Evidence of a Set of Core-Function Genes in 16 Bacillus Podoviral Genomes with Considerable Genomic Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Bacteria
2.2. Phage Discovery
2.3. DNA Sequencing
2.4. Genome Annotation
2.5. Comparative Genomics
3. Results
3.1. Phage Isolation and Genome Sequencing
3.2. Genome Relationships to Sequenced Bacteriophages
3.3. Average Nucleotide Identity
3.4. Protein Content
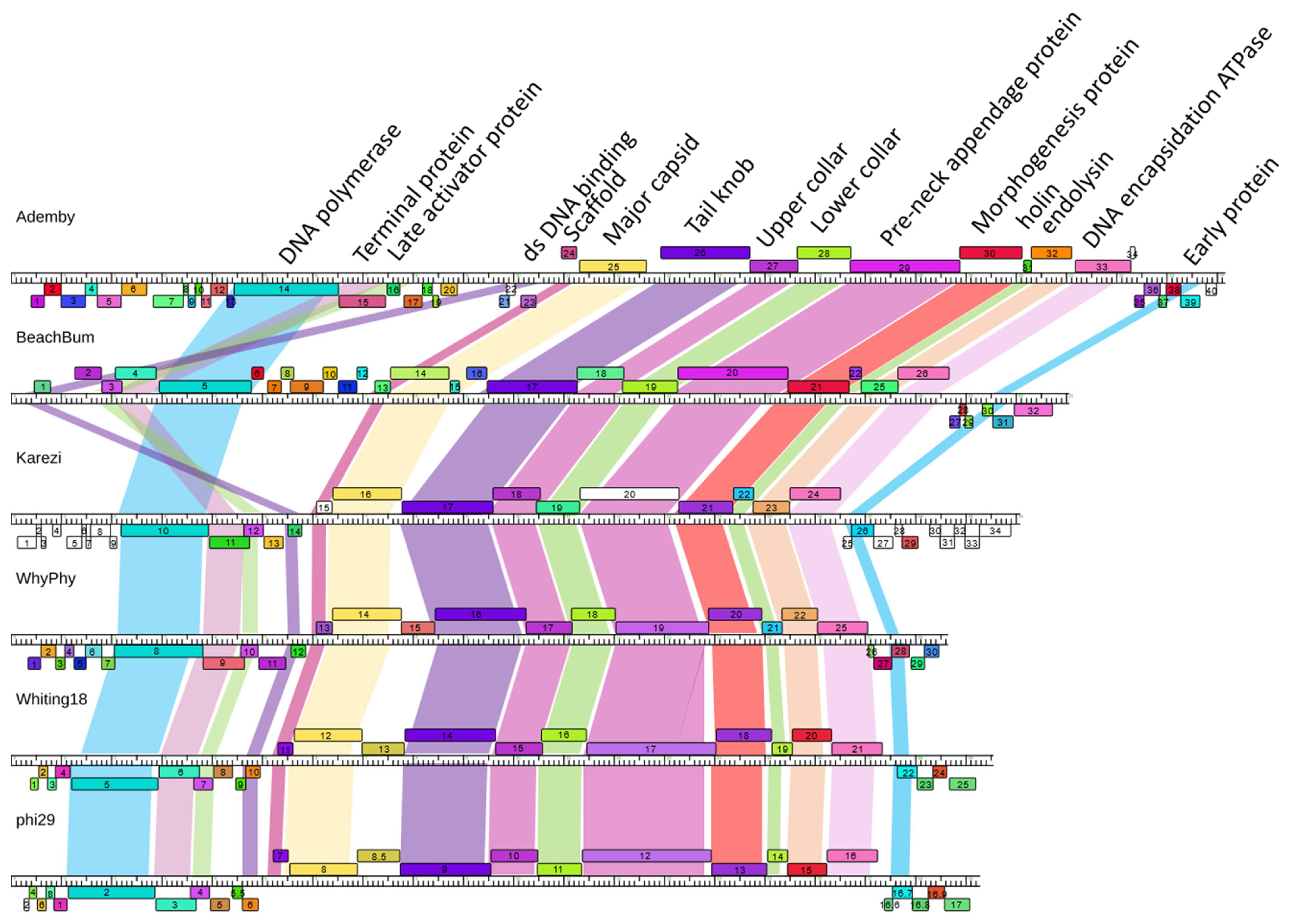
3.5. Phamerator Maps
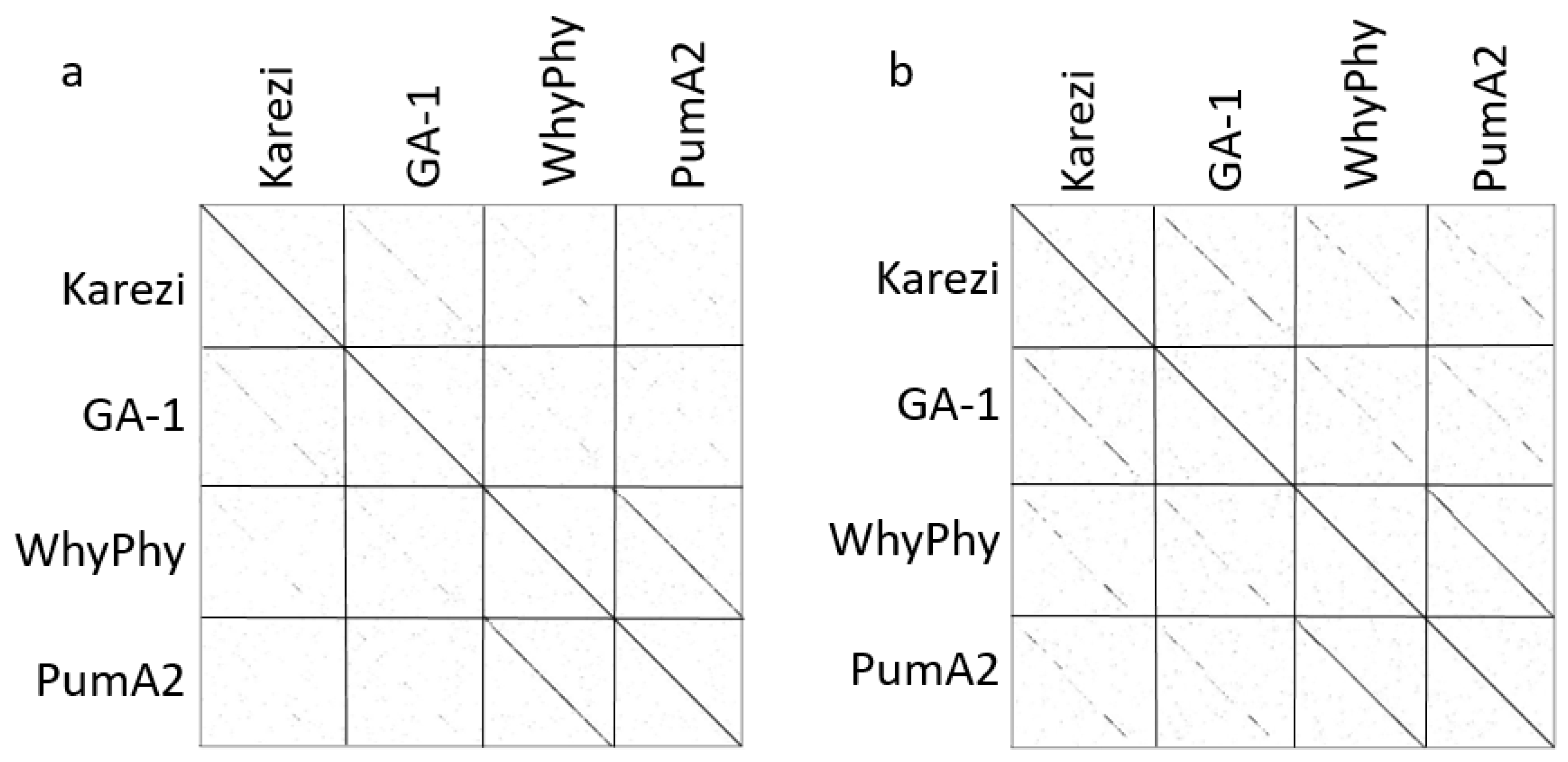
3.6. Nucleotide vs. Protein Dotplots
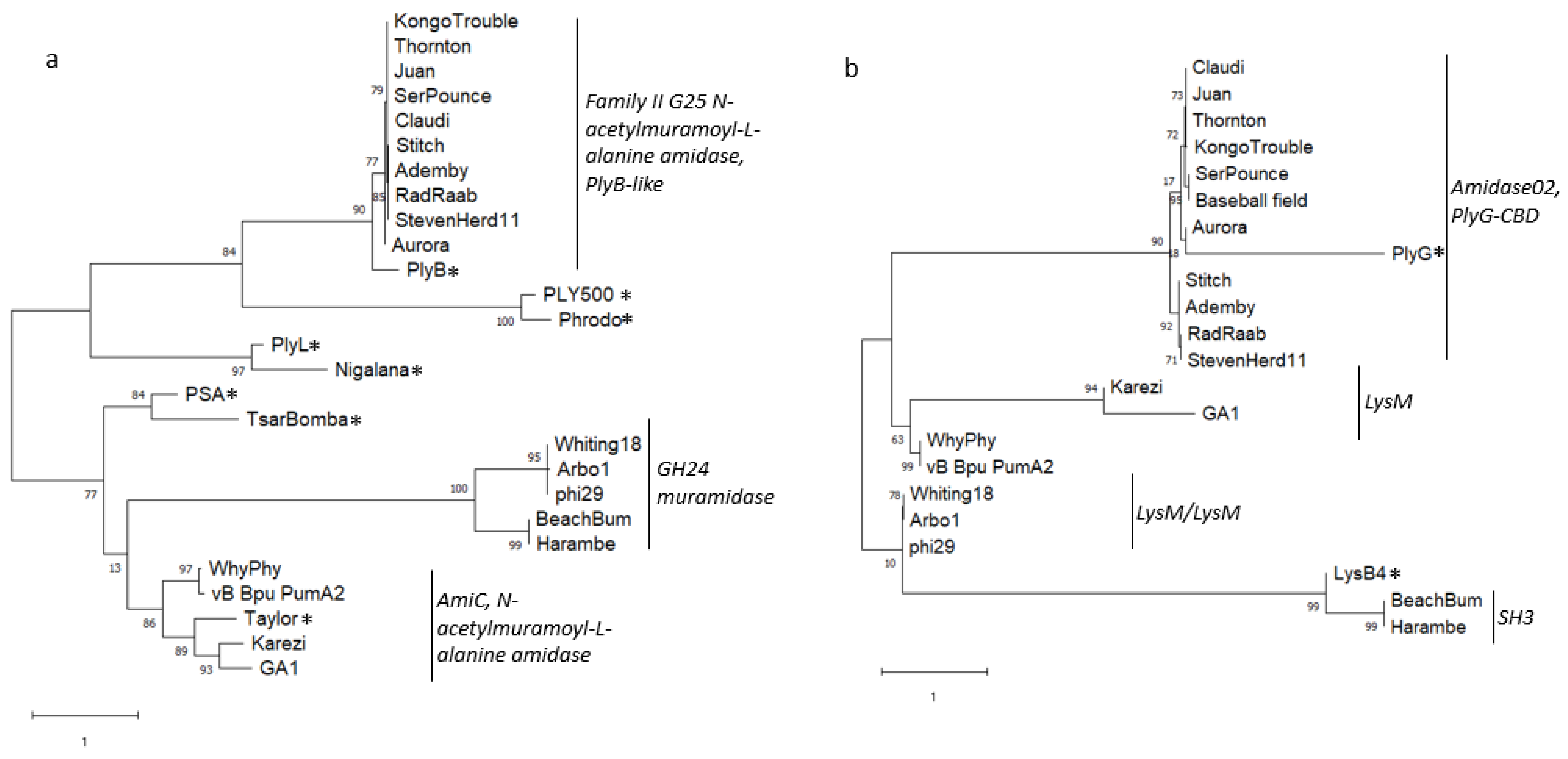
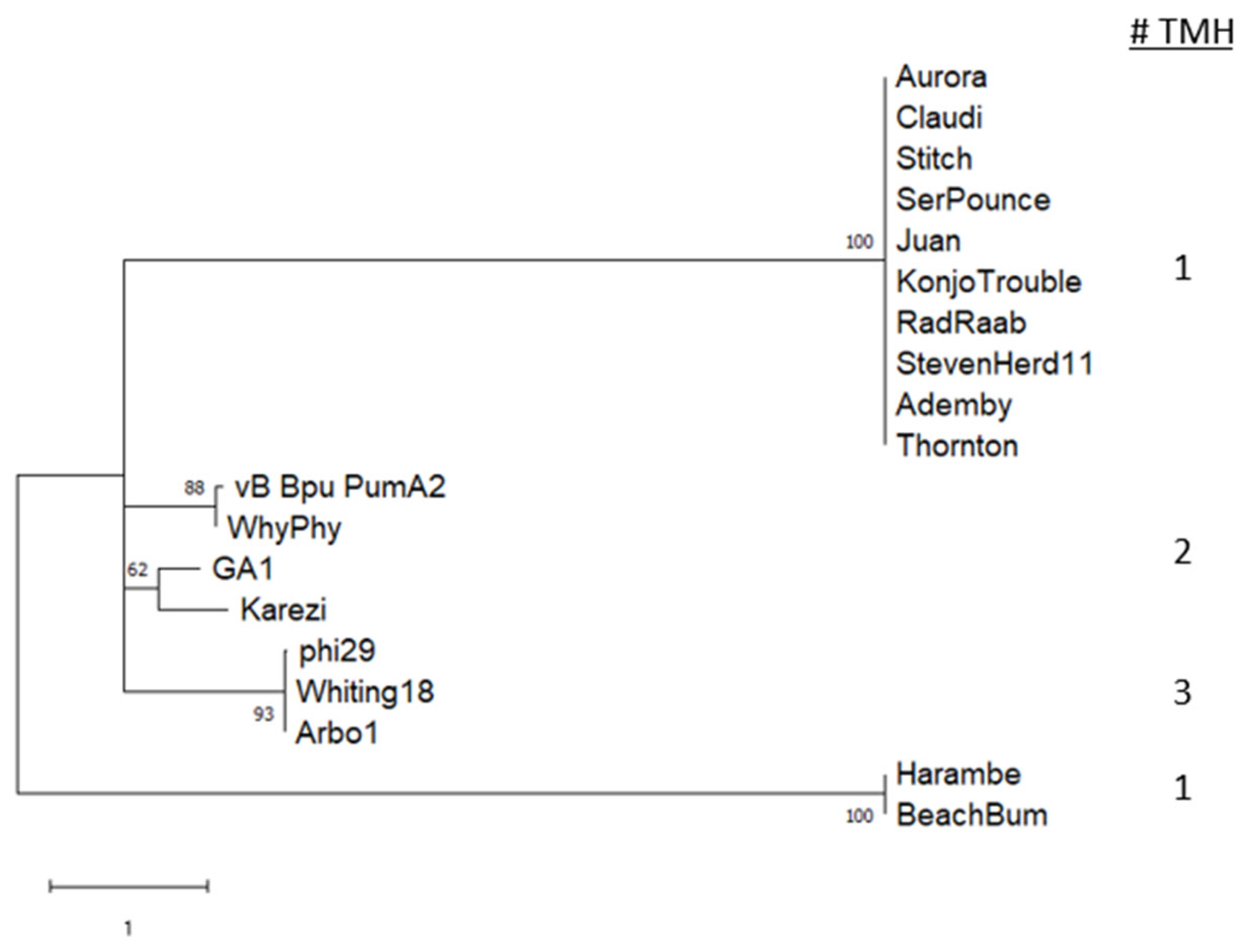
3.7. Endolysin and Holin
3.8. pRNA Prediction
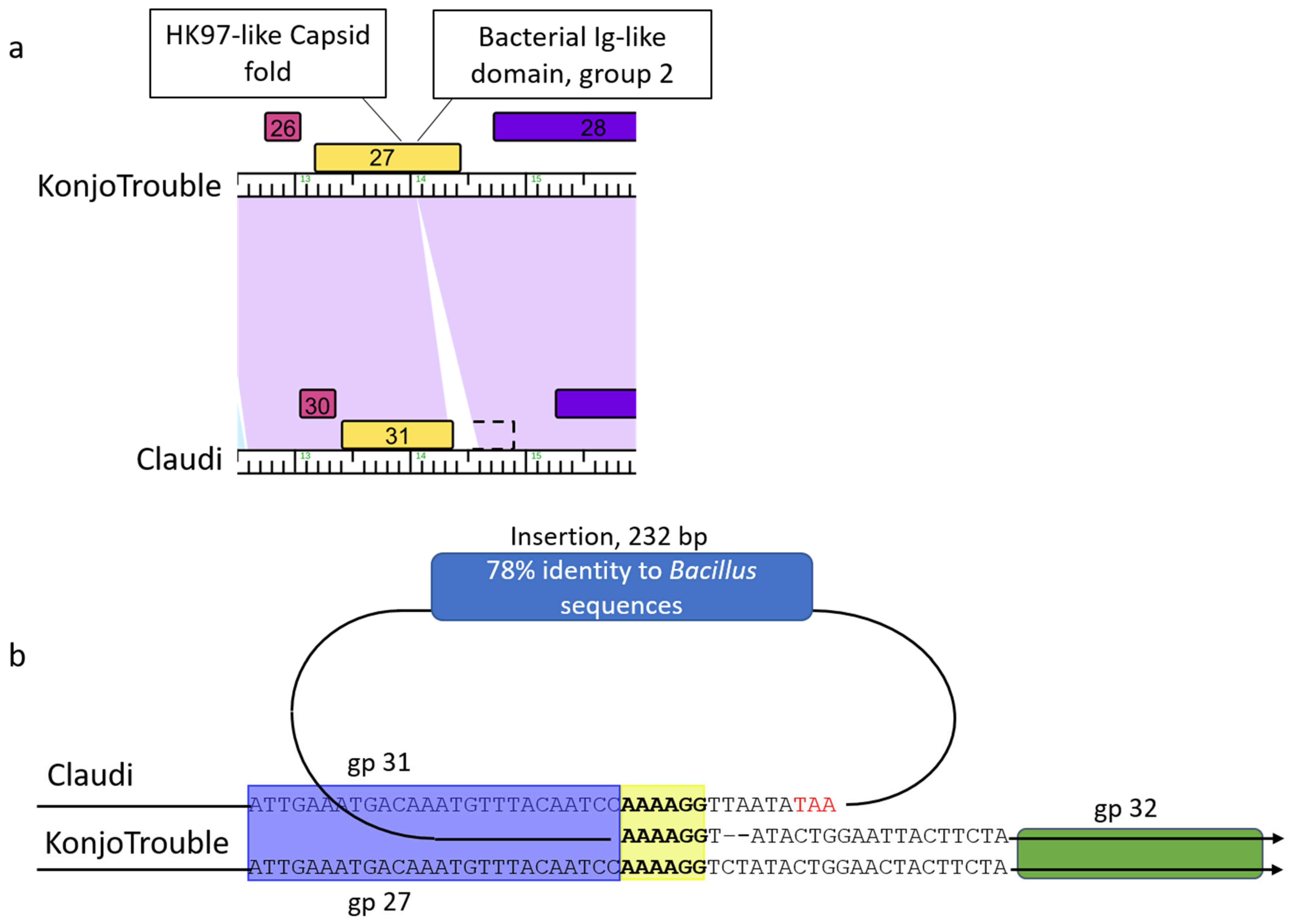
3.9. Sequence-Directed Recombination of Capsid and GNAT Family Acetyltransferase Genes
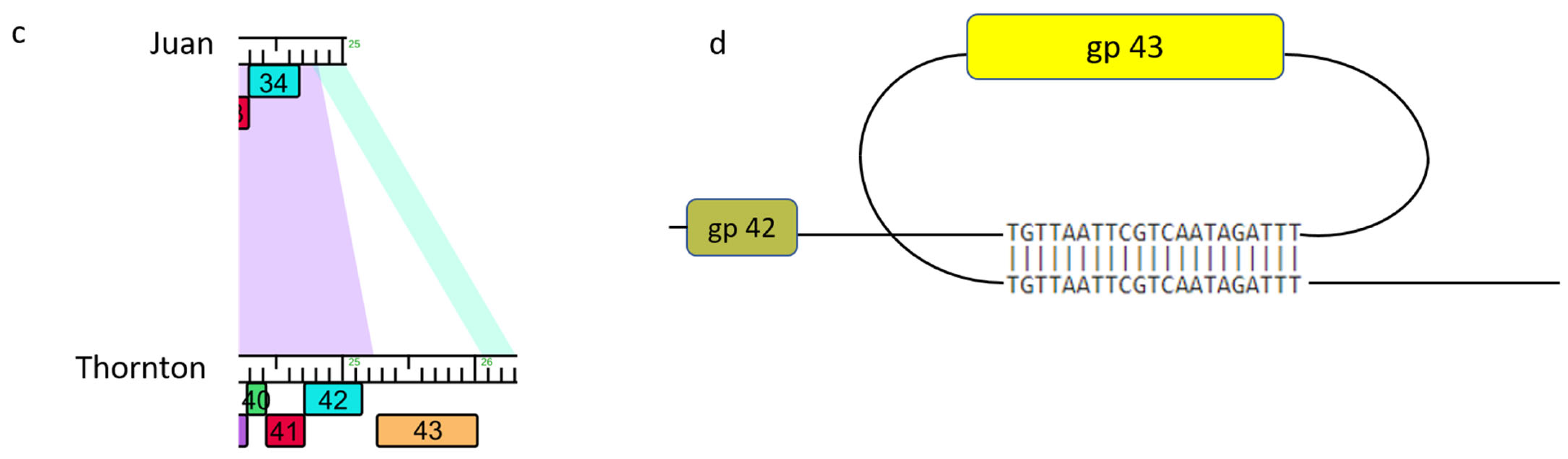
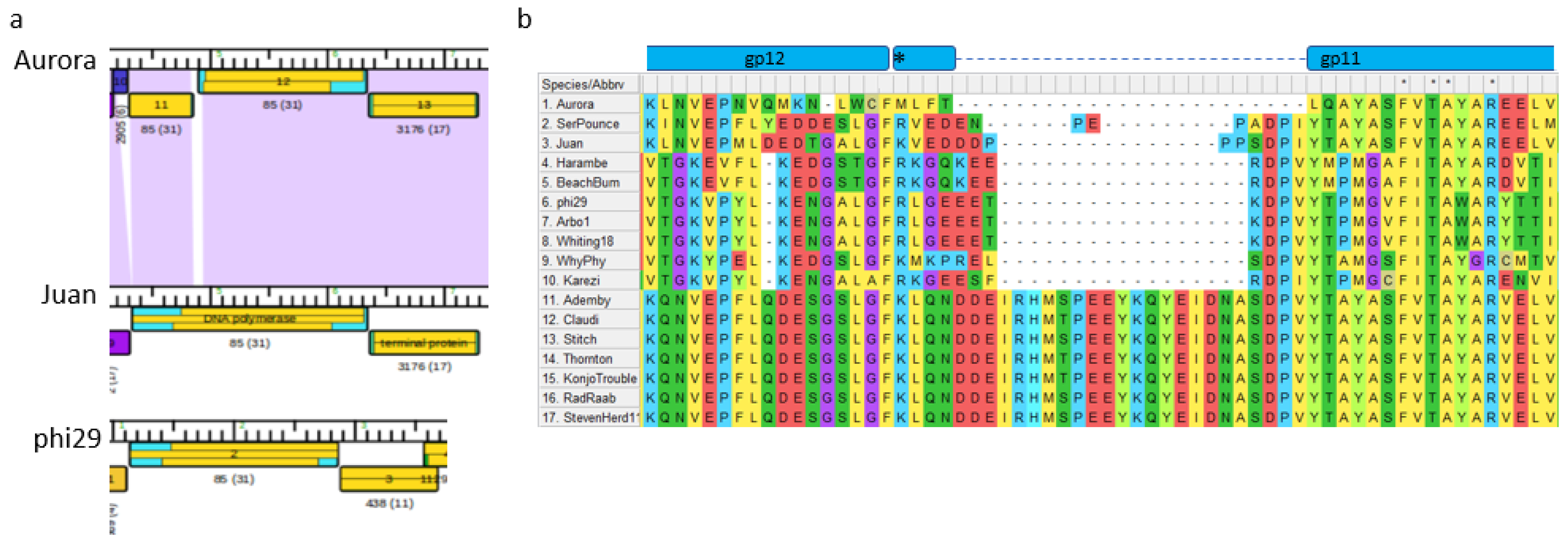
3.10. Non-Sequence-Directed Recombination Leads to Modifications to Essential DNA Polymerase
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in Aquatic Ecosystems. Microbiol. Mol. Biol. Rev. MMBR 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Jordan, T.C.; Burnett, S.H.; Carson, S.; Caruso, S.M.; Clase, K.; DeJong, R.J.; Dennehy, J.J.; Denver, D.R.; Dunbar, D.; Elgin, S.C.R.; et al. A Broadly Implementable Research Course in Phage Discovery and Genomics for First-Year Undergraduate Students. mBio 2014, 5, e01051-13. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F. Actinobacteriophages: Genomics, Dynamics, and Applications. Annu. Rev. Virol. 2020, 7, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.A.; Hatfull, G.F. PhagesDB: The Actinobacteriophage Database. Bioinform. Oxf. Engl. 2017, 33, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage Diversity, Genomics and Phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Jacobs-Sera, D.; Lawrence, J.G.; Pope, W.H.; Russell, D.A.; Ko, C.-C.; Weber, R.J.; Patel, M.C.; Germane, K.L.; Edgar, R.H.; et al. Comparative Genomic Analysis of 60 Mycobacteriophage Genomes: Genome Clustering, Gene Acquisition, and Gene Size. J. Mol. Biol. 2010, 397, 119–143. [Google Scholar] [CrossRef]
- Pope, W.H.; Bowman, C.A.; Russell, D.A.; Jacobs-Sera, D.; Asai, D.J.; Cresawn, S.G.; Jacobs, W.R.; Hendrix, R.W.; Lawrence, J.G.; Hatfull, G.F.; et al. Whole Genome Comparison of a Large Collection of Mycobacteriophages Reveals a Continuum of Phage Genetic Diversity. eLife 2015, 4, e06416. [Google Scholar] [CrossRef]
- Grose, J.H.; Casjens, S.R. Understanding the Enormous Diversity of Bacteriophages: The Tailed Phages That Infect the Bacterial Family Enterobacteriaceae. Virology 2014, 468–470, 421–443. [Google Scholar] [CrossRef]
- Goerke, C.; Pantucek, R.; Holtfreter, S.; Schulte, B.; Zink, M.; Grumann, D.; Bröker, B.M.; Doskar, J.; Wolz, C. Diversity of Prophages in Dominant Staphylococcus aureus Clonal Lineages. J. Bacteriol. 2009, 191, 3462–3468. [Google Scholar] [CrossRef]
- Ha, A.D.; Denver, D.R. Comparative Genomic Analysis of 130 Bacteriophages Infecting Bacteria in the Genus Pseudomonas. Front. Microbiol. 2018, 9, 1456. [Google Scholar] [CrossRef]
- Reilly, B.E.; Spizizen, J. Bacteriophage Deoxyribonucleate Infection of Competent Bacillus subtilis. J. Bacteriol. 1965, 89, 782–790. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses Executive Committee; Gorbalenya, A.E.; Krupovic, M.; Mushegian, A.; Kropinski, A.M.; Siddell, S.G.; Varsani, A.; Adams, M.J.; Davison, A.J.; Dutilh, B.E.; et al. The New Scope of Virus Taxonomy: Partitioning the Virosphere into 15 Hierarchical Ranks. Nat. Microbiol. 2020, 5, 668–674. [Google Scholar] [CrossRef]
- Sarkis, G.J.; Hatfull, G.F. Mycobacteriophages. Methods Mol. Biol. Clifton NJ 1998, 101, 145–173. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Gordon, D.; Abajian, C.; Green, P. Consed: A Graphical Tool for Sequence Finishing. Genome Res. 1998, 8, 195–202. [Google Scholar] [CrossRef]
- Russell, D.A. Sequencing, Assembling, and Finishing Complete Bacteriophage Genomes. Methods Mol. Biol. Clifton NJ 2018, 1681, 109–125. [Google Scholar] [CrossRef]
- Pope, W.H.; Jacobs-Sera, D. Annotation of Bacteriophage Genome Sequences Using DNA Master: An Overview. Methods Mol. Biol. Clifton NJ 2018, 1681, 217–229. [Google Scholar] [CrossRef]
- Rinehart, C.A.; Gaffney, B.; Wood, J.D.; Smith, J. PECAAN, a Phage Evidence Collection and Annotation Network. 2016. Available online: https://discover.kbrinsgd.org/login (accessed on 12 January 2023).
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying Bacterial Genes and Endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef]
- Besemer, J.; Borodovsky, M. GeneMark: Web Software for Gene Finding in Prokaryotes, Eukaryotes and Viruses. Nucleic Acids Res. 2005, 33, W451–W454. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. TRNAscan-SE On-Line: Integrating Search and Context for Analysis of Transfer RNA Genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Laslett, D.; Canback, B. ARAGORN, a Program to Detect TRNA Genes and TmRNA Genes in Nucleotide Sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The Conserved Domain Database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Stephens, A.; Nam, S.-Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at Its Core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Tsirigos, K.D.; Peters, C.; Shu, N.; Käll, L.; Elofsson, A. The TOPCONS Web Server for Consensus Prediction of Membrane Protein Topology and Signal Peptides. Nucleic Acids Res. 2015, 43, W401–W407. [Google Scholar] [CrossRef]
- Hill, A.C.; Bartley, L.E.; Schroeder, S.J. Prohead RNA: A Noncoding Viral RNA of Novel Structure and Function. Wiley Interdiscip. Rev. RNA 2016, 7, 428–437. [Google Scholar] [CrossRef]
- Gu, X.; Schroeder, S.J. Different Sequences Show Similar Quaternary Interaction Stabilities in Prohead Viral RNA Self-Assembly. J. Biol. Chem. 2011, 286, 14419–14426. [Google Scholar] [CrossRef]
- de Jong, A.; Pietersma, H.; Cordes, M.; Kuipers, O.P.; Kok, J. PePPER: A Webserver for Prediction of Prokaryote Promoter Elements and Regulons. BMC Genomics 2012, 13, 299. [Google Scholar] [CrossRef]
- Naville, M.; Ghuillot-Gaudeffroy, A.; Marchais, A.; Gautheret, D. ARNold: A Web Tool for the Prediction of Rho-Independent Transcription Terminators. RNA Biol. 2011, 8, 11–13. [Google Scholar] [CrossRef]
- Mathews, D.H.; Disney, M.D.; Childs, J.L.; Schroeder, S.J.; Zuker, M.; Turner, D.H. Incorporating Chemical Modification Constraints into a Dynamic Programming Algorithm for Prediction of RNA Secondary Structure. Proc. Natl. Acad. Sci. 2004, 101, 7287–7292. [Google Scholar] [CrossRef]
- Krumsiek, J.; Arnold, R.; Rattei, T. Gepard: A Rapid and Sensitive Tool for Creating Dotplots on Genome Scale. Bioinformatics 2007, 23, 1026–1028. [Google Scholar] [CrossRef]
- Cresawn, S.G.; Bogel, M.; Day, N.; Jacobs-Sera, D.; Hendrix, R.W.; Hatfull, G.F. Phamerator: A Bioinformatic Tool for Comparative Bacteriophage Genomics. BMC Bioinform. 2011, 12, 395. [Google Scholar] [CrossRef]
- Huson, D.H. SplitsTree: Analyzing and Visualizing Evolutionary Data. Bioinform. Oxf. Engl. 1998, 14, 68–73. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega. Curr. Protoc. Bioinform. 2014, 48, 3–13. [Google Scholar] [CrossRef]
- Chung, C.-H.; Walter, M.H.; Yang, L.; Chen, S.-C.; Winston, V.; Thomas, M.A. Predicting Genome Terminus Sequences of Bacillus cereus-Group Bacteriophage Using next Generation Sequencing Data. BMC Genomics 2017, 18, 350. [Google Scholar] [CrossRef]
- Duperier, J.; Bulpitt, M.; Bispo, F.; Greguske, E. Genome Annotations of Two Bacillus Phages, Tomato and BaseballField. Microbiol. Resour. Announc. 2021, 10, e01196-20. [Google Scholar] [CrossRef]
- Erill, I.; Caruso, S.M.; 2016 UMBC Phage Hunters. Complete Genome Sequences of Three Phi29-Like Bacillus cereus Group Podoviridae. Genome Announc. 2017, 5, e00701-17. [Google Scholar] [CrossRef]
- Redondo, R.A.F.; Kupczok, A.; Stift, G.; Bollback, J.P. Complete Genome Sequence of the Novel Phage MG-B1 Infecting Bacillus weihenstephanensis. Genome Announc. 2013, 1, e00216-13. [Google Scholar] [CrossRef]
- Bradley, D.E. The Isolation and Morphology of Some New Bacteriophages Specific for Bacillus and Acetobacter Species. J. Gen. Microbiol. 1965, 41, 233–241. [Google Scholar] [CrossRef]
- Stanton, C.R.; Rice, D.T.F.; Beer, M.; Batinovic, S.; Petrovski, S. Isolation and Characterisation of the Bundooravirus Genus and Phylogenetic Investigation of the Salasmaviridae Bacteriophages. Viruses 2021, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Ronda, C.; López, R.; García, E. Isolation and Characterization of a New Bacteriophage, Cp-1, Infecting Streptococcus Pneumoniae. J. Virol. 1981, 40, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, X.; Li, N.; Wang, J.; Yu, S.; Zeng, H.; Zhang, J.; Wu, Q.; Ding, Y. Isolation and Characterization of Bacillus Cereus Phage VB_BceP-DLc1 Reveals the Largest Member of the Φ29-Like Phages. Microorganisms 2020, 8, 1750. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Cheng, B.; Zhang, S.; Wang, N.; Fang, Y.; Zhang, Q.; Kuang, A.; Lin, L.; Ji, X.; Wei, Y. Complete Genome Sequence of the Cold-Active Bacteriophage VMY22 from Bacillus Cereus. Virus Genes 2016, 52, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Pecenková, T.; Benes, V.; Paces, J.; Vlcek, C.; Paces, V. Bacteriophage B103: Complete DNA Sequence of Its Genome and Relationship to Other Bacillus Phages. Gene 1997, 199, 157–163. [Google Scholar] [CrossRef]
- Kong, L.; Ding, Y.; Wu, Q.; Wang, J.; Zhang, J.; Li, H.; Yu, S.; Yu, P.; Gao, T.; Zeng, H.; et al. Genome Sequencing and Characterization of Three Bacillus Cereus-Specific Phages, DK1, DK2, and DK3. Arch. Virol. 2019, 164, 1927–1929. [Google Scholar] [CrossRef]
- ICTV taxonomy release, 2021 Taxon Details for Cepunavirus Cp1. Available online: https://ictv.global/taxonomy/taxondetails?taxnode_id=202100592 (accessed on 5 January 2023).
- Blanco, L.; Salas, M. Relating Structure to Function in 29 DNA Polymerase (∗). J. Biol. Chem. 1996, 271, 8509–8512. [Google Scholar] [CrossRef]
- Morais, M.C.; Choi, K.H.; Koti, J.S.; Chipman, P.R.; Anderson, D.L.; Rossmann, M.G. Conservation of the Capsid Structure in Tailed DsDNA Bacteriophages: The Pseudoatomic Structure of Φ29. Mol. Cell 2005, 18, 149–159. [Google Scholar] [CrossRef]
- Xiao, F.; Moll, W.-D.; Guo, S.; Guo, P. Binding of PRNA to the N-Terminal 14 Amino Acids of Connector Protein of Bacteriophage Phi29. Nucleic Acids Res. 2005, 33, 2640–2649. [Google Scholar] [CrossRef]
- Cohen, D.N.; Erickson, S.E.; Xiang, Y.; Rossmann, M.G.; Anderson, D.L. Multifunctional Roles of a Bacteriophage Phi 29 Morphogenetic Factor in Assembly and Infection. J. Mol. Biol. 2008, 378, 804–817. [Google Scholar] [CrossRef]
- Hermoso, J.M.; Salas, M. Protein P3 Is Linked to the DNA of Phage Phi 29 through a Phosphoester Bond between Serine and 5’-DAMP. Proc. Natl. Acad. Sci. USA 1980, 77, 6425–6428. [Google Scholar] [CrossRef]
- Loessner, M.J.; Maier, S.K.; Daubek-Puza, H.; Wendlinger, G.; Scherer, S. Three Bacillus Cereus Bacteriophage Endolysins Are Unrelated but Reveal High Homology to Cell Wall Hydrolases from Different Bacilli. J. Bacteriol. 1997, 179, 2845–2851. [Google Scholar] [CrossRef]
- Etobayeva, I.; Linden, S.B.; Alem, F.; Harb, L.; Rizkalla, L.; Mosier, P.D.; Johnson, A.A.; Temple, L.; Hakami, R.M.; Nelson, D.C. Discovery and Biochemical Characterization of PlyP56, PlyN74, and PlyTB40—Bacillus Specific Endolysins. Viruses 2018, 10, 276. [Google Scholar] [CrossRef]
- Steiner, M.; Lubitz, W.; Bläsi, U. The Missing Link in Phage Lysis of Gram-Positive Bacteria: Gene 14 of Bacillus subtilis Phage Phi 29 Encodes the Functional Homolog of Lambda S Protein. J. Bacteriol. 1993, 175, 1038–1042. [Google Scholar] [CrossRef]
- Tedin, K.; Resch, A.; Steiner, M.; Bläsi, U. Dual Translational Start Motif Evolutionarily Conserved in the Holin Gene of Bacillus subtilis Phage Phi 29. Virology 1995, 206, 479–484. [Google Scholar] [CrossRef]
- Reddy, B.L.; Saier, M.H. Topological and Phylogenetic Analyses of Bacterial Holin Families and Superfamilies. Biochim. Biophys. Acta 2013, 1828, 2654–2671. [Google Scholar] [CrossRef]
- Bläsi, U.; Chang, C.Y.; Zagotta, M.T.; Nam, K.B.; Young, R. The Lethal Lambda S Gene Encodes Its Own Inhibitor. EMBO J. 1990, 9, 981–989. [Google Scholar] [CrossRef]
- Guo, P.X.; Erickson, S.; Anderson, D. A Small Viral RNA Is Required for in Vitro Packaging of Bacteriophage Phi 29 DNA. Science 1987, 236, 690–694. [Google Scholar] [CrossRef]
- Bailey, S.; Wichitwechkarn, J.; Johnson, D.; Reilly, B.E.; Anderson, D.L.; Bodley, J.W. Phylogenetic Analysis and Secondary Structure of the Bacillus subtilis Bacteriophage RNA Required for DNA Packaging. J. Biol. Chem. 1990, 265, 22365–22370. [Google Scholar] [CrossRef]
- Schilling, T.; Hoppert, M.; Hertel, R. Genomic Analysis of the Recent Viral Isolate VB_BthP-Goe4 Reveals Increased Diversity of Φ29-Like Phages. Viruses 2018, 10, 624. [Google Scholar] [CrossRef]
- Reilly, B.E.; Nelson, R.A.; Anderson, D.L. Morphogenesis of Bacteriophage Phi 29 of Bacillus subtilis: Mapping and Functional Analysis of the Head Fiber Gene. J. Virol. 1977, 24, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Karambelkar, S.; Udupa, S.; Gowthami, V.N.; Ramachandra, S.G.; Swapna, G.; Nagaraja, V. Emergence of a Novel Immune-Evasion Strategy from an Ancestral Protein Fold in Bacteriophage Mu. Nucleic Acids Res. 2020, 48, 5294–5305. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Lázaro, J.M.; Blanco, L.; Kamtekar, S.; Berman, A.J.; Wang, J.; Steitz, T.A.; Salas, M.; de Vega, M. A Specific Subdomain in Φ29 DNA Polymerase Confers Both Processivity and Strand-Displacement Capacity. Proc. Natl. Acad. Sci. 2005, 102, 6407–6412. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A.; Blanco, L.; Parés, E.; Salas, M.; Bernad, A. Structural and Functional Analysis of Temperature-Sensitive Mutants of the Phage Phi 29 DNA Polymerase. Nucleic Acids Res. 1990, 18, 4763–4770. [Google Scholar]
- Ackermann, H.W. Tailed Bacteriophages: The Order Caudovirales. Adv. Virus Res. 1998, 51, 135–201. [Google Scholar] [CrossRef]
- Meijer, W.J.; Horcajadas, J.A.; Salas, M. Phi29 Family of Phages. Microbiol. Mol. Biol. Rev. MMBR 2001, 65, 261–287. [Google Scholar] [CrossRef]
- Petrov, V.M.; Ratnayaka, S.; Nolan, J.M.; Miller, E.S.; Karam, J.D. Genomes of the T4-Related Bacteriophages as Windows on Microbial Genome Evolution. Virol. J. 2010, 7, 292. [Google Scholar] [CrossRef]
- Grose, J.H.; Jensen, G.L.; Burnett, S.H.; Breakwell, D.P. Genomic Comparison of 93 Bacillus Phages Reveals 12 Clusters, 14 Singletons and Remarkable Diversity. BMC Genomics 2014, 15, 855. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage Endolysins as Novel Antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef]
- Loessner, M.J.; Kramer, K.; Ebel, F.; Scherer, S. C-Terminal Domains of Listeria Monocytogenes Bacteriophage Murein Hydrolases Determine Specific Recognition and High-Affinity Binding to Bacterial Cell Wall Carbohydrates. Mol. Microbiol. 2002, 44, 335–349. [Google Scholar] [CrossRef]
- Schuch, R.; Pelzek, A.J.; Nelson, D.C.; Fischetti, V.A. The PlyB Endolysin of Bacteriophage VB_BanS_Bcp1 Exhibits Broad-Spectrum Bactericidal Activity against Bacillus cereus Sensu Lato Isolates. Appl. Environ. Microbiol. 2019, 85, e00003-19. [Google Scholar] [CrossRef]



| Phage Name | Genome Length (bp) | GC% | Number of Genes | Host | GenBank ID | Inverted Repeat Ends |
|---|---|---|---|---|---|---|
| Genus: Claudivirus | ||||||
| Ademby | 24,162 | 30.7 | 40 | Btk * | OL744112.1 | ND |
| RadRaab | 23,946 | 30.6 | 34 | Btk | MF156580.1 | 8 bp ITR, 5′-AAATGTAA |
| StevenHerd11 | 23,953 | 30.6 | 37 | Btk | MK084630.1 | 11 bp ITR, 5′-AAATGTAAGGG |
| Stitch | 24,320 | 30.4 | 36 | Btk | KX349901.1 | 7 bp ITR, 5′-AAATGTA |
| KonjoTrouble | 26,061 | 30.1 | 40 | Btk | MF156578.1 | 9 bp ITR, 5′-AAATGTAAA |
| Claudi | 26,502 | 30.3 | 46 | Btk | KX349900.2 | 8 bp ITR, 5′-AAATGTAA |
| Thornton | 26,319 | 30.5 | 43 | Btk | MW348917 | 8 bp ITR, 5′-AAATGTAA |
| SerPounce | 27,206 | 30.4 | 44 | Btk | KY947509 | 8 bp ITR, 5′-AAATGTAA |
| Aurora | 25,905 | 30.6 | 40 | Btk | KX349899.2 | 5′ end is 5′-AAATGTAA, 3′ end ND |
| Juan | 25,032 | 30.6 | 34 | Btk | MF156577.1 | 9 bp ITR, 5′-AAATGTAAA |
| Genus: Harambevirus | ||||||
| BeachBum | 21,054 | 35.4 | 30 | Btk | KY921761.1 | 16 bp ITR, 5′-AAGATAGCCCCCCACC |
| Harambe | 21,684 | 35.3 | 33 | Btk | KY821088 | 16 bp ITR, 5′-AAGATAGCCCCCCACC |
| Genus: Karezivirus | ||||||
| Karezi | 20,083 | 37.3 | 34 | B. pum.* SAFR 32 | MN082625 | 7 bp ITR, 5′- AAATTAG |
| Genus: Bundooravirus | ||||||
| WhyPhy | 18,642 | 34.9 | 28 | B. pum SAFR 32 | MW419775 | 12 bp ITR, 5′-AATGTAAAGGTA |
| Genus: Salasvirus | ||||||
| Whiting18 | 19,548 | 39.6 | 25 | Bacillus sp. 203 | MW477480 | ND |
| Arbo1 | 19,362 | 39.6 | 25 | Bacillus sp. 203 | OL744111.1 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.; Saini, T.; Al Qaffas, A.; Erill, I.; Caruso, S.M.; Temple, L.; Johnson, A.A. Evidence of a Set of Core-Function Genes in 16 Bacillus Podoviral Genomes with Considerable Genomic Diversity. Viruses 2023, 15, 276. https://doi.org/10.3390/v15020276
Ismail A, Saini T, Al Qaffas A, Erill I, Caruso SM, Temple L, Johnson AA. Evidence of a Set of Core-Function Genes in 16 Bacillus Podoviral Genomes with Considerable Genomic Diversity. Viruses. 2023; 15(2):276. https://doi.org/10.3390/v15020276
Chicago/Turabian StyleIsmail, Ahmed, Tanuj Saini, Ahmed Al Qaffas, Ivan Erill, Steven M. Caruso, Louise Temple, and Allison A. Johnson. 2023. "Evidence of a Set of Core-Function Genes in 16 Bacillus Podoviral Genomes with Considerable Genomic Diversity" Viruses 15, no. 2: 276. https://doi.org/10.3390/v15020276
APA StyleIsmail, A., Saini, T., Al Qaffas, A., Erill, I., Caruso, S. M., Temple, L., & Johnson, A. A. (2023). Evidence of a Set of Core-Function Genes in 16 Bacillus Podoviral Genomes with Considerable Genomic Diversity. Viruses, 15(2), 276. https://doi.org/10.3390/v15020276






