Pepper Mild Mottle Virus: An Infectious Pathogen in Pepper Production and a Potential Indicator of Domestic Water Quality
Abstract
1. Introduction
2. The Genus Tobamovirus
3. Genome Sequence Variationof PMMoVIsolates
4. Host Range and Symptoms Associated with PMMoV
5. Mode of PMMoV Transmission in Plants
6. Global Distribution and Economic Importance of PMMoV
7. Management of Pepper Mild Mottle Virus (PMMoV)
8. Genetic and Gene Resources for Resistance Breeding against PMMoV
8.1. Diversity of L Resistance Genes and PMMoV Pathotypes
8.2. Pepper Genetic Resources with Resistance against PMMoV
9. PMMoV as an Indicator of Water Quality
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.P.; Li, Y.Y.; Li, F.; Tan, G.L. First report of natural infection of tomato by pepper mild mottle virus in China. J. Plant Pathol. 2020, 103, 363. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, A.E.; Fashogbon, B.A. Analysis of technical efficiency of pepper production among farmers under tropical conditions. Int. J. Veg. Sci. 2014, 20, 124–130. [Google Scholar] [CrossRef]
- Parisi, M.; Alioto, D.; Tripodi, P. Overview of Biotic Stresses in Pepper (Capsicum spp.): Sources of Genetic Resistance, Molecular Breeding and Genomics. Int. J. Mol. Sci. 2020, 21, 2587. [Google Scholar] [CrossRef]
- Vélez-Olmedo, J.B.; Fribourg, C.E.; Melo, F.L.; Nagata, T.; de Oliveira, A.S.; Resende, R.O. Tobamoviruses of two new species trigger resistance in pepper plants harbouring functional L alleles. J. Gen. Virol. 2021, 102, 001524. [Google Scholar] [CrossRef]
- Secrist, K.; Ali, A. FirstComplete Genome Sequence of Pepper mild mottle virus from Chili Pepper in the United States. Genome Announc. 2018, 6, e00331-18. [Google Scholar] [CrossRef]
- Kitajima, M.; Sassi, H.P.; Torrey, J.R. Pepper mild mottle virus as a water quality indicator. NPJ Clean Water 1 2018, 1, 19. [Google Scholar] [CrossRef]
- Rosario, K.; Symonds, E.M.; Sinigalliano, C.; Stewart, J.; Breitbart, M. Pepper mild mottle virus as an indicator of fecal pollution. Appl. Environ. Microbiol. 2009, 75, 7261–7267. [Google Scholar] [CrossRef]
- Adams, M.J.; Antoniw, J.F.; Kreuze, J. Virgaviridae: A new family of rod-shaped plant viruses. Arch. Virol. 2009, 154, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Choi, G.-S.; Kwon, S.-J.; Yoon, J.-Y. Complete nucleotide sequences and genome organization of two pepper mild mottle virus isolates from Capsicum annuum in South Korea. Genome Announc. 2016, 4, e00411-16. [Google Scholar] [CrossRef]
- Choi, G.-S.; Choi, S.-K.; Cho, I.-S.; Kwon, S.-J. Resistance screening to pepper mild mottle virus pathotypes in paprika cultivars. Res. Plant Dis. 2014, 20, 299–302. [Google Scholar] [CrossRef]
- Salgado-Ortíz, H.; De La Torre-Almaraz, R.; Sánchez-Navarro, J.Á.; Pallás, V. Identification and genomic characterization of a novel Tobamovirus from prickly pear cactus. Arch. Virol. 2020, 165, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Min, B.E.; Hong, J.S.; Rhie, M.J.; Kim, M.J.; Ryu, K.H. Molecular evidence supporting the confirmation of Maracuja mosaic virus as a species of the genus Tobamovirus and production of an infectious cDNA transcript. Arch. Virol. 2006, 151, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.; Rodriguez, M.C.; Venturuzzi, A.L.; Asurmendi, S. Modulation of host plant immunity by Tobamovirus proteins. Ann. Bot. 2017, 119, 737–747. [Google Scholar] [CrossRef]
- Rhie, M.J.; Min, B.E.; Hong, J.S.; Song, Y.S.; Rhu, K.H. Complete genome sequence supports bell pepper mottle virus as a species of the genus Tobamovirus. Arch. Virol. 2007, 152, 1401–1407. [Google Scholar] [CrossRef]
- Hamada, H.; Takeuchi, S.; Morita, Y.; Sawadah, H.; Kiba, A.; Hikichi, Y. Characterization of paprika mottle virus first isolated in Japan. J. Gen. Plant Pathol. 2003, 69, 99–204. [Google Scholar] [CrossRef]
- Ikeda, R.; Watanabe, E.; Watanabe, Y.; Okada, Y. Nucleotide sequence of Tobamovirus Ob which can spread systemically in N gene tobacco. J. Gen. Viol. 1993, 74, 1939–1944. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, T.; Xue, C.; Zhou, X. Characterization and complete nucleotide sequence of two isolates of tomato mosaic virus. J. Phytopathol. 2011, 160, 115–119. [Google Scholar] [CrossRef]
- Chanda, B.; Rivera, Y.; Nunziata, S.O.; Galvez, M.E.; Gilliard, A.; Ling, K.-S. Complete genome sequence of a tomato brown rugose fruit virus isolated in the United States. Microbiol. Resour. Announc. 2020, 9, e00630-20. [Google Scholar] [CrossRef]
- Padmanabhan, C.; Zheng, Y.; Li, R.; Martin, G.B.; Fei, Z.; Ling, K.-S. Complete genome sequence of a tomato-infecting tomato mottle mosaic virus in New York. Genome Announc. 2015, 3, e01523-15. [Google Scholar] [CrossRef]
- Kim, N.Y.; Lee, H.J.; Kim, N.K.; Kim, H.; Jeong, R.-D. First report of tobacco mosaic virus infecting Hosta longipes in Korea. J. Plant Pathol. 2021, 103, 341. [Google Scholar] [CrossRef]
- Solis, I.; Garcia-Arenal, F. The complete nucleotide sequence of the genomic RNA of the Tobamovirus tobacco mild green mosaic virus. Virol. 1990, 177, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Crosslin, J.M.; Hamm, P.B.; Kirk, W.W.; Hammond, R.W. Complete genomic sequence of a Tobacco rattle virus isolate from Michigan-grown potatoes. Arch. Virol. 2010, 155, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Ladipo, J.; Koenig, R.; Lesemann, D.-E. Nigerian tobacco latent virus: A new Tobamovirus from tobacco in Nigeria. Eur. J. Plant Pathol. 2003, 109, 373–379. [Google Scholar] [CrossRef]
- Ilmberger, N.; Willingmann, P.; Adam, G.; Heinze, C.A. Subgroup 1 Tobamovirus isolated from Brugmansia sp. and its Detection by RT-PCR. J. Phytopathol. 2007, 155, 326–332. [Google Scholar] [CrossRef]
- Fillmer, K.; Adkins, S.; Pongam, P.; D’Elia, T. The complete nucleotide sequence and genomic characterization of tropical soda apple mosaic virus. Arch. Virol. 2016, 161, 2317–2320. [Google Scholar] [CrossRef]
- Uehara-Ichiki, T.; Uke, A.; Hanada, K.; Hishida, A.; Nakazono-Nagaoka, E.; Kodaira, E. Scopolia mild mottle virus: A new Tobamovirus isolated from a Scopolia japonica plant in Japan. Arch. Virol. 2022, 167, 947–951. [Google Scholar] [CrossRef]
- Wylie, S.J.; Li, H.; Jones, M.G.K. Yellow tailflower mild mottle virus: A new Tobamovirus described from Anthocercislittorea (Solanaceae) in Western Australia. Arch. Virol. 2013, 159, 791–795. [Google Scholar] [CrossRef]
- Antignus, Y.; Wang, Y.; Pearlsman, M.; Lachman, O.; Lavi, N.; Gal-On, A. Biological and molecular characterization of a new cucurbit-infecting Tobamovirus. Phytopathology 2001, 91, 565–571. [Google Scholar] [CrossRef]
- Ugaki, M.; Tomiyama, M.; Kakutani, T.; Hidaka, S.; Kiguchi, T.; Nagata, R.; Sato, T.; Motoyoshi, F.; Nishiguchi, M. The complete nucleotide sequence of cucumber green mottle mosaic virus (SH strain) genomic RNA. J. Gen. Virol. 1991, 72, 1487–1495. [Google Scholar] [CrossRef]
- Orita, H.; Sakai, J.I.; Kubota, K.; Okuda, M.; Tanaka, Y.; Hanada, K.; Imamura, Y.; Nishiguchi, M.; Karasev, A.V.; Miyata, S.I.; et al. Molecular and serological characterization of Cucumber mottle virus, a new cucurbit-infecting Tobamo-like. Virus. Plant Dis. 2007, 91, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Min, B.E.; Choi, S.H.; Ryu, K.H. Completion of nucleotide sequence and generation of highly infectious transcripts to cucurbits from full-length cDNA clone of Kyuri green mottle mosaic virus. Arch. Virol. 2001, 146, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Min, B.E.; Choi, J.K.; Ryu, K.H. Genome structure and production of biologically active in vitro transcripts of cucurbit-infecting Zucchini green mottle mosaic virus. Phytopathology 2002, 92, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Lartey, R.T.; Voss, T.C.; Melcher, U. Completion of a cDNA sequence from a Tobamovirus pathogenic to crucifers. Gene 1995, 166, 331–332. [Google Scholar] [CrossRef]
- MacDonald, J.L.; Punja, Z.K.; Xiang, Y.; Bouthillier, M.J.; Reade, R.; DeYoung, R.M.; Bhagwat, B.; Betz, E.C.; Li, Y.Q.; Chen, X. First report of wasabi mottle virus causing ringspot and vein-clearing symptoms on wasabi (Wasabia japonica) in North America. Can. J. Plant Pathol. 2020, 43, 311–322. [Google Scholar] [CrossRef]
- Aguilar, I.; Sanchez, F.; Martin, A.M.; Martinez-Herrera, D.; Ponz, F. Nucleotide sequence of Chinese rape mosaic virus (oilseed rape mosaic virus), a crucifer Tobamovirus infectious on Arabidopsis thaliana. Plant Mol. Biol. 1996, 30, 191–197. [Google Scholar] [CrossRef]
- Yoshida, T.; Kitazawa, Y.; Komatsu, K.; Neriya, Y.; Ishikawa, K.; Fujita, N.; Hashimoto, M.; Maejima, K.; Yamaji, Y.; Namba, S. Complete nucleotide sequence and genome structure of a Japanese isolate of hibiscus latent Fort Pierce virus, a unique Tobamovirus that contains an internal poly(A) region in its 3′ end. Arch. Virol. 2014, 159, 3161–3165. [Google Scholar] [CrossRef]
- Srinivasan, K.G.; Min, B.E.; Ryu, K.H.; Adkins, S.; Wong, S.M. Determination of complete nucleotide sequence of Hibiscus latent Singapore virus: Evidence for the presence of an internal poly (A) tract. Arch. Virol. 2004, 150, 153–166. [Google Scholar] [CrossRef]
- Wei, K.; Gibbs, A.; Mackenzie, A. Clitoria yellow mottle virus: A Tobamovirus from Northern Australia. Australas. Plant Dis. Notes 2012, 7, 59–61. [Google Scholar] [CrossRef]
- Silver, S.; Quan, S.; Deom, C.M. Completion of the nucleotide sequence of sunn-hemp mosaic virus: A Tobamovirus pathogenic to legumes. Virus Genes 1996, 13, 83–85. [Google Scholar] [CrossRef]
- Min, B.E.; Song, Y.S.; Ryu, K.H. Complete sequence and genome structure of cactus mild mottle virus. Arch. Virol. 2009, 154, 1371–1374. [Google Scholar] [CrossRef]
- Kim, N.R.; Hong, J.S.; Song, Y.S.; Chung, B.N.; Park, J.W.; Ryu, K.H. The complete genome sequence of a member of a new species of Tobamovirus (rattail cactus necrosis-associated virus) isolated from Aporcactusflagelliformis. Arch. Virol. 2011, 157, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.A.; Hong, J.S.; Song, Y.S.; Ryu, K.H. The complete genome sequence and genome structure of frangipani mosaic virus. Arch. Virol. 2010, 155, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Ryu, K.H. The complete genome sequence and genome structure of passion fruit mosaic virus. Arch. Virol. 2011, 156, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Park, W.M. The complete nucleotide sequence and genome organization of odontoglossum ringspot Tobamovirus RNA. Arch. Virol. 1995, 140, 1577–1587. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, B. Molecular Characterization of a New Tobamovirus, Plumeria Mosaic Virus. Unpublished. 2015. Available online: https://www.ncbi.nlm.nih.gov/genomes/GenomesGroup.cgi?taxid-12234 (accessed on 8 December 2022).
- Zhang, Z.C.; Lei, C.Y.; Zhang, L.F.; Yang, X.X.; Chen, R.; Zhang, D.S. The complete nucleotide sequence of a novel Tobamovirus, Rehmannia mosaic virus. Arch. Virol. 2008, 153, 595–599. [Google Scholar] [CrossRef]
- Chavan, R.R.; Cohen, D.; Blouin, A.G.; Pearson, M.N. Characterization of the complete genome of ribgrass mosaic virus isolated from Plantago major L. from New Zealand and Actinidia spp. from China. Arch. Virol. 2012, 157, 1253–1260. [Google Scholar] [CrossRef]
- Heinze, C.; Lesemann, D.-E.; Ilmberger, N.; Willingmann, P.; Adam, G. The phylogenetic structure of the cluster of Tobamovirus species serologically related to ribgrass mosaic virus (RMV) and the sequence of Streptocarpus flower break virus (SFBV). Arch. Virol. 2005, 151, 763–774. [Google Scholar] [CrossRef]
- Adkins, S.; D’Elia, T.; Fillmer, K.; Pongam, P.; Baker, C.A. Biological and genomic characterization of a novel Tobamovirus infecting Hoya spp. Plant Dis. 2018, 102, 2571–2577. [Google Scholar] [CrossRef]
- Koenig, R. Polyhedral Plant Viruses with Monopartite RNA Genomes. In The Plant Viruses. The Viruses; Koenig, R., Ed.; Springer: Boston, MA, USA, 1988. [Google Scholar] [CrossRef]
- Berendsen, S.M.H.; Schravesande, W.E.W. Complete genome sequence of a novel genotype of pepper mild mottle virus infecting pepper in Chile. Microbiol 2020, 9, e01183-20. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, T.; Wu, Y.; An, M. Complete genome sequence of a pepper mild mottle virus isolate from Northeast China. Genome Announc. 2018, 6, e01500-17. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Garcia-Luque, I.; de la Cruz, A.; Wicke, B.; Avila-Rincon, M.J.; Serra, M.T.; Castresana, C.; Diaz-Ruiz, J.R. Nucleotide sequence of the genomic RNA of pepper mild mottle virus, a resistance-breaking Tobamovirus in pepper. J. Gen. Virol. 1991, 72, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Park, J.-S.; Han, J.-Y.; Gong, J.-S.; Park, C.-H.; Kim, J.-K.; Seo, E.-Y.; Domier, L.L.; Hammond, J.; Lim, H.-S. New Korean isolates of pepper mild mottle virus (PMMoV) differ in symptom severity and subcellular localization of the 126 kDa protein. Virus Genes 2017, 53, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Hong, J.-S.; Kim, M.-J.; Ha, J.-H.; Choi, G.-S.; Choi, J.-K.; Ryu, K.-H. Molecular characterization of pepper mild mottle virus Kr strain. Plant Pathol. J. 2005, 21, 361–368. [Google Scholar] [CrossRef]
- Velasco, L.; Janssen, D.; Ruiz-Garcia, L.; Segundo, E.; Cuadrado, I.M. The complete nucleotide sequence and development of a differential detection assay for a pepper mild mottle virus (PMMoV) isolate that overcomes L3 resistance in pepper. J. Virol. 2002, 106, 135–140. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Inoue-Nagata, A.K.; Nagata, T. Complete genome nucleotide sequence of PMMoV in Brazil. Trop Plant Pathol. 2010, 35, 373–376. [Google Scholar]
- Rialch, N.; Sharma, V.; Sharma, A.; Sharma, P.N. Characterization and complete nucleotide sequencing of Pepper Mild Mottle Virus infecting Bell Pepper in India. Phytoparasitica 2015, 43, 327–337. [Google Scholar] [CrossRef]
- Leon, Y.; Mejias, A.; Rodriguez-Roman, E.; Marys, E. Molecular and Biological Characterization of Pepper Mild Mottle Virus Isolates from Venezuela (Unpublished). 2015. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KU312319. (accessed on 8 December 2022).
- Liu, X.; Tan, Q.; Chen, H.; Liu, M.; Dai, L.; Xiao, Q. Cloning and Sequence Analysis of the Complete Nucleotide Sequence of Pepper Mild Mottle Virus Isolates from Hunan (Unpublished). 2015. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KP345899 (accessed on 8 December 2022).
- Wang, X.; Liu, F.; Zhou, G.; Li, X.-H.; Li, Z. Detection and molecular characterization of pepper mild mottle virus in China. J. Phytopathol. 2006, 154, 755–757. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Hou, H.; Xu, Q.; An, M. Identification, Whole-Genome Sequencing and Phylogenetic Analysis of Pepper Mild Mottle Virus Fengcheng Isolate (Unpublished). 2016. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KU646837 (accessed on 8 December 2022).
- Han, K.; Zheng, H.; Ji, M.; Cui, W.; Hu, S.; Peng, J.; Zhao, J.; Lu, Y.; Lin, L.; Liu, Y.; et al. A single amino acid in coat protein of Pepper mild mottle virus determines its subcellular localization and the chlorosis symptom on leaves of pepper. J. Gen. Virol. 2020, 101, 565–570. [Google Scholar] [CrossRef]
- Kirita, M.; Akutsu, K.; Watanabe, Y.; Tsuda, S. Nucleotide Sequence of the Japanese Isolate of Pepper Mild Mottle Tobamovirus (TMV-P) RNA. Jpn. J. Phytopathol. 1997, 63, 373–376. [Google Scholar] [CrossRef]
- Hamada, H.; Tomita, R.; Iwadate, Y.; Kobayashi, K.; Munemura, I.; Takeuchi, S.; Hikichi, Y.; Suzuki, K. Cooperative effect of two amino acid mutations in the coat protein of Pepper mild mottle virus overcomes L 3 -mediated resistance in Capsicum plants. Virus Genes 2006, 34, 205–214. [Google Scholar] [CrossRef]
- Hagiwara, K.; Ichiki, T.U.; Ogawa, Y.; Omura, T.; Tsuda, S. A single amino acid substitution in 126-kDa protein of pepper mild mottle virus associates with symptoms attenuation in pepper; the complete nucleotide sequence of an attenuated strain, C-1421. Arch. Virol. 2002, 147, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.U.; Nagaoka, E.N.; Hagiwara, K.; Uchikawa, K.; Tsuda, S.; Omura, T. Integration of mutations responsible for the attenuated phenotype of pepper mild mottle virus strains results in a symptomless cross-protecting strain. Arch. Virol. 2005, 150, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Genda, Y.; Kanda, A.; Hamada, H.; Sato, K.; Ohnishi, J.; Tsuda, S. Two amino acid substitutions in the coat protein of pepper mild mottle virus are responsible for overcoming the L4Gene-mediated resistance in Capsicum spp. Phytopathology 2007, 9, 787–793. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Yoon, J.-Y.; Cho, I.-S.; Choi, S.-K.; Choi, G.-S. Phylogenetic analyses of pepper mild mottle virus and Cucumber mosaic virus isolated from Rorippapalustris. Res. Plant Dis. 2016, 22, 25–31. [Google Scholar] [CrossRef]
- Moreno-Perez, M.G.; Garcia-Luque, I.; Fraile, A.; Garcia-Arenal, F. Mutations that determine resistance breaking in a plant RNA virus have pleiotropic effects on its fitness that depend on the host environment and on the type, single or mixed, of infection. J. Virol. 2016, 90, 9128–9137. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.S.; Yang, L.Y.; Anane, R.F.; Chen, Z.L.; Yang, Y.H.; Chen, L.; Sun, Y.; Zhao, M.F. First report of pepper mild mottle virus in Parispolyphylla var. yunnanensis in China. Plant Dis. 2019, 103, 3289. [Google Scholar] [CrossRef]
- Kim, S.-W.; Jeong, Y.; Yang, K.-Y.; Jeong, R.-D. First report of natural infection of Dracaena braunii by pepper mild mottle virus in Korea. J. Plant Pathol. 2022, 1, 1579. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, S.-H.; Choi, H.-S.; Kim, M.-K.; Kwak, H.-R.; Kim, J.-S.; Nam, M.; Cho, J.-D.; Cho, I.-S.; Choi, G.-S. 2007–2011 Characteristics of plant virus infections on crop samples submitted from agricultural places. Res. Plant Dis. 2012, 18, 277–289. [Google Scholar] [CrossRef]
- Jones, R.A.C. Disease Pandemics and Major Epidemics Arising from New Encounters between Indigenous Viruses and Introduced Crops. Viruses 2020, 12, 1388. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Naidu, R.A. Global Dimensions of Plant Virus Diseases: Current Status and Future Perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef]
- McKinney, H.H. Two strains of tobacco mosaic virus, one which is seed borne in an etch-immune pungent pepper. Plant Dis. Rep. 1952, 36, 184–187. [Google Scholar]
- Wetter, C.; Conti, M.; Altschuh, D.; Tabillion, R.; van Regenmortel, M.H.V. Pepper mild mottle virus, a Tobamovirus infecting pepper cultivars in Sicily. Phytopathology 1984, 74, 405–410. [Google Scholar] [CrossRef]
- Adkins, S.; Lamb, E.M.; Roberts, P.D.; Gooch, M.D.; Breman, L.; Shuler, K.D. Identification of pepper mild mottle virus in commercial bell pepper in Florida. Plant Health Prog. 2007, 4, 26. [Google Scholar] [CrossRef]
- Martínez-Ochoa, N.; Langston, D.B.; Mullis, S.W.; Flanders, J.T. First report of pepper mild mottle virus in jalapeno pepper in Georgia. Plant Health Prog. 2003, 4, 26. [Google Scholar] [CrossRef]
- Hur, O.-S.; Kwak, H.-R.; Ro, N.-Y.; Choi, Y.; Lee, S.; Hwang, A.; Kim, B.; Kim, S.-H.; Hahn, B.-S. Resistance Screening of Capsicum Germplasm to Pepper Mild Mottle Virus (PMMoV) Pathotypes P1,2 and P1,2,3. Korean J. Breed. 2022, 54, 1–7. [Google Scholar] [CrossRef]
- Moury, B.; Verdin, E. Viruses of Pepper Crops in the Mediterranean Basin. Viruses and Virus Diseases of Vegetables in the Mediterranean Basin. Adv. Virus Res. 2012, 127–162. [Google Scholar] [CrossRef]
- Antignus, Y.; Lachman, O.; Pearlsman, M.; Maslenin, L.; Rosner, A. A New Pathotype of Pepper mild mottle virus (PMMoV) Overcomes the L4 Resistance Genotype of Pepper Cultivars. Plant Dis. 2008, 92, 1033–1037. [Google Scholar] [CrossRef]
- Hamada, H.; Takeuchi, S.; Kiba, A.; Tsuda, S.; Hikichi, Y.; Okuno, T. Amino acid changes in pepper mild mottle virus coat protein that affect L3 gene-mediated resistance in pepper. J. Gen. Plant Pathol. 2002, 68, 155–162. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, H.; Liu, Y.; Huang, C.; Yuan, C.; Chen, Z.; Li, K.; Larkin, R.M.; Chen, J.; Kuang, H. Two tobamovirus multiplication proteins 2A homologs in tobacco determine asymptomatic response to tobacco mosaic virus. Plant Physiol. 2021, 187, 2674–2690. [Google Scholar] [CrossRef]
- Matsunaga, H.; Saito, T.; Hirai, M.; Nunome, T.; Yoshida, T. DNA markers linked to pepper mild mottle virus (PMMoV) resistant locus (L4) in Capsicum. J. Japan. Soc. Hort. 2003, 72, 218–220. [Google Scholar] [CrossRef]
- Yang, H.-B.; Liu, W.Y.; Knag, W.-H.; Jahn, M.; Knag, B.-C. Development of SNP markers linked to the L locus in Capsicum spp. A comparative genetic analysis. Mol. Breed. 2009, 24, 433–446. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, J.H.; Yoo, J.H.; Cho, H.J.; Kim, B.D. Development of a sequence characteristic amplified region marker linked to the L4 locus conferring broad spectrum resistance to tobamoviruses in pepper plants. Mol. Cells 2008, 25, 205–210. [Google Scholar] [PubMed]
- Sugita, T.; Yamaguchi, K.; Sugimura, Y.; Nagata, R. Development of SCAR markers linked to L3 gene in Capsicum. Breed. Sci. 2004, 54, 111–115. [Google Scholar] [CrossRef]
- Nankar, A.N.; Todorova, V.; Tringovska, I.; Pasev, G.; Radeva-Ivanova, V.; Ivanova, V.; Kostova, D.A. Step towards Balkan Capsicum annuum L. core collection: Phenotypic and biochemical characterization of 180 accessions for agronomic, fruit quality, and virus resistance traits. PLoS ONE 2020, 15, e0237741. [Google Scholar] [CrossRef]
- Svoboda, J.; Cervena, G.; Rodova, J.; Jokes, M. First report of pepper miled mottle virus in seeds produced in the Czech Republic-Short communication. Plant Prot. Sci. 2006, 42, 34–37. [Google Scholar] [CrossRef]
- Filipić, A.; Dobnik, D.; Tušek Žnidarič, M.; Žegura, B.; Štern, A.; Primc, G.; Mozetič, M.; Ravnikar, M.; Žel, J.; Gutierrez Aguirre, I. Inactivation of Pepper Mild Mottle Virus in Water by Cold Atmospheric Plasma. Front. Microbiol. 2021, 12, 618209. [Google Scholar] [CrossRef]
- Colson, P.; Richet, H.; Desnues, C.; Balique, F.; Moal, V.; Grob, J.-J.; Berbis, P.; Lecoq, H.; Harle, J.-R.; Berland, Y.; et al. Pepper mild mottle virus, a plant virus associated with specific immune responses, fever, abdominal pains, and pruritus in humans. PLoS ONE 2010, 5, e10041. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, V.; Geetanjali, A.S. Role of pepper mild mottle virus as a tracking tool for fecal pollution in aquatic environments. Arch. Microbiol. 2022, 204, 513. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, S.; Shindo, J.; Sherchand, J.B.; Haramoto, E. Virological quality of irrigation water sources and pepper mild mottle virus and tobacco mosaic virus as index of pathogenic virus contamination level. Food Environ. Virol. 2018, 10, 107–120. [Google Scholar] [CrossRef]
- Zhang, T.; Breitbart, M.; Lee, W.H.; Run, J.Q.; Wei, C.L.; Soh, S.W.L.; Hibberd, M.L.; Liu, E.T.; Rohwer, F.; Ruan, Y. RNA viral community in human feces: Prevalence of plant pathogenic viruses. PLoS Biol. 2006, 4, e3. [Google Scholar] [CrossRef]
- Kuroda, K.; Nakada, N.; Hanamoto, S.; Inaba, M.; Katayama, H.; Do, A.T.; Nga, T.T.; Oguma, K.; Hayashi, T.; Takizawa, S. Pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: Comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Sci. Total Environ. 2015, 15, 287–298. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Kishida, N.; Konno, Y.; Katayama, H.; Asami, M.; Akiba, M. Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl. Environ. Microbiol. 2013, 79, 7413–7418. [Google Scholar] [CrossRef]
- Hamza, I.A.; Jurzik, L.; Uberla, K.; Wilhelm, M. Evaluation of pepper mild mottle virus, human picobirnavirus and Torque teno virus as indicators of fecal contamination in river water. Water Res. 2011, 45, 1358–1368. [Google Scholar] [CrossRef]
- Otaki, Y.; Otaki, M.; Chaminda, T.; Kishimoto, Y.; Nakazawa, Y.; Gimhana, K. Hygiene risk of waterborne pathogenic viruses in rural communities using onsite sanitation systems and shallow dug wells. Sci. Total Environ. 2020, 752, 141775. [Google Scholar] [CrossRef]
- Bačnik, K.; Kutnjak, D.; Pecman, A.; Mehle, N.; Tušek Žnidarič, M.; Gutiérrez, A.I.; Ravnikar, M. Viromics and infectivity analysis reveal the release of infective plant viruses from wastewater into the environment. Water Res. 2020, 177, 115628. [Google Scholar] [CrossRef]
- Anderson-Coughlin, B.L.; Craighead, S.; Kelly, A.; Gartley, S.; Vanore, A.; Johnson, G.; Jiang, C.; Haymaker, J.; White, C.; Foust, D.; et al. Enteric Viruses and pepper mild mottle virus show significant correlation in select mid-atlantic agricultural waters. Appl. Environ. Microbiol. 2021, 87, e0021121. [Google Scholar] [CrossRef]
- Canh, V.D.; Torii, S.; Furumai, H.; Katayama, H. Application of capsid integrity (RT-)qPCR to assessing occurrence of intact viruses in surface water and tap water in Japan. Water Res. 2021, 189, 116674. [Google Scholar] [CrossRef]
- Bonanno Ferraro, G.; Suffredini, E.; Mancini, P.; Veneri, C.; Iaconelli, M.; Bonadonna, L.; Montagna, M.T.; De Gi-glio, O.; La Rosa, G. Pepper Mild Mottle Virus as Indicator of Pollution: Assessment of Prevalence and Concentration in Different Water Environments in Italy. Food Environ. 2021, 13, 117–125. [Google Scholar] [CrossRef]
- González-Fernández, A.; Symonds, E.M.; Gallard-Gongora, J.F.; Mull, B.; Lukasik, J.O.; Navarro, P.R.; Aguilar, A.B.; Peraud, J.; Brown, M.L.; Alvarado, D.M.; et al. Relationships among microbial indicators of fecal pollution, microbial source tracking markers, and pathogens in Costa Rican coastal waters. Water Res. 2021, 188, 116507. [Google Scholar] [CrossRef]
- Ferraro, G.B.; Suffredini, E.; Mancini, P.; Veneri, C.; Iaconelli, M.; Bonadonna, L.; Montagna, M.T.; De Giglio, O.; La Rosa, G. Pepper mild mottle virus in different water matrices. Eur. J. Public Health 2020, 30, ckaa116. [Google Scholar] [CrossRef]
- Aguado-García, Y.; Taboada, B.; Morán, P.; Rivera-Gutiérrez1, X.; Serrano-Vázquez, A.; Iša1, P.; Rojas-Velázquez, L.; Pérez-Juárez, H.; López1, S.; Torres, J.; et al. Tobamoviruses can be frequently present in the oropharynx and gut of infants during their first year of life. Sci. Rep. 2020, 10, 13595. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, N.; Matsushita, T.; Matsui, Y.; Koriki, S. Suitability of pepper mild mottle virus as a human enteric virus surrogate for assessing the efficacy of thermal or free-chlorine disinfection processes by using infectivity assays and enhanced viability PCR. Water Res. 2020, 186, 116409. [Google Scholar] [CrossRef] [PubMed]
- Hamza, H.; Rizk, N.M.; Gad, M.A.; Hamza, I.A. Pepper mild mottle virus in wastewater in Egypt: A potential indicator of wastewater pollution and the efficiency of the treatment process. Adv. Virol. 2019, 164, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Thakali, O.; Sherchand, J.B.; Haramoto, E. Detection of Pathogenic Viruses, Pathogen Indicators, and Fecal-Source Markers within Tanker Water and Their Sources in the Kathmandu Valley, Nepal. Pathogens 2019, 8, 81. [Google Scholar] [CrossRef]
- Van Zyl, W.B.; Zhou, N.A.; Wolfaardt, M.; Matsapola, P.N.; Ngwana, F.B.; Symonds, E.M.; Fagnant-Sperati, C.S.; Shirai, J.H.; Kossik, A.L.; Beck, N.K.; et al. Detection of potentially pathogenic enteric viruses in environmental samples from Kenya using the bag-mediated fltration system. Water Supply 2019, 19, 1668–1676. [Google Scholar] [CrossRef]
- Gyawali, P.; Croucher, D.; Ahmed, W.; Devane, M.; Hewitt, J. Evaluation of pepper mild mottle virus as an indicator of human faecal pollution in shellfish and growing waters. Water Res. 2019, 154, 370–376. [Google Scholar] [CrossRef]
- Hata, A.; Hanamoto, S.; Ihara, M.; Shirasaka, Y.; Yamashita, N.; Tanaka, H. Comprehensive Study on Enteric Viruses and Indicators in Surface Water in Kyoto, Japan, During 2014-2015 Season. Food Environ. Virol. 2018, 10, 353–364. [Google Scholar] [CrossRef]
- Kato, R.; Asami, T.; Utagawa, E.; Furumai, H.; Katayama, H. Pepper mild mottle virus as a process indicator at drinking water treatment plants employing coagulation-sedimentation, rapid sand filtration, ozonation, and biological activated carbon treatments in Japan. Water Res. 2018, 132, 61–70. [Google Scholar] [CrossRef]
- Saeidi, N.; Gu, X.; Tran, N.H.; Goh, S.G.; Kitajima, M.; Kushmaro, A.; Schmitz, B.W.; Gin, K.Y. Occurrence of traditional and alternative fecal indicators in tropical urban environments under different land use patterns. Appl. Environ. Microbiol. 2018, 84, e00287-18. [Google Scholar] [CrossRef]
- Symonds, E.M.; Young, S.; Verbyla, M.E.; McQuaig-Ulrich, S.M.; Ross, E.; Jiménez, J.A.; Harwood, V.J.; Breitbart, M. Microbial source tracking in shellfish harvesting waters in the Gulf of Nicoya, Costa Rica. Water Res. 2017, 111, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rosiles-González, G.; Ávila-Torres, G.; Moreno-Valenzuela, O.A.; Acosta-González, G.; Leal-Bautista, R.M.; Grimaldo-Hernández, C.D.; Brown, J.K.; Chaidez-Quiroz, C.; Betancourt, W.Q.; Gerba, C.P.; et al. Occurrence of pepper mild mottle virus (PMMoV) in Groundwater from a Karst Aquifer System in the Yucatan Peninsula, Mexico. Food Environ. Virol. 2017, 9, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.M.; Sinigalliano, C.; Gidley, M.; Ahmed, W.; McQuaig-Ulrich, S.M.; Breitbart, M. Faecal pollution along the southeastern coast of Florida and insight into the use of pepper mild mottle virus as an indicator. J. Appl. Microbiol. 2016, 121, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.W.; Kitajima, M.; Campillo, M.E.; Gerba, C.P.; Pepper, I.L. Virus Reduction during Advanced Bardenpho and Conventional Wastewater Treatment Processes. Environ. Sci. Technol. 2016, 50, 9524–9532. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Iker, B.C.; Pepper, I.L.; Gerba, C.P. Relative Abundance and Treatment Reduction of Viruses during Wastewater Treatment Processes--identification of Potential Viral Indicators. Sci Total Environ. 2014, 488–489, 290–296. [Google Scholar] [CrossRef]
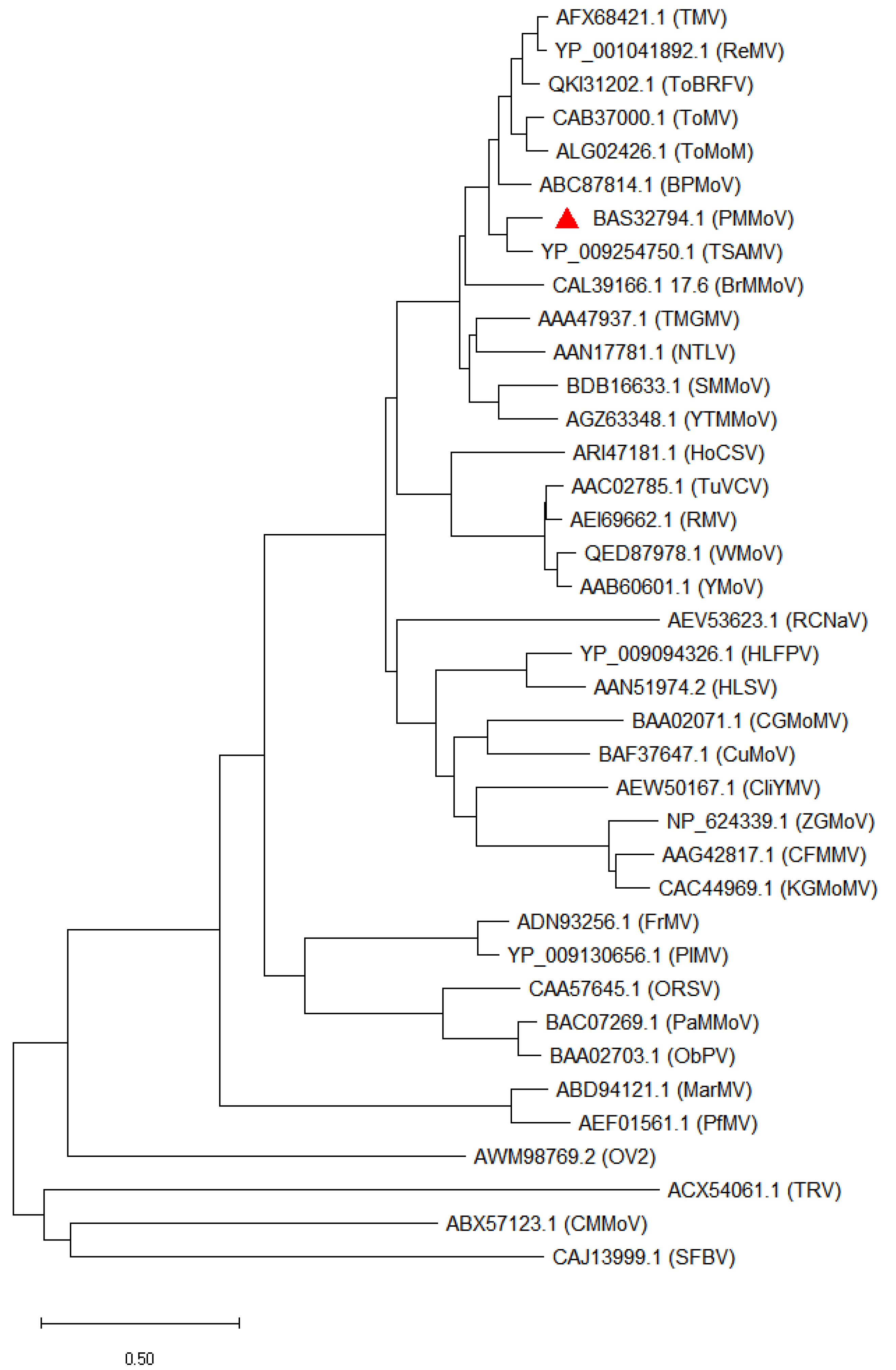
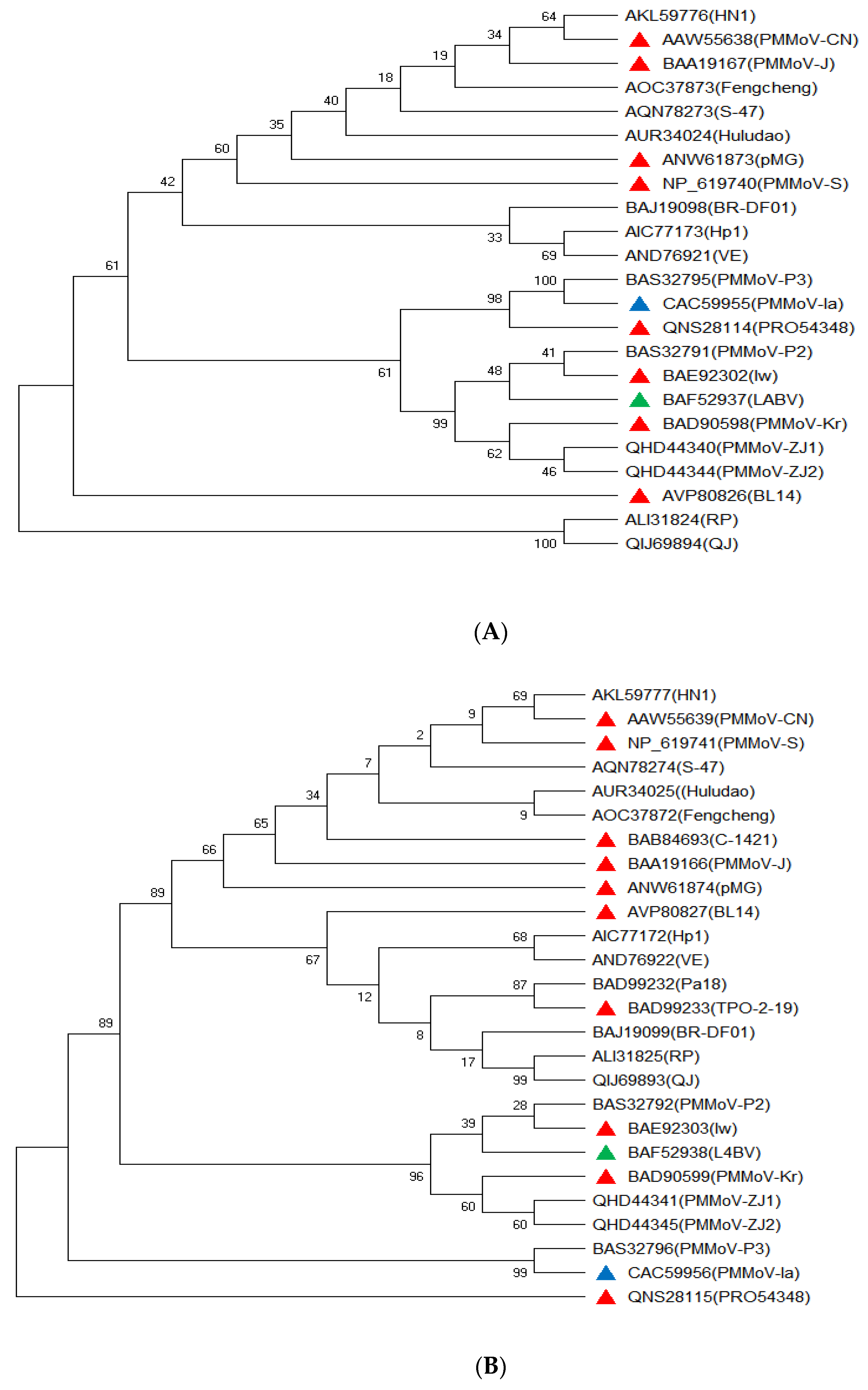
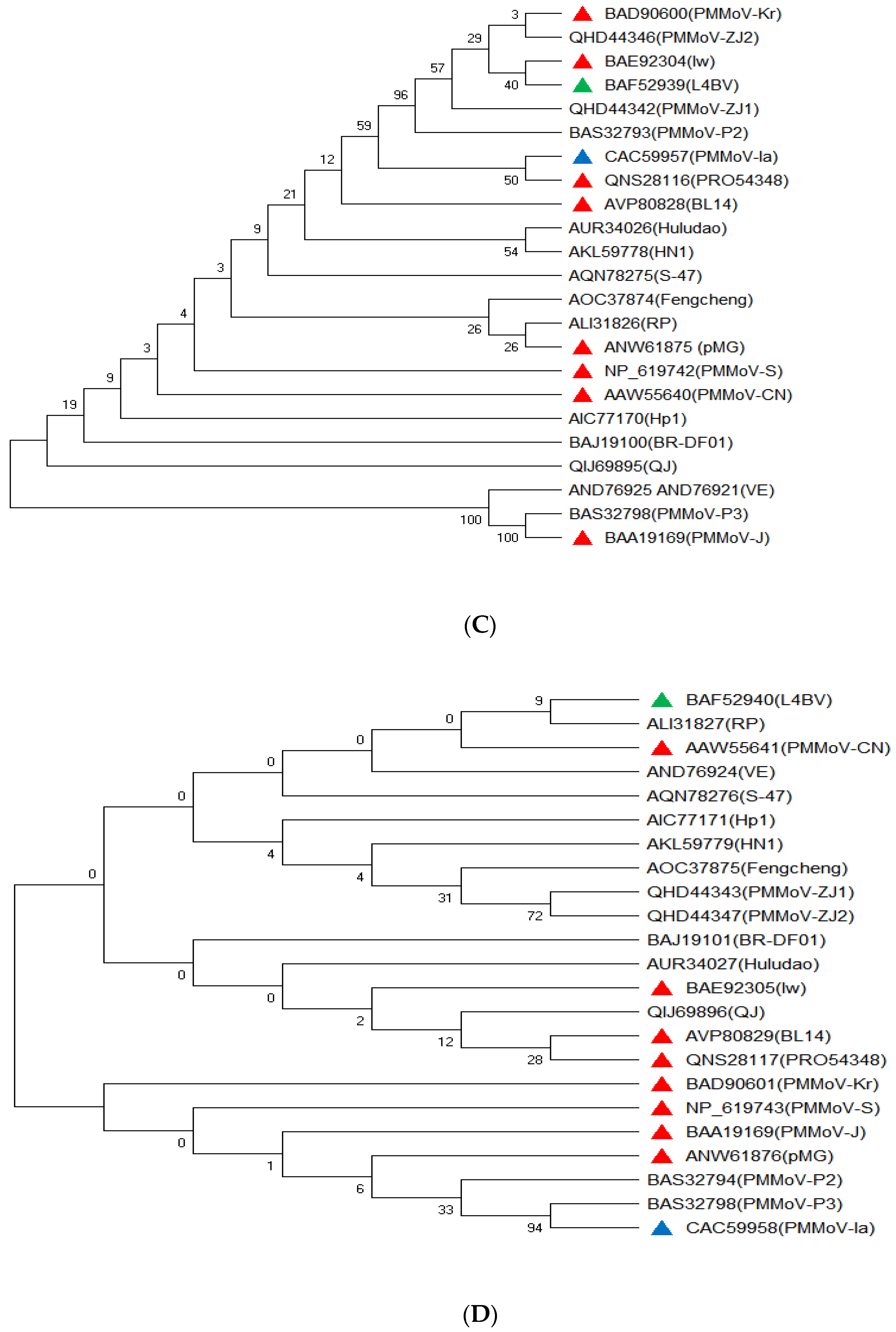


| Name of Virus | Short Name | Representative Isolate | GeneBank Accession | Nucleotide Length (kb) | Host Common Name | Host Taxonomic Name | Host Family | Reference |
|---|---|---|---|---|---|---|---|---|
| Pepper mild mottle virus | PMMoV | PMMoV-p2 | LC082099 | 6.356 | Paprika | Capsicum annuum | Solanaceae | [10] |
| Bell pepper motile virus | BPMoV | BPMoV-P1 | DQ355023 | 6.375 | Eggplant | Solanum melongena | Solanaceae | [15] |
| Paprika mild mottle virus | PaMMoV | PaMMoV-J | ABO89381 | 6.525 | Sweet pepper | Capsicum annuum L. | Solanaceae | [16] |
| Obuda pepper virus | ObPV | ObPV-Ob | D13438 | 6.507 | Tobacco | Nicotiana tabacum cv. Xanthi nc. | Solanaceae | [17] |
| Tomato mosaic virus | ToMV | S1 | AJ132845 | 6.384 | Tomato | Solanum spp. | Solanaceae | [18] |
| Tomato brown rugose fruit virus | ToBRFV | ToBRFV-CA18-01 | MT002973 | 6.389 | Tomato | Solanum lycopersicum | Solanaceae | [19] |
| Tomato mottle mosaic virus | ToMoMV | ToMMV_NY-13 | KT810183 | 6.398 | Tomato | Solanum lycopersicum | Solanaceae | [20] |
| Tobacco mosaic virus | TMV | TMV-variant 1 | V01408 | 6.395 | Tobacco | Nicotiana spp. | Solanaceae | [21] |
| Tobacco mild green mosaic virus | TMGMV | - | M34077 | 6.355 | Tobacco | Nicotiana tabacum | Solanaceae | [22] |
| Tobacco rattle virus | TRV | TRV MI-1 RNA-1 | GQ903771 | 6.791 | Potatoes | Solanaceae | [23] | |
| Tobacco latent virus | NTLV | AY137775 | 1.415 | Tobacco | Nicotiana species | Solanaceae | [24] | |
| Brugmansia mild mottle virus | BrMMoV | BrMMoV-2373 | AM398436 | 6.381 | Brugmansia | Angel’s trampet | Solanaceae | [25] |
| Tropical soda apple mosaic virus | TSAMV | TSAMV-Okeechobee | NC030229 | 6.350 | Tropical soda apple | Solanum viarum | Solanaceae | [26] |
| Scopolia mild mottle virus | SMMoV | SMMoV-Kyo35 | LC643028 | 6.350 | Japanese belladonna | Scopolia japonica | Solanaceae | [27] |
| Yello tailflower mild mottle virus | YTMMoV | YTMMV-Cervantes | KF495564 | 6.379 | Yellow tailflower | Anthocercislittorea | Solanaceae | [28] |
| Cucumber fruit mottle mosaic virus | CFMMV | CFMMV | AF321057 | 6.562 | Cucumber | Cucumis sativus | Cucubitaceae | [29] |
| Cucumber green mottle mosaic virus | CGMoMV | CGMMV-SH | D12505 | 6.424 | Muskmelon | Cucumis melo | Cucubitaceae | [30] |
| Cucumber mottle virus | CuMoV | CMoV | AB261167 | 6.485 | Cucumber | Cucumis sativus | Cucubitaceae | [31] |
| Kyuri green mottle mosaic virus | KGMoMV | KGMoMV-C1 | AJ295948 | 6.514 | Cucumber | Cucumis sativus L. | Cucubitaceae | [32] |
| Zucchini green mottle mosaic virus | ZGMoV | ZGMMV-K | NC003878 | 6.513 | Zucchini squash | Cucurbita pepo L. zucchini | Cucubitaceae | [33] |
| Turnip vein-clearing virus | TuVCV | TuVCV-OSU | U03387 | 6.311 | Turnip | Brassica rapa | Brassicaceae | [34] |
| Wasabi mottle virus | WMoV | WMoV-SFU2 | MK431779 | 6.297 | Wasabi | Wasabi japonica (Miq) Matsum | Brassicaceae | [35] |
| Youcai mosaic virus/Oilseed rape mosaic virus | YMoV | U30944 | 6.303 | Rapeseed | Brassica napus | Brassicaceae | [36] | |
| Hibiscus latent Fort Pierce virus | HLFPV | HLFPV-J | NC025381 | 6.431 | Hibiscus | Hibiscus spp. | Malvaceae | [37] |
| Hibiscus latent Singapore virus | HLSV | Singapore | AF395898 | 6.485 | Hibiscus | Hibiscus rsa-sinensis | Malvaceae | [38] |
| Clitoria yellow mottle virus | CliYMV | Larrimah | JN566124 | 6.514 | Butterfly peas | Clitoriaternatea | Fabaceae | [39] |
| Sunn-hemp mosaic virus | SHMV | SHMV | U47034 | 4.683 | Sunn-hemp | Crotalaria juncea | Fabaceae | [40] |
| Cactus mild mottle virus | CMMoV | CMMoV-Kr | EU043335 | 6.449 | Diseased grafted cactus | Gymnocalyciummihanovichii | Cactaceae | [41] |
| Opuntia virus 2 | OV2 | Nopal_hec Mex | MF434821 | 6.4.53 | Prickly pear (mixed sample) | Opuntia sp. | Cactaceae | [12] |
| Rattail cactus necrosis-associated virus | RCNaV | RCNaV | JF729471 | 6.506 | Ratilcatus | Aporocactusflagelliformis | Cactaceae | [42] |
| Frangipani mosaic virus | FrMV | FrMV-P | HM026454 | 6.643 | Frangipani | Plumeria rubra f. acustifolia | Apocynaceae | [43] |
| Maracuja mosaic virus | MarMV | MarMV | DQ356949 | 6.794 | Passion fruit | Passiflora edulis Sims ‘Flavicarpa’ | Passifloraceae | [13] |
| Passion fruit mosaic virus | PfMV | PfMV | HQ389540 | 6.791 | Passion fruit | Passiflora incarnata L. | Passifloraceae | [44] |
| Odontoglossum ringspot virus | ORSV | ORSV-Cy | X82130 | 6.618 | Tobacco | Nicotiana tabacum | Orchidaceae | [45] |
| Plumeira mosaic virus | PlMV | Plu-Ind-1 | NC026816 | 6.688 | Frangipani | Plumeria rubra f. acustifolia | Apocynaceae | [46] |
| Rehmannia mosaic virus | ReMV | Henan | NC009041 | 6.395 | Rehmannia | Rehmanniaglutinosa Libosch | Orobanchacee | [47] |
| Ribgrass mosaic virus | RMV | Kons 1105-R14 | HQ667979 | 6.311 | Rigbgrass | Plantago major L. | Plantaginaceae | [48] |
| Streptocarpus flower break virus | SFBV | SFBV | AM040955 | 6.279 | Streptocarpus | Streptocarpus spp. | Gesneriaceae | [49] |
| Hoya chlorotic spot virus | HoCSV | 12-415 | KX434725 | 6.386 | Hoya wayetii | Hoya spp. | Asclepiadaceae | [50] |
| PMMoV Isolate | Country | Accession Number | Sequence Length (kb) | Protein ID (183 kDa) | Host | Reference |
|---|---|---|---|---|---|---|
| PMMoV-P2 | Republic of Korea | LC082099 | 6.356 | BAS32791 | Capsicum spp. | [10] |
| PMMoV-P3 | Republic of Korea | LC082100 | 6.356 | BAS32795 | Capsicum spp. | [10] |
| S-47 | Republic of Korea | KX399390 | 6.356 | AQN78273 | Capsicum spp. | [55] |
| J-47 | Republic of Korea | KX399389 | 6.356 | AQN78269 | Capsicum spp. | [55] |
| PMMoV-Kr | Republic of Korea | AB126003 | 6.356 | BAD90598 | Capsicum spp. | [56] |
| PMMoV-Ia | Spain | AJ308228 | 6.357 | CAC59955 | Capsicum spp. | [57] |
| BR-DF01 | Brazil | AB550911 | 6.356 | BAJ19098 | _ | [58] |
| Hp1 | India | KJ631123 | 6.356 | AIC77173 | Capsicum spp. | [59] |
| VE | Venezuela | KU312319 | 6.356 | AND76921 | Capsicum spp. | [60] |
| PMMoV-S | Spain | NC003630 | 6.357 | NP_619740 | Capsicum spp. | [54] |
| Huludao | China | MG515725 | 6.356 | AUR34024 | Capsicum spp. | [53] |
| HN1 | China | KP345899 | 6.356 | AKL59776 | Capsicum spp. | [61] |
| PMMoV-CN | China | AY859497 | 6.356 | AAW55638 | Capsicum spp. | [62] |
| Fengcheng | China | KU646837 | 6.356 | AOC37873 | Capsicum spp. | [63] |
| PMMoV-ZJ1 | China | MN616926 | 6.356 | QHD44340 | Capsicum spp. | [64] |
| PmmoV-ZJ2 | China | MN616927 | 6.357 | QHD44344 | Capsicum spp. | [64] |
| PMMoV-J | Japan | AB000709 | 6.357 | BAA19167 | Capsicum spp. | [65] |
| Iw | Japan | AB254821 | 6.356 | BAE92302 | Capsicum spp. | [66] |
| BL14 | USA | MH063882 | 6.353 | AVP80826 | Capsicum spp. | [4] |
| C-1421 | Japan | AB069853 | 6.357 | BAB84693 | Capsicum spp. | [67] |
| Pa18 | Japan | AB113116 | 6.356 | BAD99232 | Capsicum spp. | [68] |
| TPO-2-19 | Japan | AB113117 | 6.356 | BAD99233 | Capsicum spp. | [68] |
| PRO54348 | Chile | MT385868 | 6.356 | QNS28114 | Capsicum spp. | [52] |
| L4BV | Japan | AB276030 | 6.356 | BAF52937 | Capsicum spp. | [69] |
| RP | Republic of Korea | KR108206 | 6.356 | AL131824 | Rorippa palustris | [70] |
| pMG | Spain | KX063611 | 6.361 | ANW61873 | Capsicum spp. | [71] |
| QJ | China | MK784568 | 6.357 | QIJ69894 | P. polyphylla | [72] |
| Marker | Primer | Primer Sequence (5′–3′) | Primer Size (bp) | Type | Resistance | Reference |
|---|---|---|---|---|---|---|
| AP-7 | CGTACTGTGGCTCAAAACTC | - | - | L4 | ||
| SCAR | AP-8 | ATTCGCACCGTTTAGCCCGT | - | - | L4 | [86] |
| 087H3T7150F | CATGATTACATTTTATGTTGC | Co-dominant | L4 | |||
| 087H3T7 | 087H3T7150R | AAAAGGAAGGTTCTCATTGTT | 150 | L4 | [87] | |
| 087H3T7F | CCTTTGCCTGCATTATTCTTG | L4 | ||||
| 087H3T7 | 087H3T7R | GCCCAAATTTATTCCCAAATGC | 440 | Co-dominant | L4 | [87] |
| 060I2END-2F | GCACATCAGCAGGTTTAGTACG | L4 | ||||
| 060I2END | 060I2END-2R | CCAACTGTCAAACCTCGGTT | 751 | Co-dominant | L4 | [87] |
| 158K24HRMF | CAGATTAAGTGTTCAAAATGAGTGATG | Co-dominant | L4 | |||
| 158K24HRM | 158K24HRMR | TGATTCCATGAAAATAAATTGTAAAGA | 125 | L4 | [87] | |
| F | AAGGGGCGTTCTTGAGCCAA | - | L4 | |||
| L4SC340 | R | TCCATGGAGTTGTTCTGCAT | 340 | - | L4 | [88] |
| PMF1 | CTGCAGAACAACAATGGCACG | Co-dominant | L3 | |||
| PMFR11269 | PMR1 | GCTTCCTCCTCTGCAGTCC | 268 | L3 | [89] | |
| PMF2 | GCCAAAATGGTAATTG | Co-dominant | L3 | |||
| PMFR11283 | PMF1 | GCTTCCTCCTCTGCAGTCC | 283 | L3 | [89] |
| Germplasm (Name or Accession) * | Pepper Type | Resistance Genotype | PMMoV Pathotype | Reaction | Response | Screening Method | Reference |
|---|---|---|---|---|---|---|---|
| Easy | C. annuum | L4L4 | P1.2 and P1.2.3 | NS/- | R | Bioassay and genetic markers | [11] |
| Magnipico | C. annuum | L4L4 | P1.2 and P1.2.3 | NS/ | R | Bioassay and genetic markers | [11] |
| Orange glory | C. annuum | L4L3 | P1.2 and P1.2.3 | NS/ | R | Bioassay and genetic markers | [11] |
| Scirocco F1 | C. annuum | L4L3 | P1.2 and P1.2.3 | NS/ | R | Bioassay and genetic markers | [11] |
| Special F1 | C. annuum | L4L1 | P1.2 and P1.2.3 | NS/ | R | Bioassay and genetic markers | [11] |
| IT261210 * | C. chinense | PMMoV-1.2.3 | Nl/- | R | Bioassay and RT-PCR | [81] | |
| IT261211 * | C. chinense | PMMoV-1.2.3 | Nl/- | R | Bioassay and RT | [81] | |
| IT261426 * | C. chinense | PMMoV-1.2.3 | Nl/- | R | Bioassay and RT | [81] | |
| IT261431 * | C. chinense | PMMoV-1.2.3 | Nl/- | R | Bioassay and RT | [81] | |
| IT261442 * | C. chinense | PMMoV-1.2.3 | Nl/- | R | Bioassay and RT | [81] | |
| IT284050 * | C. chinense | PMMoV-1.2.3 | Nl/- | R | Bioassay and RT | [81] | |
| PI 152225 * | C. chinense | L3 | PMMoV-P1.2 | Nl/ | R | Mechanical and biological characterization | [91] |
| PI 260429 * | C. chinense | L4 | PMMoV-P1.2 | Nl/ | R | Mechanical and biological characterization | [91] |
| PI260429 * | C. Chacoense | L4 | PMMoV-1.2.3 | - | R | SCAR DNA marker | [86] |
| SA185 * | C. Chacoense | L4 | PMMoV | - | R | SCAR DNA marker | [86] |
| Susan | C. annuum | L4 | PMMoV | - | R | SCAR DNA marker | [86] |
| Special | C. annuum | L4 | PMMoV-1.2.3 | - | R | SCAR DNA marker | [86] |
| AP-PM01 * | C. annuum | L4 | PMMoV | - | R | SCAR DNA marker | [86] |
| AP-PM02 * | C. annuum | L4 | PMMoV | - | R | SCAR DNA marker | [86] |
| AP-PM03 * | C. annuum | L4 | PMMoV-1.2.3 | - | R | SCAR DNA marker | [86] |
| AP-PM04 * | C. annuum | L4 | PMMoV | - | R | SCAR DNA marker | [86] |
| AP-PM05 * | C. annuum | L4 | PMMoV | - | R | SCAR DNA marker | [86] |
| AP-PM06 * | C. annuum | L4 | PMMoV | - | R | SCAR DNA marker | [86] |
| Kyouyutaka | C. annuum | L1 | PMMoV-1.2 | - | R | SCAR marker | [89] |
| Tosahikari D | L1 | PMMoV-1.2 | - | R | SCAR marker | [89] | |
| Tabasco | C. frutescens | L2 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| PI159236 * | C. chinense | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Berumasari | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Himukamidori | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| T-143 * | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Tosahime R | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Spirit | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Mihata 1 | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Sarara | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Miogi | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Fiesta | C. annuum | L3 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| PI260429 | C. chacoense | L4 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Leira | C. annuum | L4 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Special | C. annuum | L4 | PMMoV-1.2/1.2.3 | - | R | SCAR marker | [89] |
| Study Location | Year | Sample Source | Detection Method | Key Findings | References |
|---|---|---|---|---|---|
| Atlantic, USA | 2021 | Surface and reclaimed water | RT-qPCR | 1. PMMoV detected more in reclaimed water than in surface water samples. 2. Water salinity affected the detection of PMMoV and other enteric viruses. | [102] |
| Japan | 2021 | Surface and tap water | RT-qPCR and SD-CDDP-(RT-qPCR | 1. PMMoV detected in surface water. 2. Intact PMMoV was more common than intact human pathogenic viruses. | [103] |
| Italy | 2021 | Raw and treated sewage, river, estuarine, bathing water, groundwater, and drinking water | Nested RT-qPCR and sequencing | 1. PMMoV detected in both treated and untreated sewage, river, estuarine water, bathing water, and groundwater samples. 2. No PMMoV detected in drinking water. | [104] |
| Costa Rica | 2021 | River and ocean discharge sites | RT-qPCR | 1. PMMoV and HF183 detected in all river samples and in >89% of ocean samples. | [105] |
| Slovenia | 2021 | PMMoV -containing plant homogenates and PMMoV-free homogenates | Test plant infectivity assays, transmission electron microscopy, RT-PCR- and RT-droplet digital PCR | 1. PMMoV is a very resilient water-transmissible Tobamovirus and can survive transit through the human gut. 2. CAP is a useful water treatment tool for inactivation of pathogenic viruses, including PMMoVand other enteric viruses. | [92] |
| Japan | 2021 | Groundwater (well water) | Quantitative microbial risk assessment, membrane filtration method | 1. PMMoV detected in well water in high based only on the horizontal distance as the PMMoV concentration decreased rapidly as distance increased. | [100] |
| Italy | 2020 | Urban wastewaters, treated effluents, surface water, estuarine, seawater, groundwater, and drinking water | Nested RT-PCR and TaqMan-based qPCR | 1. PMMoV detected in wastewater, treated sewage, river, estuarine, bathing water, and groundwater samples. 2. No PMMoV detected in drinking water samples. 3. PMMoV is ubiquitous throughout the water cycle with different concentrations. | [106] |
| Mexico | 2020 | Fecal, oropharyngeal (gastrointestinal) samples. | NextSeq500 Illumina platform | 1. PMMoV, in addition to tropical soda apple mosaic virus and opuntia virus 2, were the most common species detected in fecal and oropharynx samples. | [107] |
| Slovenia | 2020 | Influents and effluents ofwastewater treatment plants | RT-qPCR | 1. High-diversity plant viruses, especially tobamoviruses, were detected in wastewater treatment plant influents and effluents. | [101] |
| Japan | 2020 | Human enteric viruses and PMMoV | PMA-PCR, PMA-Enhancer-PCR, PMAxx-PCR, and PMAxx-Enhancer-PCR | 1. PMMoV was more resistant to heat treatments and could be a potential surrogate for some enteric viruses in thermal disinfection processes. 2. The PMMoV was comparatively much more resistant to chlorine treatment. | [108] |
| Egypt | 2020 | Influent and effluentwastewater | g qRT-PCR | 1. PMMoV was detected in both influent and effluent samples and no clear seasonality of detection was found. 2. PMMoV can be used as a fecal indicator of wastewater contamination and a process indicator for the performance of the treatment process. | [109] |
| Nepal | 2019 | Tanker water | qPCR | 1. PMMoV together with tobacco mosaic virus was detected in tanker water. | [110] |
| Kenya | 2019 | Wastewater and wastewater-impacted surface waters | RT-PCR | 1. PMMoV and other enteroviruses were detected in all samples and could be used as indicators in fecal contaminated sites. | [111] |
| New Zealand | 2019 | Nonhuman fecal matter; influent wastewater, and fish-growing waters | RT-qPCR | 1. Certain nonhuman fecal samples (seagull, Canada goose, black swan, and dog) were positive for PMMoV. 2. PMMoV detected in shellfish and shellfish-growing water samples. | [112] |
| Japan | 2018 | Surface water | Conventional plaque assay, RT-qPCR | 1. PMMoVdetected in the surface water samples regardless of season and location, and is useful as an indicator for water contamination. | [113] |
| Japan | 2018 | Water | Taqman-based RT-qPCR | 1. PMMoV detected in raw water throughput the year and can serve as a treatment process indicator of enteric viruses. | [114] |
| Singapore | 2018 | Water from different water bodies | Hollow fiber ultrafiltration, ImProm-II reverse transcription system (Promega), qPCR | 1. PMMoV detected in the water sample and can be used as a suitable indicator of fecal pollution in tropical surface waters. | [115] |
| Kathmandu Valley, Nepal. | 2018 | Irrigation water sources | Electronegative membrane-vortex method and TaqMan (MGB)-based qPCR assays | 1. PMMoV (and TMV) detected in all types of irrigation water sources and is a potential indicator to elucidate pathogenic virus levels in environmental samples. 2. Seasons had good correspondence with the presence of pathogenic virus types. | [95] |
| Costa Rica | 2017 | Fecal matter of animals, domestic wastewater, and surface water | RT-qPCR | 1. PMMoV is a useful domestic wastewater-associated marker, with high concentrations and 100% sensitivity and specificity. 2. PMMoV markers were not detected in any surface water samples. | [116] |
| Mexico | 2017 | Groundwater | RT-PCR and cloning | 1. PMMoV RNA detected in most samples with gene sequences sharing 99–100% of nucleotide identity with other PMMoV sequences. 2. No significant correlation observed between PMMoV occurrences by season or water type. | [117] |
| Southeastern Florida | 2016 | Surface water samples from inlets, exposed to runoff and septic seepage, and coastal sites, exposed to ocean outfalls | RT-qPCR | 1. PMMoV detected more frequently than other microbial source tracking markers. | [118] |
| Southern Arizona | 2016 | Wastewater | TaqMan-based qPCR | 1. PMMoV in addition to AiV, AdV, JCPyV and BKPyV were detected in the samples and are potential viral markers for human fecal contamination. 2. Frequency of PMMoV detection was less influenced by seasonal variation. | [119] |
| Hanoi, Vietnam | 2015 | Surface water, wastewater, groundwater, tap water, and bottled water | qPCR | 1. PMMoV detected in many surface water samples and in all wastewater samples in high concentration. 2. PMMoV is useful as a sensitive fecal indicator for evaluating the potential occurrence of pathogenic viruses. 3. No PMMoV detection in tap water and bottled water samples. | [97] |
| Southern Arizona | 2014 | Wastewater samples | RT-qPCR TaqMan-based quantitative PCR (qPCR) assays | 1. PMMoV (and AiV) detected in both influent and effluent water. 2. PMMoV can be used as potential indicator of wastewater reclamation. 3. No significant seasonal change in concentration of PMMoV was recorded. | [120] |
| Japan | 2013 | Drinking water sources | qPCR | 1. Significant difference in the occurrence of PMMoV observed among geographical regions but not a seasonal difference. 2. PMMoV strains were diverse in the water sources. | [98] |
| Germany | 2011 | Rivers, influents and effluents of wastewater; animal (including human) stool. | Quantitative real time (RT-) PCR | 1. PMMoV highly detected in all river water samples, while frequently of other viruses (HAdV and HPyV, TTV and hPBV) were less detected. 2.PMMoV could be a promising indicator of fecal pollution in surface water. | [99] |
| USA | 2010 | Commercialized food products containing peppers; human stool | RT-PCR, sequencing, and electron microscopy | 1. PMMoV in feces can infect host plants and is viable after passing through the gut. 2. Individuals (humans) positive for PMMoV showed symptoms such as pain in the stomach and mild fever. | [93] |
| USA | 2009 | Raw sewage, treated wastewater, seawater exposed to wastewater, and fecal samples and intestinal homogenates from a wide variety of animals | qPCR | 1. PMMoV was present in all wastewater and some seawater samples but at higher concentrations in raw sewage and has a potential utility as an indicator of human fecal pollution. 2. Though ubiquitous in human feces, PMMoV was not detected in the majority of animal fecal samples tested (except chicken and seagull samples). 3. PMMoV was not found in nonpolluted seawater samples but could be detected in surface seawater. | [8] |
| San Diego, California, United States | 2006 | Fecal samples from two healthy human individuals | RT-PCR | 1. PMMoV detected in human fecal samples and high concentration of its viron particles observed in the samples. 2. The vast majority of the viral sequences showed similarity to plant pathogenic RNA viruses. 3. PMMV was also detected in some fecal samples from healthy individuals. 4. A number of pepper-based foods were tested positive for PMMV, which suggests a dietary origin for the virus. 5. PMMV derived from fecal matter is infectious to host plants. | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochar, K.; Ko, H.-C.; Woo, H.-J.; Hahn, B.-S.; Hur, O. Pepper Mild Mottle Virus: An Infectious Pathogen in Pepper Production and a Potential Indicator of Domestic Water Quality. Viruses 2023, 15, 282. https://doi.org/10.3390/v15020282
Ochar K, Ko H-C, Woo H-J, Hahn B-S, Hur O. Pepper Mild Mottle Virus: An Infectious Pathogen in Pepper Production and a Potential Indicator of Domestic Water Quality. Viruses. 2023; 15(2):282. https://doi.org/10.3390/v15020282
Chicago/Turabian StyleOchar, Kingsley, Ho-Cheol Ko, Hee-Jong Woo, Bum-Soo Hahn, and Onsook Hur. 2023. "Pepper Mild Mottle Virus: An Infectious Pathogen in Pepper Production and a Potential Indicator of Domestic Water Quality" Viruses 15, no. 2: 282. https://doi.org/10.3390/v15020282
APA StyleOchar, K., Ko, H.-C., Woo, H.-J., Hahn, B.-S., & Hur, O. (2023). Pepper Mild Mottle Virus: An Infectious Pathogen in Pepper Production and a Potential Indicator of Domestic Water Quality. Viruses, 15(2), 282. https://doi.org/10.3390/v15020282






