Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients
Abstract
1. Introduction
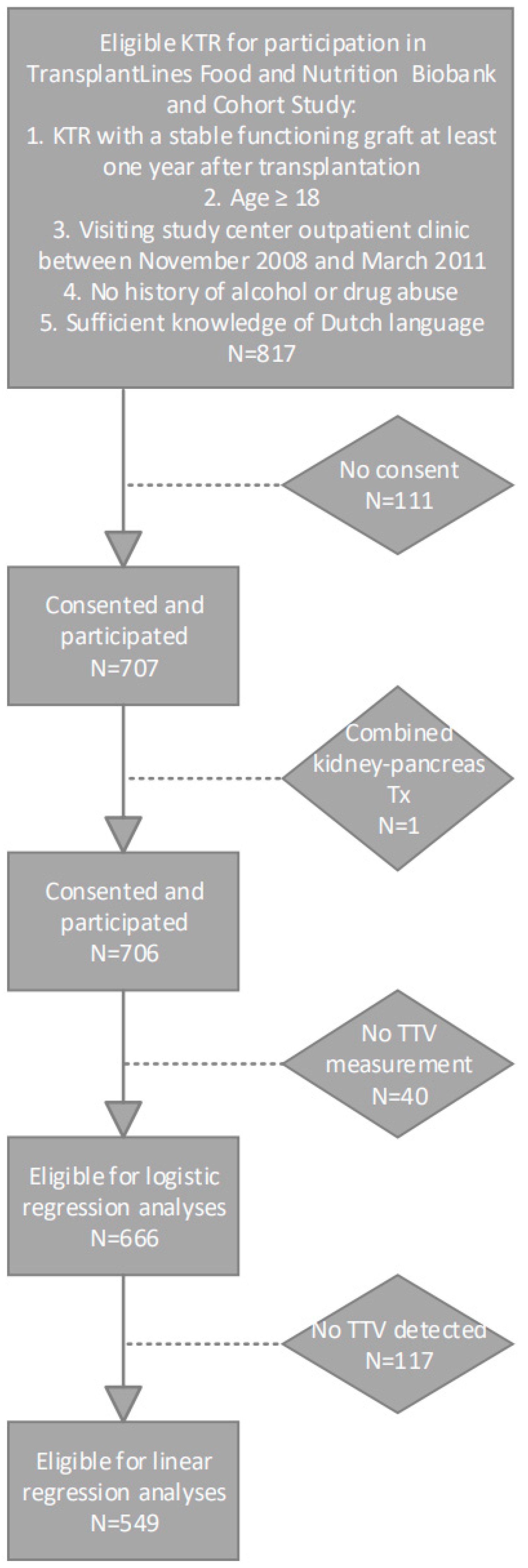
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Covariates
2.3. TTV Load Measurements
2.4. Assessment of Smoking Status
2.5. Assessment of Alcohol Intake
2.6. Data Analysis
2.7. Population Characteristics
2.8. Logistic Regression Analyses
2.9. Linear Regression Analyses
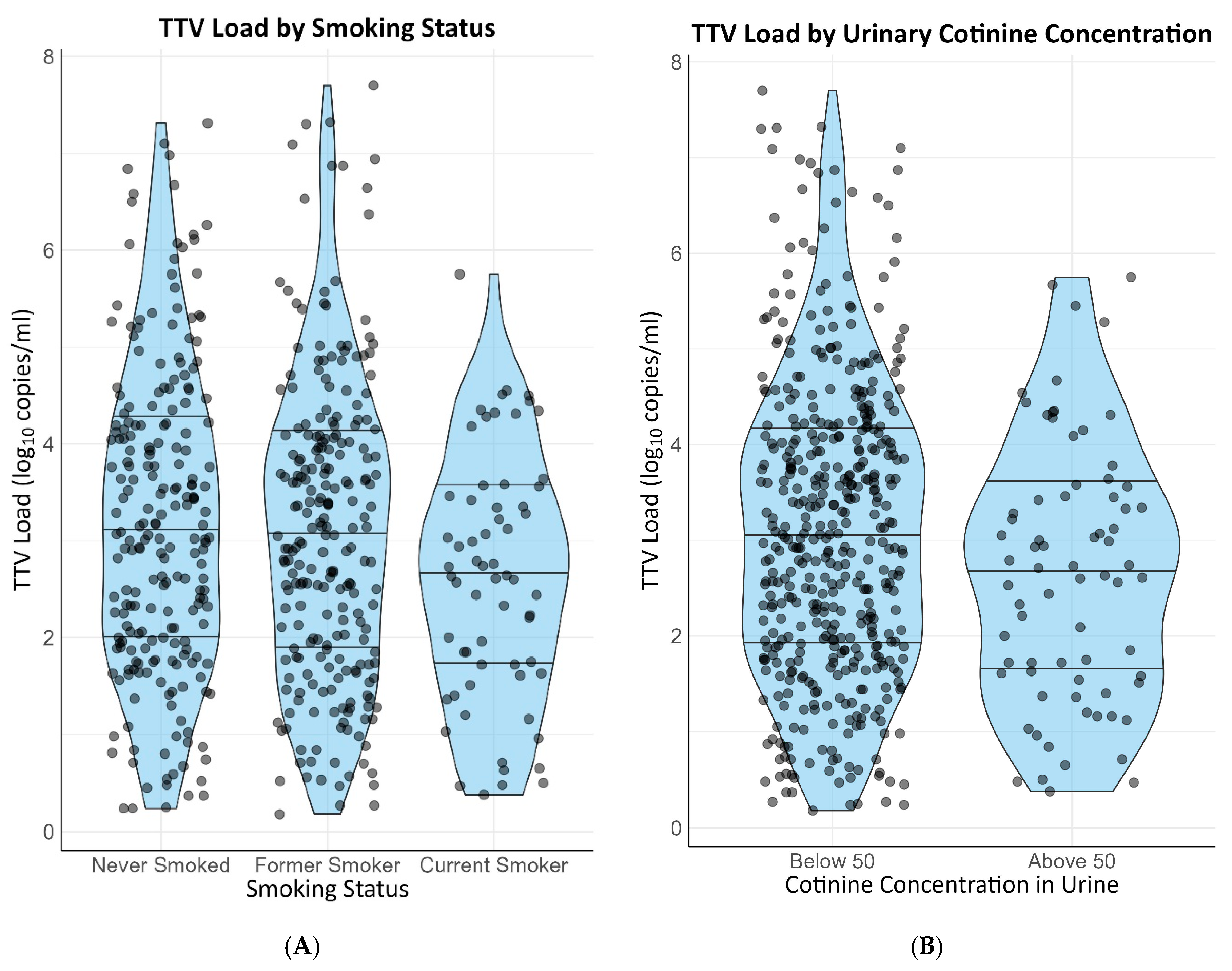
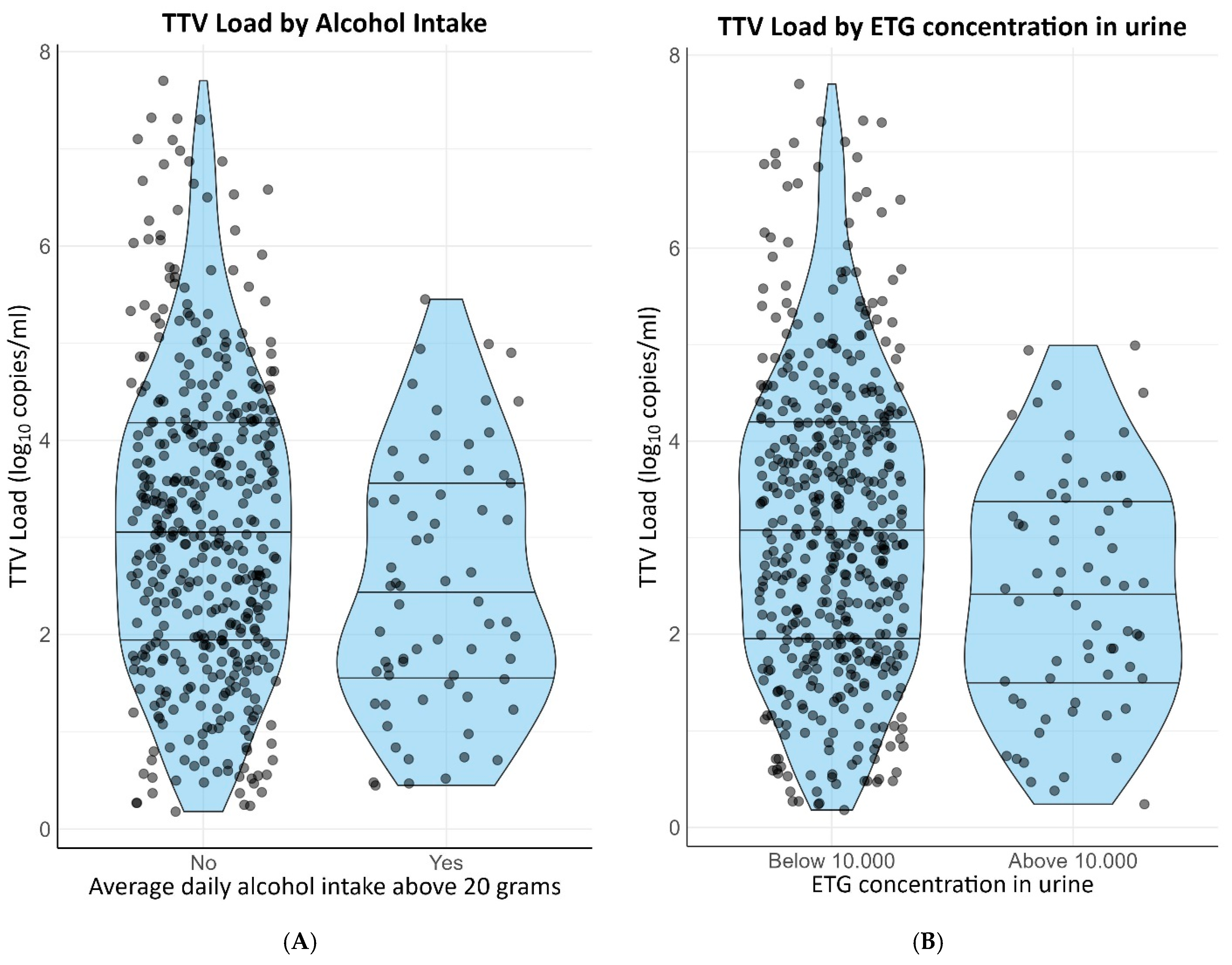
3. Results
3.1. Population Characteristics
3.2. Logistic Regression Analyses
3.3. Linear Regression Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Andrews, L.M.; Li, Y.; De Winter, B.C.M.; Shi, Y.Y.; Baan, C.C.; Van Gelder, T.; Hesselink, D.A. Pharmacokinetic Considerations Related to Therapeutic Drug Monitoring of Tacrolimus in Kidney Transplant Patients. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Anglicheau, D. Precision Transplant Medicine: Biomarkers to the Rescue. J. Am. Soc. Nephrol. 2018, 29, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, E.V.; Trofimov, D.Y.; Tonevitsky, A.G.; Ilinsky, V.V.; Korostin, D.O.; Rebrikov, D.V. Torque Teno Virus (TTV) Distribution in Healthy Russian Population. Virol. J. 2009, 6, 134. [Google Scholar] [CrossRef]
- De Vlaminck, I.; Kiran, K.K.; Strehl, C.; Kohli, B.; Neff, N.F.; Okamoto, J.; Snyder, T.M.; Weill, D.; Bernstein, D.; Valantine, H.A.; et al. Temporal Response of the Human Virome to Immunosuppression and Antiviral Therapy. Cell 2013, 155, 1178–1187. [Google Scholar] [CrossRef]
- Focosi, D.; Antonelli, G.; Pistello, M.; Maggi, F. Torquetenovirus: The Human Virome from Bench to Bedside. Clin. Microbiol. Infect. 2016, 22, 589–593. [Google Scholar] [CrossRef]
- Jaksch, P.; Görzer, I.; Puchhammer-Stöckl, E.; Bond, G. Integrated Immunologic Monitoring in Solid Organ Transplantation: The Road Towards Torque Teno Virus-Guided Immunosuppression. Transplantation 2022, 106, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.; Velay, A.; Gantner, P.; Bausson, J.; Filipputtu, A.; Freitag, R.; Moulin, B.; Caillard, S.; Fafi-Kremer, S. Torquetenovirus Viremia for Early Prediction of Graft Rejection after Kidney Transplantation. J. Infect. 2019, 79, 56–60. [Google Scholar] [CrossRef]
- Strassl, R.; Schiemann, M.; Doberer, K.; Görzer, I.; Puchhammer-Stöckl, E.; Eskandary, F.; Kikić, Ž.; Gualdoni, G.A.; Vossen, M.G.; Rasoul-Rockenschaub, S.; et al. Quantification of Torque Teno Virus Viremia as a Prospective Biomarker for Infectious Disease in Kidney Allograft Recipients. J. Infect. Dis. 2018, 218, 1191–1199. [Google Scholar] [CrossRef]
- Strassl, R.; Doberer, K.; Rasoul-Rockenschaub, S.; Herkner, H.; Görzer, I.; Kläger, J.P.; Schmidt, R.; Haslacher, H.; Schiemann, M.; Eskandary, F.A.; et al. Torque Teno Virus for Risk Stratification of Acute Biopsyproven Alloreactivity in Kidney Transplant Recipients. J. Infect. Dis. 2019, 219, 1934–1939. [Google Scholar] [CrossRef]
- Doberer, K.; Schiemann, M.; Strassl, R.; Haupenthal, F.; Dermuth, F.; Görzer, I.; Eskandary, F.; Reindl-Schwaighofer, R.; Kikić, Ž.; Puchhammer-Stöckl, E.; et al. Torque Teno Virus for Risk Stratification of Graft Rejection and Infection in Kidney Transplant Recipients—A Prospective Observational Trial. Am. J. Transplant. 2020, 20, 2081–2090. [Google Scholar] [CrossRef]
- Doberer, K.; Haupenthal, F.; Nackenhorst, M.; Bauernfeind, F.; Dermuth, F.; Eigenschink, M.; Schiemann, M.; Kläger, J.; Görzer, I.; Eskandary, F.; et al. Torque Teno Virus Load Is Associated with Subclinical Alloreactivity in Kidney Transplant Recipients: A Prospective Observational Trial. Transplantation 2021, 105, 2112–2118. [Google Scholar] [CrossRef]
- van Rijn, A.L.; Roos, R.; Dekker, F.W.; Rotmans, J.I.; Feltkamp, M.C.W. Torque Teno Virus Load as Marker of Rejection and Infection in Solid Organ Transplantation—A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2023, 33, e2393. [Google Scholar] [CrossRef]
- Gore, E.J.; Gomes-neto, A.W.; Wang, L.; Bakker, S.J.L.; Niesters, H.G.M.; de Joode, A.A.E.; Verschuuren, E.A.M.; Westra, J.; Van Leer-Buter, C. Torquetenovirus Serum Load and Long-Term Outcomes in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 440. [Google Scholar] [CrossRef]
- Barr, T.; Helms, C.; Grant, K.; Messaoudi, I. Opposing Effects of Alcohol on the Immune System. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 4, 242–251. [Google Scholar] [CrossRef]
- Romeo, J.; Würnberg, J.; Nova, E.; Díaz, L.E.; Gómez-Martinez, S.; Marcos, A. Moderate Alcohol Consumption and the Immune System: A Review. Br. J. Nutr. 2007, 98, S111–S115. [Google Scholar] [CrossRef]
- Díaz, L.E.; Montero, A.; González-Gross, M.; Vallejo, A.I.; Romeo, J.; Marcos, A. Influence of Alcohol Consumption on Immunological Status: A Review. Eur. J. Clin. Nutr. 2002, 56, S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of Cigarette Smoking on Immune Responsiveness: Up and down or Upside Down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Gomes-Neto, A.W.; Van Londen, M.; Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and Design of TransplantLines: A Prospective Cohort Study and Biobank of Solid Organ Transplant Recipients. BMJ Open 2018, 8, e24502. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2016, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.M.; Kromhout, D. Reproducibility and Relative Validity of the Short Questionnaire to Assess Health-Enhancing Physical Activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Campbell, N.; Gaston, A.; Gray, C.; Rush, E.; Maddison, R.; Prapavessis, H. The Short Questionnaire to Assess Health-Enhancing (SQUASH) Physical Activity in Adolescents: A Validation Using Doubly Labeled Water. J. Phys. Act. Health 2016, 13, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Kulifaj, D.; Durgueil-Lariviere, B.; Meynier, F.; Munteanu, E.; Pichon, N.; Dubé, M.; Joannes, M.; Essig, M.; Hantz, S.; Barranger, C.; et al. Development of a Standardized Real Time PCR for Torque Teno Viruses (TTV) Viral Load Detection and Quantification: A New Tool for Immune Monitoring. J. Clin. Virol. 2018, 105, 118–127. [Google Scholar] [CrossRef]
- Macera, L.; Spezia, P.G.; Medici, C.; Rofi, E.; Del Re, M.; Focosi, D.; Mazzetti, P.; Navarro, D.; Antonelli, G.; Danesi, R.; et al. Comparative Evaluation of Molecular Methods for the Quantitative Measure of Torquetenovirus Viremia, the New Surrogate Marker of Immune Competence. J. Med. Virol. 2022, 94, 491–498. [Google Scholar] [CrossRef] [PubMed]
- ISO15189:2022; Medical Laboratories—Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2022.
- Feunekes, G.I.J.; Van Staveren, W.A.; De Vries, J.H.M.; Burema, J.; Hautvast, J.G.A.J. Relative and Biomarker-Based Validity of a Food-Frequency Questionnaire Estimating Intake of Fats and Cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Rijksinstituut voor Volksgezondheid en Milieu. Dutch Food Composition Database, Nederlands Voedingsstoffenbestand (NEVO) Version: 2006; Rijksinstituut voor Volksgezondheid en Milieu: Bilthoven, The Netherlands, 2006. [Google Scholar]
- Böttcher, M.; Beck, O.; Helander, A. Evaluation of a New Immunoassay for Urinary Ethyl Glucuronide Testing. Alcohol Alcohol. 2008, 43, 46–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 1 December 2023).
- Spandole-dinu, S.; Cr, A.; Radu, I.; Nica, S.; Toma, M.; Alexiu, O.A.; Iorga, C.S.; Berca, L.; Nica, R. Prevalence of Human Anelloviruses in Romanian Healthy Subjects and Patients with Common Pathologies. BMC Infect. Dis. 2018, 18, 334. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Diamond, J.M.; Chehoud, C.; Chang, B.; Kotzin, J.J.; Young, J.C.; Imai, I.; Haas, A.R.; Cantu, E.; Lederer, D.J.; et al. The Perioperative Lung Transplant Virome: Torque Teno Viruses Are Elevated in Donor Lungs and Show Divergent Dynamics in Primary Graft Dysfunction. Am. J. Transplant. 2017, 17, 1313–1324. [Google Scholar] [CrossRef]
- Focosi, D.; Macera, L.; Boggi, U.; Nelli, L.C.; Maggi, F. Short-Term Kinetics of Torque Teno Virus Viraemia after Induction Immunosuppression Confirm T Lymphocytes as the Main Replication-Competent Cells. J. Gen. Virol. 2015, 96, 115–117. [Google Scholar] [CrossRef]
- Rezahosseini, O.; Drabe, C.H.; Sørensen, S.S.; Rasmussen, A.; Perch, M.; Ostrowski, S.R.; Nielsen, S.D. Torque-Teno Virus Viral Load as a Potential Endogenous Marker of Immune Function in Solid Organ Transplantation. Transplant. Rev. 2019, 33, 137–144. [Google Scholar] [CrossRef]
- Spandole, S.; Cimponeriu, D.; Berca, L.M.; Mihăescu, G. Human Anelloviruses: An Update of Molecular, Epidemiological and Clinical Aspects. Arch. Virol. 2015, 160, 893–908. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Ruiz-Merlo, T.; Parra, P.; López-Medrano, F.; San Juan, R.; Polanco, N.; Andrés, A.; Navarro, D.; et al. Monitoring of Alphatorquevirus DNA Levels for the Prediction of Immunosuppression-Related Complications after Kidney Transplantation. Am. J. Transplant. 2019, 19, 1139–1149. [Google Scholar] [CrossRef]
- Gardiner, B.J.; Lee, S.J.; Cristiano, Y.; Levvey, B.J.; Sullivan, L.C.; Snell, G.I.; Peleg, A.Y.; Westall, G.P. Evaluation of Quantiferon ®-Monitor as a Biomarker of Immunosuppression and Predictor of Infection in Lung Transplant Recipients. Transpl. Infect. Dis. 2021, 23, e13550. [Google Scholar] [CrossRef] [PubMed]
- Charan, N.; Lavanya, N.; Praveen, B.; Praveen, A.; Sridevi, A.; Narasimha, G. Antiviral Activity of Antimony and Arsenic Oxides. Biochem. Biophys. Res. Commun. 2012, 4, 687–689. [Google Scholar]
- Zhang, A.; Zhao, L.; Li, N.; Duan, H.; Liu, H. Crossm Reproductive and Respiratory Syndrome Virus Replication by the Cyclic GMP/Protein Kinase G and NF-κB. J. Virol. 2017, 91, e01866-16. [Google Scholar] [CrossRef] [PubMed]
- Ellaby, S.; David, W.A.L. Formaldehyde as an Antiviral Agent Against Virus of Pieris Bra & Cue a Granulosis. J. Invertebr. Pathol. 1969, 14, 96–101. [Google Scholar]
- Frumence, E.; Roche, M.; Guiraud, P. Cadmium Reduces the Ef Fi Ciency of Sindbis Virus Replication in Human Cells and Promotes Their Survival by Inhibiting Apoptosis. Biochem. Biophys. Rep. 2016, 8, 151–156. [Google Scholar] [CrossRef]
- Pollack, T.M.; Duong, H.T.; Pham, T.T.; Do, C.D.; Colby, D. Cigarette Smoking Is Associated with High HIV Viral Load among Adults Presenting for Antiretroviral Therapy in Vietnam. PLoS ONE 2017, 12, e0173534. [Google Scholar] [CrossRef]
- Hashida, T.; Masuda, S.; Uemoto, S.; Saito, H.; Tanaka, K.; Inui, K. ichi Pharmacokinetic and Prognostic Significance of Intestinal MDR1 Expression in Recipients of Living-Donor Liver Transplantation. Clin. Pharmacol. Ther. 2001, 69, 308–316. [Google Scholar] [CrossRef]
- Saeki, T.; Ueda, K.; Tanigawara, Y.; Hori, R.; Komano, T. Human P-Glycoprotein Transports Cyclosporin A and FK506. J. Biol. Chem. 1993, 268, 6077–6080. [Google Scholar] [CrossRef]
- He, X.M.; Zhou, Y.; Xu, M.Z.; Li, Y.; Li, H.Q.; Li, W.Y. Effects of Long-Term Smoking on the Activity and MRNA Expression of CYP Isozymes in Rats. J. Thorac. Dis. 2015, 7, 1725–1731. [Google Scholar] [CrossRef]
- Singh, R.; Farmer, P.B. Liquid Chromatography-Electrospray Ionization-Mass Spectrometry: The Future of DNA Adduct Detection. Carcinogenesis 2006, 27, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and Chemical Carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Henn, S.A.; Succop, P.; Talaska, G.; Anderson, K.; Hecht, S.S.; Gross, M. Carcinogen-DNA Adducts Are Increased in the Exfoliated Urothelial Cells of Wives of Smokers: Biological Monitoring of Passive Smoke Exposure. Polycycl. Aromat. Compd. 2004, 24, 475–485. [Google Scholar] [CrossRef]
- Redondo, N.; Navarro, D.; Aguado, J.M.; Fernández-Ruiz, M. Viruses, Friends, and Foes: The Case of Torque Teno Virus and the Net State of Immunosuppression. Transpl. Infect. Dis. 2022, 24, e13778. [Google Scholar] [CrossRef] [PubMed]
- Sheet, F. Personalisation of Immunosuppression by Monitoring Viral Load Post Kidney Transplantation—A Randomised Controlled Phase II Trial|TTV Guide TX Project|Fact Sheet|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/896932 (accessed on 1 December 2023).
- Gottlieb, J.; Reuss, A.; Mayer, K.; Weide, K.; Schade-Brittinger, C.; Hoyer, S.; Jaksch, P. Viral Load-Guided Immunosuppression after Lung Transplantation (VIGILung)—Study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 48. [Google Scholar] [CrossRef] [PubMed]
| Variable | Undetectable TTV (n = 117) | Detectable TTV (n = 549) | p-Value |
|---|---|---|---|
| TTV load (log10 copies/mL), mean (SD) | NA | 3.05 (1.53) | NA |
| Clinical characteristics | |||
| Female sex, n (%) | 60 (51.3) | 228 (41.5) | 0.05 |
| Age (years), mean (SD) | 48.5 (14.3) | 54.0 (12.26) | <0.001 |
| Height (cm), mean (SD) | 172 (10) | 174 (10) | 0.13 |
| Weight (kg), mean (SD) | 77.3 (15.1) | 81.1 (16.5) | 0.02 |
| BMI (kg/m2), mean (SD) | 26.0 (4.3) | 26.8 (4.8) | 0.09 |
| Systolic blood pressure (mmHg), mean (SD) | 134 (18) | 137 (17.4) | 0.10 |
| History of diabetes, n (%) | 22 (18.8) | 138 (25.1) | 0.15 |
| Transplantation characteristics | |||
| Living donor, n (%) | 45 (38.5) | 187 (34.1) | 0.36 |
| Age of the donor (years), mean (SD) | 40.6 (15.3) | 43.5 (15.4) | 0.08 |
| Female sex of the donor, n (%) | 58 (53.7) | 257 (47.3) | 0.23 |
| Time since transplantation (years), median [IQR] | 7.1 [4.1, 12.4] | 5.1 [1.7, 11.4] | 0.009 |
| Positive CMV serostatus of the donor, n (%) | 43 (42.6) | 243 (47.3) | 0.46 |
| Positive CMV serostatus of the recipient, n (%) | 24 (30.8) | 147 (40.9) | 0.22 |
| Laboratory measurements | |||
| Hemoglobin (mmol/L), mean (SD) | 8.2 (1.1) | 8.3 (1.1) | 0.80 |
| Leukocyte count (109/L), mean (SD) | 8.0 (2.4) | 8.2 (2.6) | 0.43 |
| Trombocyte count (109/L), mean (SD) | 241.8 (72.4) | 234.9 (74.5) | 0.57 |
| Creatine (umol/L), median [IQR] | 115 [91, 144] | 126 [102, 163] | 0.01 |
| eGFR creatine (mL/min/1.73 m2), mean (SD) | 57.4 (22.3) | 51.3 (19.9) | 0.003 |
| Cystatin C (mg/L), median [IQR] | 1.46 [1.22, 2.00] | 1.71 [1.35, 2.28] | 0.004 |
| eGFR cystatin (mL/min/1.73 m2), mean (SD) | 46.4 (20.4) | 30.0 (18.2) | 0.001 |
| HS-CRP (mg/dL), median [IQR] | 1.40 [0.65, 3.60] | 1.60 [0.70, 4.60] | 0.40 |
| Albumin (g/L), mean (SD) | 43.3 (2.9) | 42.9 (3.0) | 0.21 |
| Total urinary protein excretion (g/24 h), median [IQR] | 0.2 [0.0, 0.3] | 0.2 [0.0, 0.4] | 0.13 |
| Lifestyle factors | |||
| SQUASH score, median [IQR] | 5700 [2780, 9060] | 4935 [1920, 7500] | 0.23 |
| Average alcohol intake (grams/day), median [IQR] | 4.1 [0.3, 11.8] | 2.3 [0.0, 10.6] | 0.22 |
| Average daily alcohol intake above 20 g, n (%) | 13 (12.5) | 66 (13.2) | 0.85 |
| Ethyl glucuronide concentration in urine above 10.000 ug/L, n (%) | 18 (15.5) | 66 (12.5) | 0.38 |
| Smoking behavior according to questionnaire | 0.42 | ||
| 42 (38.9) | 221 (42.6) | |
| 48 (44.4) | 235 (45.3) | |
| 18 (16.7) | 63 (12.1) | |
| Smoking behavior correct for urinary cotinine above 0 ng/L | 0.50 | ||
| 41 (37.6) | 203 (39.7) | |
| 41 (37.6) | 207 (40.5) | |
| 27 (24.8) | 101 (19.8) | |
| Cotinine concentration in urine above 0 ng/L, n (%) | 26 (22.4) | 86 (16.3) | 0.11 |
| Medication | |||
| Calcineurin inhibitor usage | <0.001 | ||
| 75 (64.1) | 214 (39.0) | |
| 24 (20.5) | 235 (42.8) | |
| 18 (15.4) | 100 (18.2) | |
| Prednisolone usage, n (%) | 115 (98.3) | 545 (99.3) | 0.31 |
| Proliferation inhibitor usage, n (%) | 104 (88.9) | 451 (82.1) | 0.08 |
| mTOR inhibitor usage, n (%) | 3 (2.6) | 19 (3.5) | 0.62 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| St. β [95% CI] | p-Value | St. β [95% CI] | p-Value | |
| Female sex | 0.00 [−0.17–0.17] | 0.99 | −0.04 [−0.20–0.12] | 0.64 |
| Age | 0.07 [−0.02–0.15] | 0.12 | 0.10 [0.02–0.18] | 0.02 |
| Time since transplantation [log10] | −0.24 [−0.32–−0.16] | <0.001 | −0.18 [−0.27–−0.10] | <0.001 |
| eGFR cystatin | −0.21 [−0.29–−0.13] | <0.001 | −0.15 [−0.23–−0.06] | 0.001 |
| Calcineurin inhibitor usage | ||||
| Reference | Reference | ||
| 0.46 [0.28–0.64] | <0.001 | 0.26 [0.07–0.45] | 0.008 |
| 0.66 [0.43–0.89] | <0.001 | 0.46 [0.21–0.71] | <0.001 |
| Smoking variables | ||||
| Smoking questionnaire | ||||
| Reference | Reference | ||
| −0.06 [−0.24–0.13] | 0.55 | −0.10 [−0.28–0.08] | 0.26 |
| −0.38 [−0.66–−0.10] | 0.008 | −0.40 [−0.66–−0.13] | 0.004 |
| Smoking behavior corrected for urinary cotinine above 50 ng/L | ||||
| Reference | Reference | ||
| −0.02 [−0.21–0.18] | 0.87 | −0.07 [−0.26–0.12] | 0.48 |
| −0.25 [−0.50–0.00] | 0.048 | −0.30 [−0.54–−0.06] | 0.01 |
| Smoking behavior corrected for urinary cotinine above 0 ng/L | ||||
| Reference | Reference | ||
| −0.01 [−0.08–0.19] | 0.91 | −0.06 [−0.25–0.13] | 0.52 |
| −0.25 [−0.49–−0.01] | 0.04 | −0.29 [−0.52–−0.06] | 0.01 |
| Active smoking and urinary cotinine above 50 ng/L | −0.39 [−0.68–−0.10] | 0.008 | −0.39 [−0.67–−0.12] | 0.005 |
| Active smoking and urinary cotinine above 0 ng/L | −0.40 [−0.68–−0.12] | 0.005 | −0.39 [−0.65–−0.13] | 0.004 |
| Cotinine concentration in urine above 50 ng/L | −0.29 [−0.54–−0.04] | 0.02 | −0.32 [−0.55–−0.08] | 0.009 |
| Cotinine concentration in urine above 0 ng/L | −0.28 [−0.51–−0.04] | 0.02 | −0.30 [−0.52–−0.08] | 0.008 |
| Alcohol variables | ||||
| Average daily alcohol intake above 20 g/day | −0.40 [−0.66–−0.14] | 0.003 | −0.33 [−0.58–−0.08] | 0.009 |
| Ethyl glucuronide concentration in urine above 10.000 ug/L | −0.45 [−0.71–−0.20] | 0.001 | −0.35 [−0.60–−0.11] | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doorenbos, C.S.E.; Jonker, J.; Hao, J.; Gore, E.J.; Kremer, D.; Knobbe, T.J.; de Joode, A.A.E.; Sanders, J.S.F.; Thaunat, O.; Niesters, H.G.M.; et al. Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients. Viruses 2023, 15, 2387. https://doi.org/10.3390/v15122387
Doorenbos CSE, Jonker J, Hao J, Gore EJ, Kremer D, Knobbe TJ, de Joode AAE, Sanders JSF, Thaunat O, Niesters HGM, et al. Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients. Viruses. 2023; 15(12):2387. https://doi.org/10.3390/v15122387
Chicago/Turabian StyleDoorenbos, Caecilia S. E., Jip Jonker, Jiasi Hao, Edmund J. Gore, Daan Kremer, Tim J. Knobbe, Anoek A. E. de Joode, Jan Stephan F. Sanders, Olivier Thaunat, Hubert G. M. Niesters, and et al. 2023. "Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients" Viruses 15, no. 12: 2387. https://doi.org/10.3390/v15122387
APA StyleDoorenbos, C. S. E., Jonker, J., Hao, J., Gore, E. J., Kremer, D., Knobbe, T. J., de Joode, A. A. E., Sanders, J. S. F., Thaunat, O., Niesters, H. G. M., Van Leer-Buter, C. C., & Bakker, S. J. L. (2023). Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients. Viruses, 15(12), 2387. https://doi.org/10.3390/v15122387





