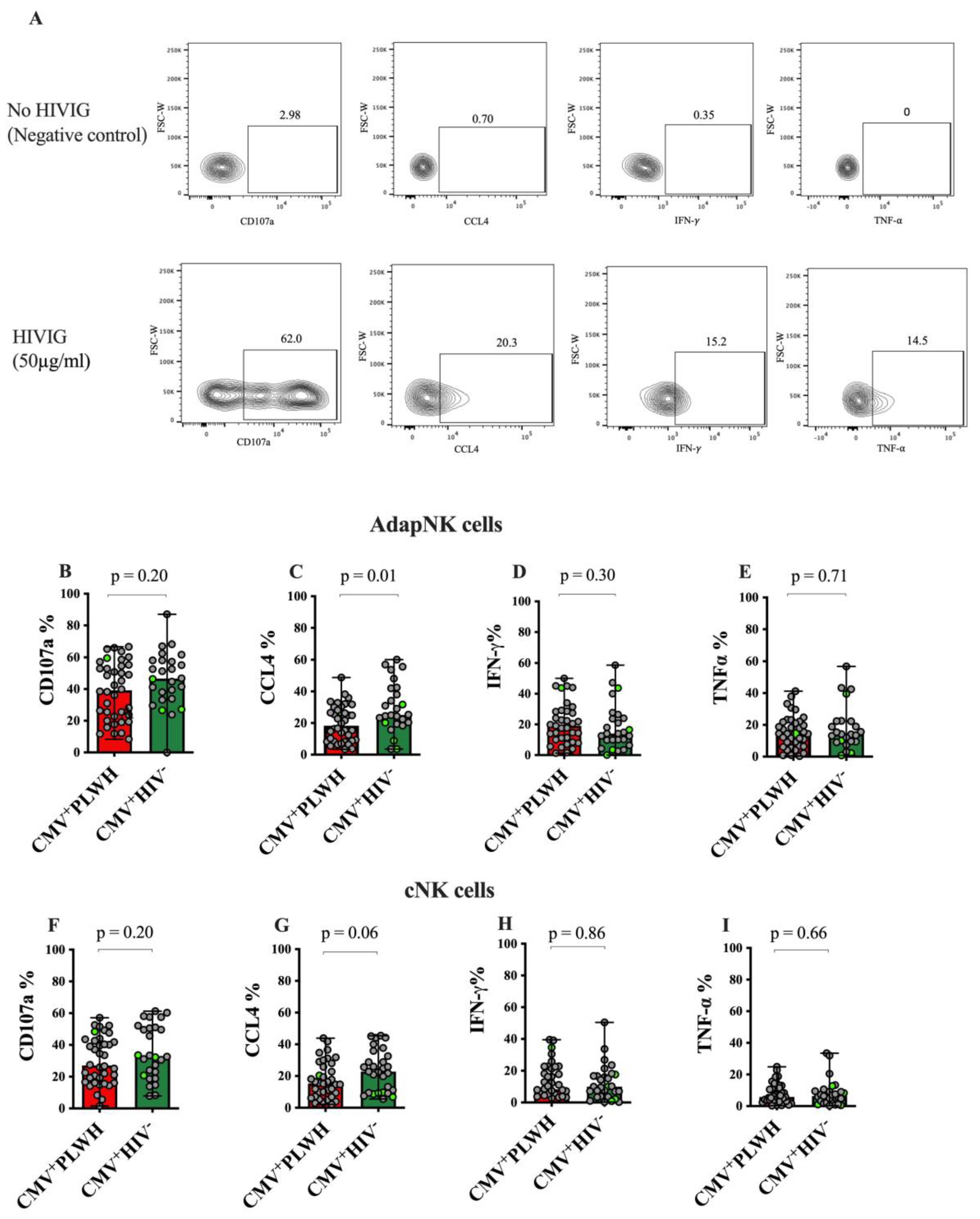
3.3. Adaptive NK and Conventional NK Cell Function in CMV+PLWH and CMV Mono-Infected Subjects
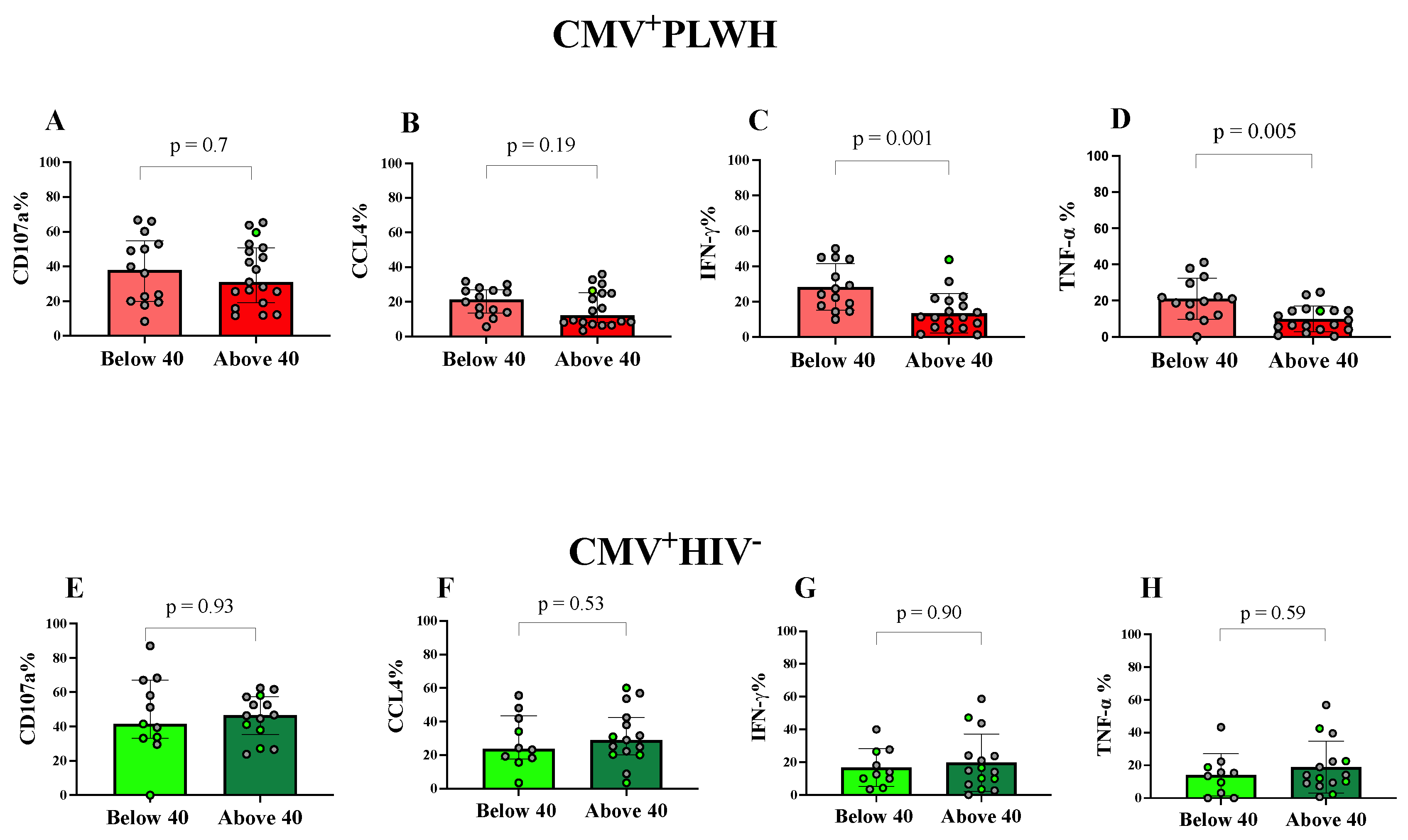
3.4. Effect of Age and Time on ART on NKG2C+CD57+CD56dim Adaptive NK Cell Functions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 1989, 47, 187–376. [Google Scholar] [PubMed]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Mujal, A.M.; Delconte, R.B.; Sun, J.C. Natural Killer Cells: From Innate to Adaptive Features. Annu. Rev. Immunol. 2021, 39, 417–447. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar]
- Romagnani, C.; Juelke, K.; Falco, M.; Morandi, B.; D’Agostino, A.; Costa, R.; Ratto, G.; Forte, G.; Carrega, P.; Lui, G.; et al. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 2007, 178, 4947–4955. [Google Scholar] [CrossRef]
- Shilling, H.G.; McQueen, K.L.; Cheng, N.W.; Shizuru, J.A.; Negrin, R.S.; Parham, P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood 2003, 101, 3730–3740. [Google Scholar] [CrossRef] [PubMed]
- Huntington, N.D.; Legrand, N.; Alves, N.L.; Jaron, B.; Weijer, K.; Plet, A.; Corcuff, E.; Mortier, E.; Jacques, Y.; Spits, H.; et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 2009, 206, 25–34. [Google Scholar] [CrossRef]
- Bjorkstrom, N.K.; Ljunggren, H.G.; Sandberg, J.K. CD56 negative NK cells: Origin, function, and role in chronic viral disease. Trends Immunol. 2010, 31, 401–406. [Google Scholar]
- Beziat, V.; Descours, B.; Parizot, C.; Debre, P.; Vieillard, V. NK cell terminal differentiation: Correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One 2010, 5, e11966. [Google Scholar]
- Mavilio, D.; Lombardo, G.; Benjamin, J.; Kim, D.; Follman, D.; Marcenaro, E.; O’Shea, M.A.; Kinter, A.; Kovacs, C.; Moretta, A.; et al. Characterization of CD56−/CD16+ natural killer (NK) cells: A highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 2005, 102, 2886–2891. [Google Scholar]
- Milush, J.M.; Lopez-Verges, S.; York, V.A.; Deeks, S.G.; Martin, J.N.; Hecht, F.M.; Lanier, L.L.; Nixon, D.F. CD56negCD16(+) NK cells are activated mature NK cells with impaired effector function during HIV-1 infection. Retrovirology 2013, 10, 158. [Google Scholar] [PubMed]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [PubMed]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Gianella, S.; Massanella, M.; Wertheim, J.O.; Smith, D.M. The Sordid Affair Between Human Herpesvirus and HIV. J. Infect. Dis. 2015, 212, 845–852. [Google Scholar] [PubMed]
- Emery, V.C. Investigation of CMV disease in immunocompromised patients. J. Clin. Pathol. 2001, 54, 84–88. [Google Scholar]
- Steininger, C.; Puchhammer-Stockl, E.; Popow-Kraupp, T. Cytomegalovirus disease in the era of highly active antiretroviral therapy (HAART). J. Clin. Virol. 2006, 37, 1–9. [Google Scholar] [CrossRef]
- Patel, R.; Kennedy, O.J.; Clarke, E.; Geretti, A.; Nilsen, A.; Lautenschlager, S.; Green, J.; Donders, G.; van der Meijden, W.; Gomberg, M.; et al. 2017 European guidelines for the management of genital herpes. Int. J. STD AIDS 2017, 28, 1366–1379. [Google Scholar] [CrossRef]
- Deayton, J.R.; Prof Sabin, C.A.; Johnson, M.A.; Emery, V.C.; Wilson, P.; Griffiths, P.D. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet 2004, 363, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Filteau, S.; Rowland-Jones, S. Cytomegalovirus Infection May Contribute to the Reduced Immune Function, Growth, Development, and Health of HIV-Exposed, Uninfected African Children. Front. Immunol. 2016, 7, 257. [Google Scholar] [CrossRef]
- Freeman, M.L.; Lederman, M.M.; Gianella, S. Partners in Crime: The Role of CMV in Immune Dysregulation and Clinical Outcome During HIV Infection. Curr. HIV/AIDS Rep. 2016, 13, 10–19. [Google Scholar]
- Hunt, P.W. HIV and inflammation: Mechanisms and consequences. Curr. HIV/AIDS Rep. 2012, 9, 139–147. [Google Scholar] [PubMed]
- Routy, J.P.; Royston, L.; Isnard, S. Aging With Grace for People Living With HIV: Strategies to Overcome Leaky Gut and Cytomegalovirus Coinfection. J. Acquir. Immune Defic. Syndr. 2022, 89 (Suppl. S1), S29–S33. [Google Scholar]
- Isnard, S.; Ramendra, R.; Lin, J.; Kant, S.; Fombuena, B.; Ouyang, J.; Peng, X.; El Far, M.; Tremblay, C.; Bernard, N.F.; et al. Anti-cytomegalovirus Immunoglobulin G Is Linked to CD4 T-cell Count Decay in Human Immunodeficiency Virus (HIV) Elite Controllers. Clin. Infect. Dis. 2021, 73, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Angulo, A.; Vilches, C.; Gomez-Lozano, N.; Malats, N.; Lopez-Botet, M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef]
- Guma, M.; Budt, M.; Saez, A.; Brckalo, T.; Hengel, H.; Angulo, A.; Lopez-Botet, M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood 2006, 107, 3624–3631. [Google Scholar] [PubMed]
- Lopez-Verges, S.; Milush, J.M.; Pandey, S.; York, V.A.; Arakawa-Hoyt, J.; Pircher, H.; Norris, P.J.; Nixon, D.F.; Lanier, L.L. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010, 116, 3865–3874. [Google Scholar] [PubMed]
- Lopez-Verges, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.M.; Norris, P.J.; et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar]
- Chang, C.; Rodriguez, A.; Carretero, M.; Lopez-Botet, M.; Phillips, J.H.; Lanier, L.L. Molecular characterization of human CD94: A type II membrane glycoprotein related to the C-type lectin superfamily. Eur. J. Immunol. 1995, 25, 2433–2437. [Google Scholar] [CrossRef]
- Hammer, Q.; Ruckert, T.; Romagnani, C. Natural killer cell specificity for viral infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- Hearps, A.C.; Agius, P.A.; Zhou, J.; Brunt, S.; Chachage, M.; Angelovich, T.A.; Cameron, P.U.; Giles, M.; Price, P.; Elliott, J.; et al. Persistence of Activated and Adaptive-Like NK Cells in HIV(+) Individuals despite 2 Years of Suppressive Combination Antiretroviral Therapy. Front. Immunol. 2017, 8, 731. [Google Scholar] [CrossRef]
- Schlums, H.; Cichocki, F.; Tesi, B.; Theorell, J.; Beziat, V.; Holmes, T.D.; Han, H.; Chiang, S.C.; Foley, B.; Mattsson, K.; et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015, 42, 443–456. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef]
- Peppa, D.; Pedroza-Pacheco, I.; Pellegrino, P.; Williams, I.; Maini, M.K.; Borrow, P. Adaptive Reconfiguration of Natural Killer Cells in HIV-1 Infection. Front. Immunol. 2018, 9, 474. [Google Scholar] [PubMed]
- Zhang, T.; Scott, J.M.; Hwang, I.; Kim, S. Cutting edge: Antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J. Immunol. 2013, 190, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Beziat, V.; Liu, L.L.; Malmberg, J.A.; Ivarsson, M.A.; Sohlberg, E.; Bjorklund, A.T.; Retiere, C.; Sverremark-Ekstrom, E.; Traherne, J.; Ljungman, P.; et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 2013, 121, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Vilches, C.; Angulo, A.; Lopez-Botet, M. Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: A different perspective of the host-pathogen interaction. Eur. J. Immunol. 2013, 43, 1133–1141. [Google Scholar] [CrossRef]
- Heath, J.; Newhook, N.; Comeau, E.; Gallant, M.; Fudge, N.; Grant, M. NKG2C(+)CD57(+) Natural Killer Cell Expansion Parallels Cytomegalovirus-Specific CD8(+) T Cell Evolution towards Senescence. J. Immunol. Res. 2016, 2016, 7470124. [Google Scholar] [CrossRef] [PubMed]
- Brunetta, E.; Fogli, M.; Varchetta, S.; Bozzo, L.; Hudspeth, K.L.; Marcenaro, E.; Moretta, A.; Mavilio, D. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. Aids 2010, 24, 27–34. [Google Scholar] [CrossRef]
- Mela, C.M.; Burton, C.T.; Imami, N.; Nelson, M.; Steel, A.; Gazzard, B.G.; Gotch, F.M.; Goodier, M.R. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: Lack of reversion with highly active antiretroviral therapy. Aids 2005, 19, 1761–1769. [Google Scholar] [PubMed]
- Durand, M.; Chartrand-Lefebvre, C.; Baril, J.G.; Trottier, S.; Trottier, B.; Harris, M.; Walmsley, S.; Conway, B.; Wong, A.; Routy, J.P.; et al. The Canadian HIV and aging cohort study—Determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: Rationale and study protocol. BMC Infect. Dis. 2017, 17, 611. [Google Scholar] [CrossRef]
- Mehraj, V.; Cox, J.; Lebouche, B.; Costiniuk, C.; Cao, W.; Li, T.; Ponte, R.; Thomas, R.; Szabo, J.; Baril, J.G.; et al. Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J. Int. AIDS Soc. 2018, 21, e25034. [Google Scholar] [CrossRef]
- Cao, W.; Mehraj, V.; Trottier, B.; Baril, J.G.; Leblanc, R.; Lebouche, B.; Cox, J.; Tremblay, C.; Lu, W.; Singer, J.; et al. Early Initiation Rather Than Prolonged Duration of Antiretroviral Therapy in HIV Infection Contributes to the Normalization of CD8 T-Cell Counts. Clin. Infect. Dis. 2016, 62, 250–257. [Google Scholar]
- Cummins, L.M.; Weinhold, K.J.; Matthews, T.J.; Langlois, A.J.; Perno, C.F.; Condie, R.M.; Allain, J.P. Preparation and characterization of an intravenous solution of IgG from human immunodeficiency virus-seropositive donors. Blood 1991, 77, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, F.P.; Kant, S.; Barbe, A.; Routy, J.P.; Bruneau, J.; Lebouche, B.; Tremblay, C.; Pazgier, M.; Finzi, A.; Bernard, N.F. Antibody-Dependent Cellular Cytotoxicity-Competent Antibodies against HIV-1-Infected Cells in Plasma from HIV-Infected Subjects. MBio 2019, 10, e02690-19. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Zhang, N.; Routy, J.P.; Tremblay, C.; Thomas, R.; Szabo, J.; Cote, P.; Trottier, B.; LeBlanc, R.; Rouleau, D.; et al. Quantifying Anti-HIV Envelope-Specific Antibodies in Plasma from HIV Infected Individuals. Viruses 2019, 11, 487. [Google Scholar] [PubMed]
- Trkola, A.; Matthews, J.; Gordon, C.; Ketas, T.; Moore, J.P. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J. Virol. 1999, 73, 8966–8974. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.N.; Andreotti, P.E.; Dawson, J.R.; Cresswell, P. Natural killing target antigens as inducers of interferon: Studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. J. Immunol. 1985, 134, 971–976. [Google Scholar]
- Imbeault, M.; Giguere, K.; Ouellet, M.; Tremblay, M.J. Exon level transcriptomic profiling of HIV-1-infected CD4(+) T cells reveals virus-induced genes and host environment favorable for viral replication. PLoS Pathog. 2012, 8, e1002861. [Google Scholar] [CrossRef]
- Veillette, M.; Coutu, M.; Richard, J.; Batraville, L.A.; Dagher, O.; Bernard, N.; Tremblay, C.; Kaufmann, D.E.; Roger, M.; Finzi, A. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J. Virol. 2015, 89, 545–551. [Google Scholar] [CrossRef]
- Veillette, M.; Desormeaux, A.; Medjahed, H.; Gharsallah, N.E.; Coutu, M.; Baalwa, J.; Guan, Y.; Lewis, G.; Ferrari, G.; Hahn, B.H.; et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J. Virol. 2014, 88, 2633–2644. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Martelli, S.; Tan, S.W.; Simoni, Y.; Chong, M.L.; Yap, S.H.; Newell, E.W.; Pender, S.L.F.; Kamarulzaman, A.; Rajasuriar, R.; et al. Adaptive NKG2C(+)CD57(+) Natural Killer Cell and Tim-3 Expression During Viral Infections. Front. Immunol. 2018, 9, 686. [Google Scholar]
- Wagner, J.A.; Fehniger, T.A. Human Adaptive Natural Killer Cells: Beyond NKG2C. Trends Immunol. 2016, 37, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Zhang, T.; Scott, J.M.; Kim, A.R.; Lee, T.; Kakarla, T.; Kim, A.; Sunwoo, J.B.; Kim, S. Identification of human NK cells that are deficient for signaling adaptor FcRgamma and specialized for antibody-dependent immune functions. Int. Immunol. 2012, 24, 793–802. [Google Scholar] [CrossRef]
- Zhou, J.; Amran, F.S.; Kramski, M.; Angelovich, T.A.; Elliott, J.; Hearps, A.C.; Price, P.; Jaworowski, A. An NK Cell Population Lacking FcRgamma Is Expanded in Chronically Infected HIV Patients. J. Immunol. 2015, 194, 4688–4697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Landskron, J.; Ask, E.H.; Enqvist, M.; Sohlberg, E.; Traherne, J.A.; Hammer, Q.; Goodridge, J.P.; Larsson, S.; Jayaraman, J.; et al. Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep. 2016, 15, 1088–1099. [Google Scholar] [CrossRef]
- Vivier, E.; Ackerly, M.; Rochet, N.; Anderson, P. Structure and function of the CD16:zeta:gamma complex expressed on human natural-killer cells. Int. J. Cancer Suppl. 1992, 7, 11–14. [Google Scholar] [PubMed]
- Vivier, E.; Morin, P.; O’Brien, C.; Druker, B.; Schlossman, S.F.; Anderson, P. Tyrosine phosphorylation of the Fc gamma RIII(CD16): Zeta complex in human natural killer cells. Induction by antibody-dependent cytotoxicity but not by natural killing. J. Immunol. 1991, 146, 206–210. [Google Scholar] [PubMed]
- Shah, S.V.; Manickam, C.; Ram, D.R.; Kroll, K.; Itell, H.; Permar, S.R.; Barouch, D.H.; Klatt, N.R.; Reeves, R.K. CMV Primes Functional Alternative Signaling in Adaptive Deltag NK Cells but Is Subverted by Lentivirus Infection in Rhesus Macaques. Cell Rep. 2018, 25, 2766–2774.e3. [Google Scholar]
- Gao, F.; Zhou, Z.; Lin, Y.; Shu, G.; Yin, G.; Zhang, T. Biology and Clinical Relevance of HCMV-Associated Adaptive NK Cells. Front. Immunol. 2022, 13, 830396. [Google Scholar] [CrossRef]
- Luetke-Eversloh, M.; Hammer, Q.; Durek, P.; Nordstrom, K.; Gasparoni, G.; Pink, M.; Hamann, A.; Walter, J.; Chang, H.D.; Dong, J.; et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014, 10, e1004441. [Google Scholar]
- Tesi, B.; Schlums, H.; Cichocki, F.; Bryceson, Y.T. Epigenetic Regulation of Adaptive NK Cell Diversification. Trends Immunol. 2016, 37, 451–461. [Google Scholar] [CrossRef]

| CHACS | PI Cohort | HIV Uninfected | Significance of between Group Comparisons | |||||
|---|---|---|---|---|---|---|---|---|
| CMV + PLWH (n = 127) Group 1 | CMV + HIV− (n = 37) Group 2 | CMV + PLWH (n = 28) Group 3 | CMV + PLWH (n = 15) Group 4 | CMV + HIV− (n = 22) Group 5 | 1 vs. 2 2 1 vs. 3 3 1 vs. 4 4 1 vs. 5 5 | 2 vs. 3 6 2 vs. 4 7 2 vs. 5 8 | 3 vs. 4 9 3 vs. 5 10 4 vs. 5 11 | |
| Age 1 | 55.7 (51.1, 59.3) | 57.7 (50.8, 62.4) | 33.2 (30.8, 38.2) | 54.1 (52.9, 60.1) | 31.5 (25.2, 36.6) | p > 0.99 2 p < 0.0001 3 p > 0.99 4 p < 0.0001 5 | p < 0.001 6 p > 0.98 7 p > 0.0001 8 | p < 0.0001 9 p > 0.99 10 p < 0.0001 11 |
| Sex, n (%) ● Male ● Female | 117 (92.1) 10 (7.9) | 28 (75.77) 9 (24.3) 4 | 26 (92.8) 2 (7.1) | 14 (93.3) 1 (6.7) | 17 (77.3) 5 (22.7) | p = 0.016 2 p > 0.99 3 p > 0.99 4 p = 0.05 5 | p < 0.1 6 p > 0.25 7 p > 0.99 8 | p > 0.99 9 p = 0.22 10 p = 0.47 11 |
| Duration of infection (years) 1 | Unknown | N.A. | 2.2 (1.6, 2.7) | 1.9 (1.8, 2.0) | N.A. | p = 0.15 9 | ||
| Viral load (HIV RNA copies/mL of plasma) | <50 | N.A. | <50 | <50 | N.A. | |||
| CD4+ count (cells/mm3) 1 | 575 (411, 723) | N.D. | 650 (500, 926.5) | 700 (600.5, 814) | N.D. | p = 0.14 3 p = 0.053 4 | p > 0.99 9 | |
| CD4+/CD8+ ratio 1 | 0.8 (0.54, 1.1) | N.D. | 1 (0.7, 1.3) | 1.1 (0.9, 1.5) | N.D. | p = 0.12 3 p = 0.006 4 | p = 0.6 9 | |
| Years on ART 1 | 16.0 (8.6, 19.1) | N.A. | 1.4 (1.0, 2.2) | 1.94 (1.8, 2.0) | N.A. | p < 0.0001 3 p < 0.0001 4 | p > 0.99 9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsulami, K.; Dupuy, F.P.; Gilbert, L.; Messier-Peet, M.; Durand, M.; Tremblay, C.; Routy, J.-P.; Bruneau, J.; Baril, J.-G.; Trottier, B.; et al. The Frequency and Function of NKG2C+CD57+ Adaptive NK Cells in Cytomagalovirus Co-Infected People Living with HIV Decline with Duration of Antiretroviral Therapy. Viruses 2023, 15, 323. https://doi.org/10.3390/v15020323
Alsulami K, Dupuy FP, Gilbert L, Messier-Peet M, Durand M, Tremblay C, Routy J-P, Bruneau J, Baril J-G, Trottier B, et al. The Frequency and Function of NKG2C+CD57+ Adaptive NK Cells in Cytomagalovirus Co-Infected People Living with HIV Decline with Duration of Antiretroviral Therapy. Viruses. 2023; 15(2):323. https://doi.org/10.3390/v15020323
Chicago/Turabian StyleAlsulami, Khlood, Franck P. Dupuy, Louise Gilbert, Marc Messier-Peet, Madeleine Durand, Cécile Tremblay, Jean-Pierre Routy, Julie Bruneau, Jean-Guy Baril, Benoit Trottier, and et al. 2023. "The Frequency and Function of NKG2C+CD57+ Adaptive NK Cells in Cytomagalovirus Co-Infected People Living with HIV Decline with Duration of Antiretroviral Therapy" Viruses 15, no. 2: 323. https://doi.org/10.3390/v15020323
APA StyleAlsulami, K., Dupuy, F. P., Gilbert, L., Messier-Peet, M., Durand, M., Tremblay, C., Routy, J.-P., Bruneau, J., Baril, J.-G., Trottier, B., & Bernard, N. F. (2023). The Frequency and Function of NKG2C+CD57+ Adaptive NK Cells in Cytomagalovirus Co-Infected People Living with HIV Decline with Duration of Antiretroviral Therapy. Viruses, 15(2), 323. https://doi.org/10.3390/v15020323






