Bone Mineral Density and Trabecular Bone Score Changes throughout Menopause in Women with HIV
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Subjects
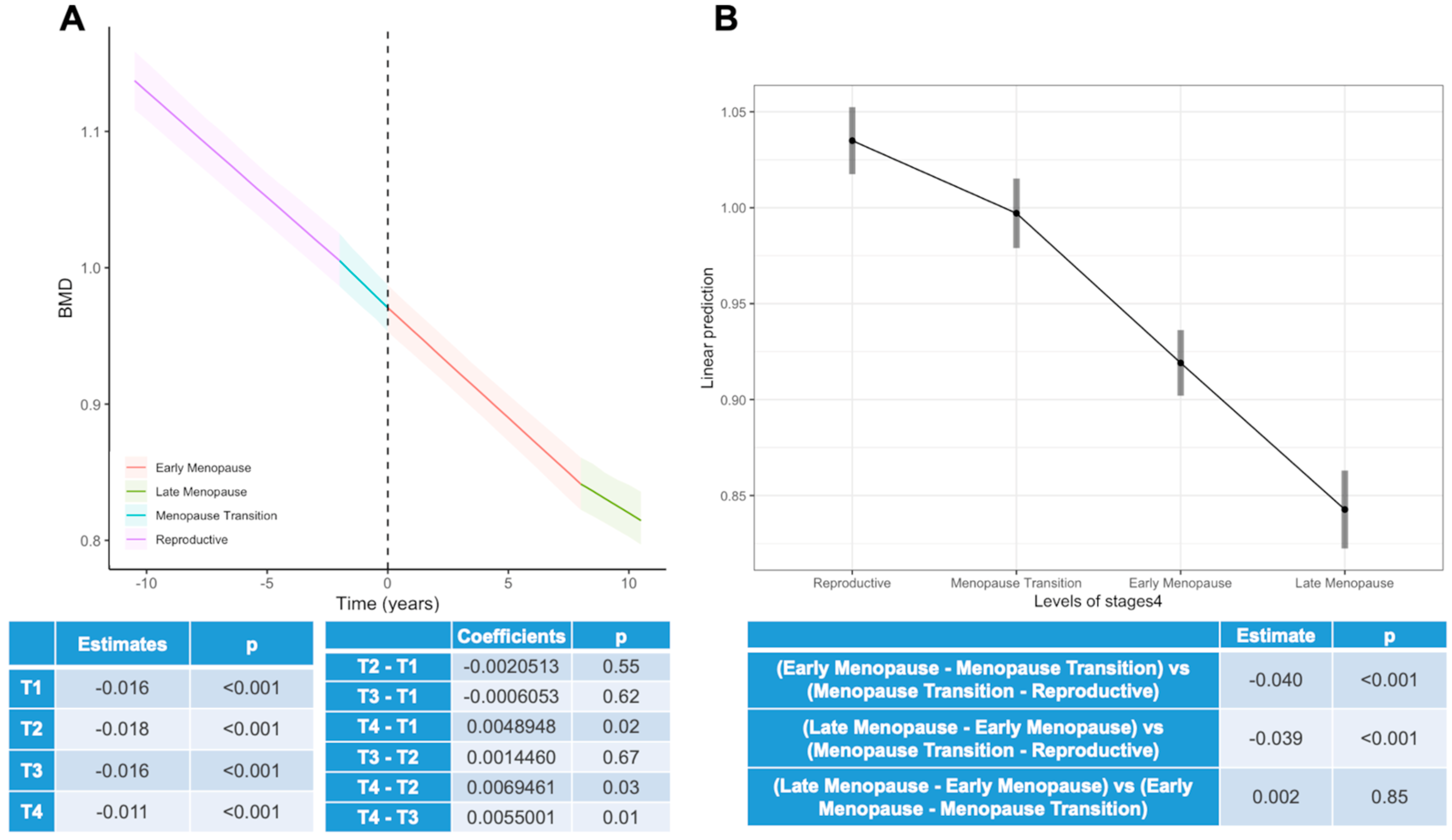
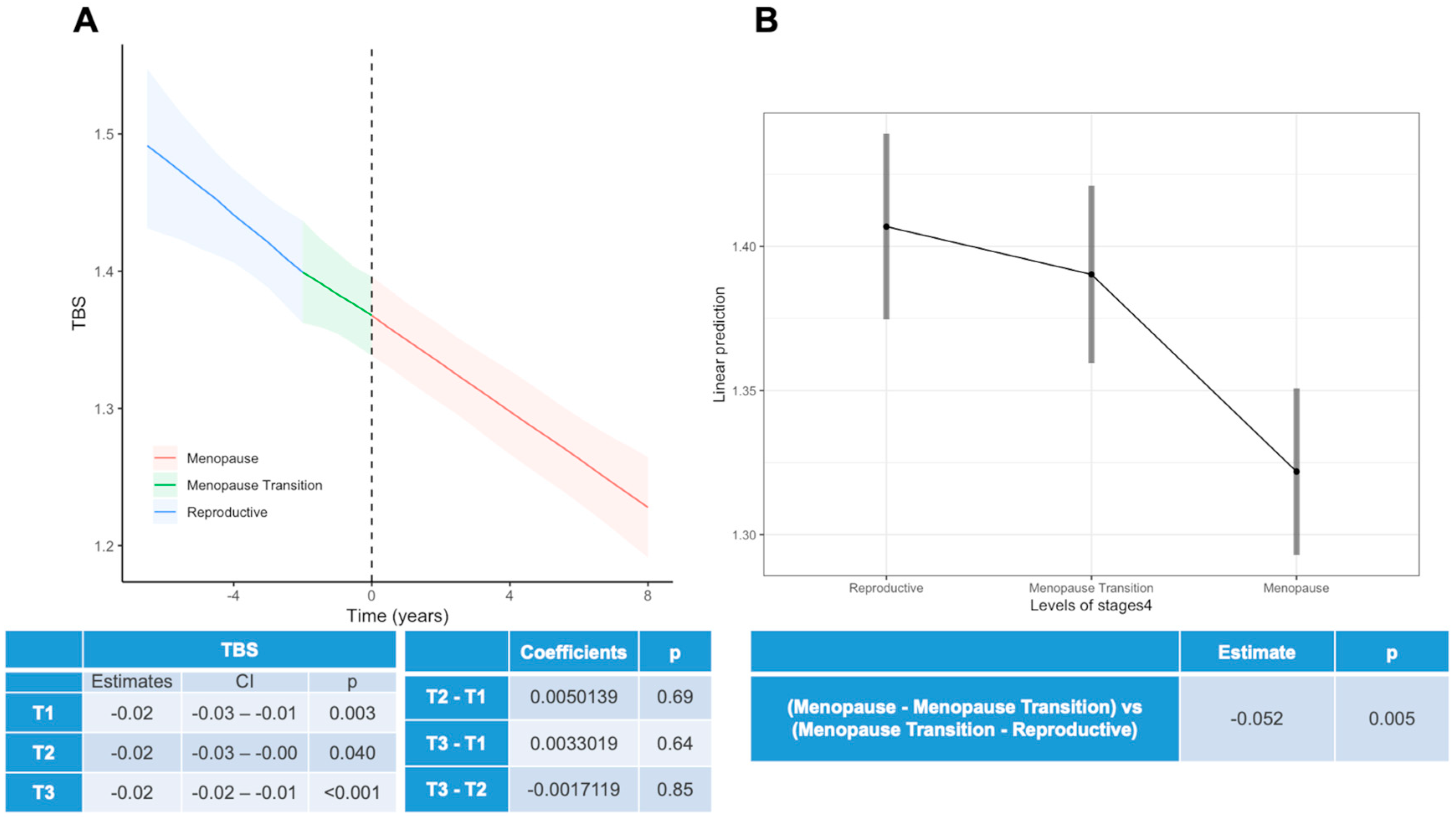
- “Reproductive Period” (T1): up to 2 years before menopausal transition.
- “Menopause Transition Period” (T2): from 2 years before menopause to menopause.
- “Early Menopause Period” (T3): from menopause onset to 6 years post-menopause.
- “Late Menopause Period” (T4): from 6 years post-menopause onwards.
2.2. Covariates and Outcomes
2.3. Bone Quantity and Bone Quality Assessments
2.4. Statistical Analysis
3. Results
3.1. Study Population
- “Reproductive Period”: 630 (32.2%) BMD and 40 (20.5%) TBS data points.
- “Menopause Transition Period”: 301 (15.4%) BMD and 49 (25.1%) TBS data points.
- “Early Menopause Period”: 878 (44.9%) BMD and 104 (53.3%) TBS data points.
- “Late Menopause Period”: 145 (7.4%) BMD and 2 (1.0%) TBS data points.
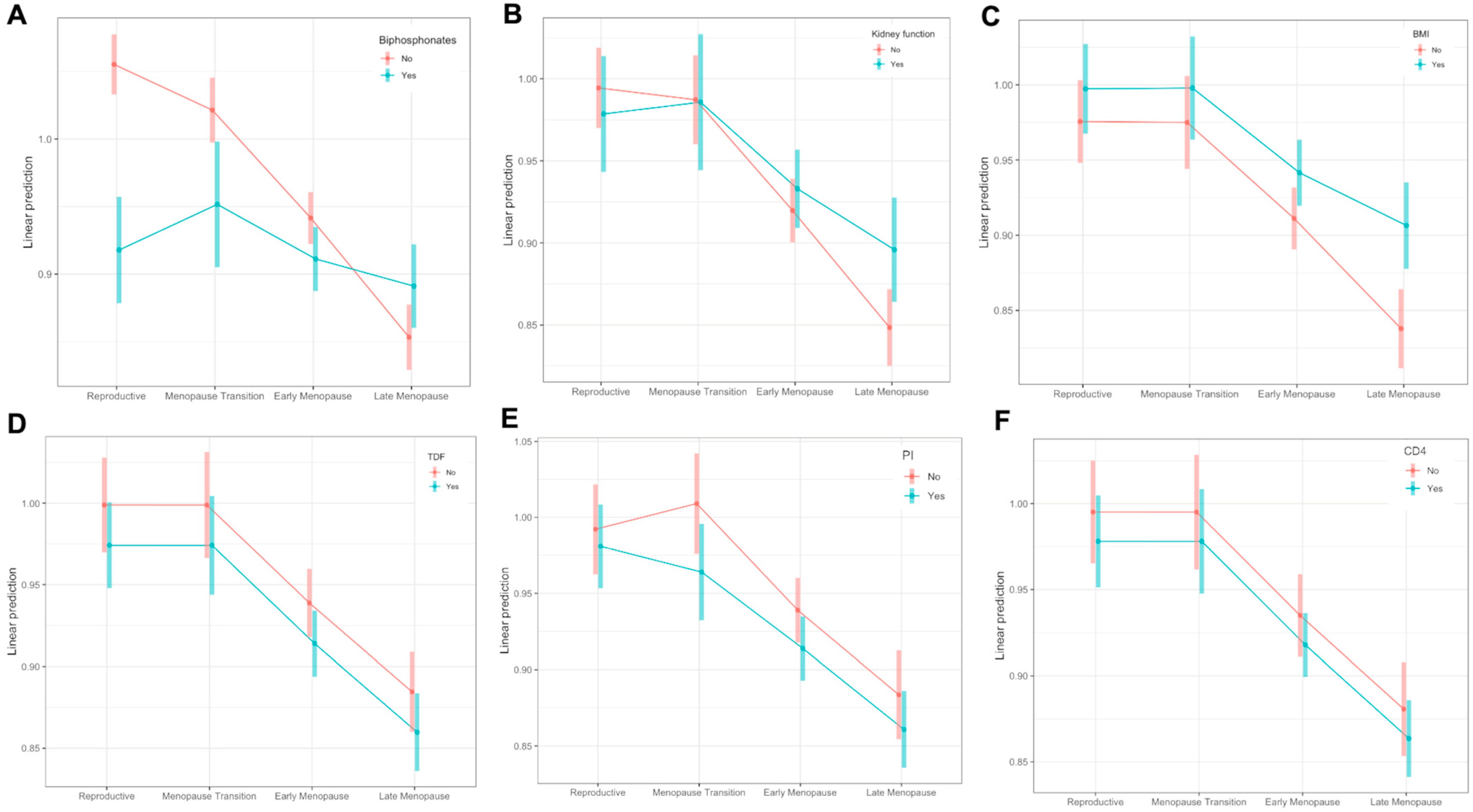
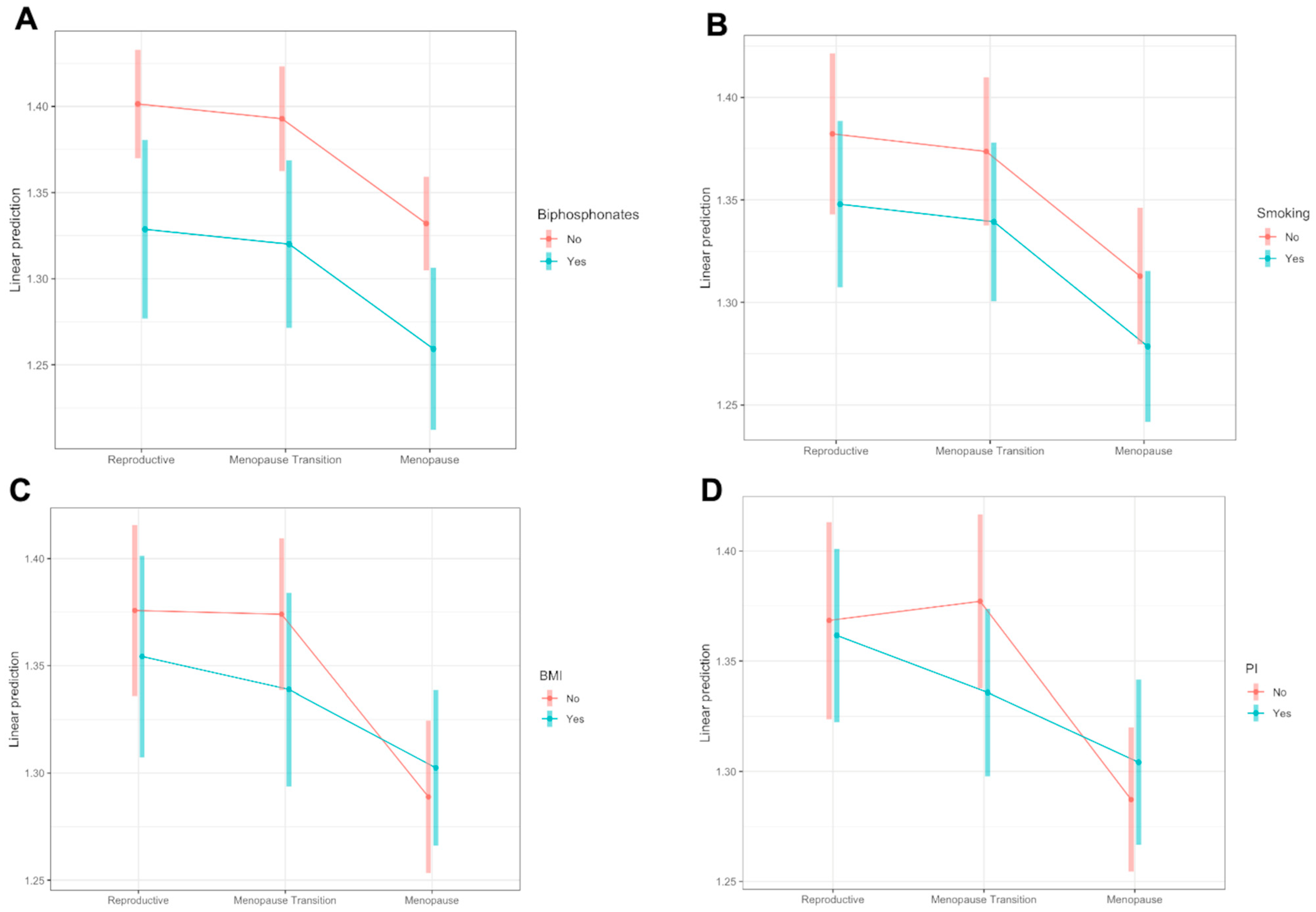
3.2. Changes throughout Menopause
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.; Schrager, S.; Dalby, J.; Torell, E.; Hampton, A. Menopause. Prim. Care 2018, 45, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.L. The Menopausal Transition. Obstet. Gynecol. Clin. N. Am. 2017, 44, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.T.; Shane, E.S. Low bone-mineral density in patients with HIV: Pathogenesis and clinical significance. Curr. Opin. Endocrinol. Diabetes 2006, 13, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Hoy, J. Bone Disease in HIV: Recommendations for Screening and Management in the Older Patient. Drugs Aging 2015, 32, 549–558. [Google Scholar] [CrossRef]
- Brown, T.; Qaqish, R. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis. AIDS 2006, 20, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- McComsey, G.A.; Kitch, D.; Daar, E.S.; Tierney, C.; Jahed, N.C.; Tebas, P.; Myers, L.; Melbourne, K.; Ha, B.; Sax, P.E. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACT. J. Infect. Dis. 2011, 203, 1791–1801. [Google Scholar] [CrossRef]
- Yin, M.T.; Brown, T.T. HIV and Bone Complications: Understudied Populations and New Management Strategies. Curr. HIV/AIDS Rep. 2016, 13, 349–358. [Google Scholar] [CrossRef]
- Güerri-Fernández, R.; Lerma-Chippirraz, E.; Marron, A.F.; García-Giralt, N.; Villar-García, J.; Soldado-Folgado, J.; González-Mena, A.; Trenchs-Rodríguez, M.; Guelar, A.; Díez-Pérez, A.; et al. Bone density, microarchitecture, and tissue quality after 1 year of treatment with tenofovir disoproxil fumarate. Aids 2018, 32, 913–920. [Google Scholar] [CrossRef]
- Stellbrink, H.-J.; Orkin, C.; Arribas, J.R.; Compston, J.; Gerstoft, J.; Van Wijngaerden, E.; Lazzarin, A.; Rizzardini, G.; Sprenger, H.G.; Lambert, J.; et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010, 51, 963–972. [Google Scholar] [CrossRef]
- McComsey, G.A.; Tebas, P.; Shane, E.; Yin, M.T.; Overton, E.T.; Huang, J.S.; Aldrovandi, G.M.; Cardoso, S.W.; Santana, J.L.; Brown, T.T. Bone disease in HIV infection: A practical review and recommendations for HIV care providers. Clin. Infect. Dis. 2010, 51, 937–946. [Google Scholar] [CrossRef]
- Chan, H.L.Y.; Buti, M.; Lim, Y.-S.; Agarwal, K.; Marcellin, P.; Brunetto, M.; Chuang, W.-L.; A Janssen, H.L.; Fung, S.; Izumi, N.; et al. Long-term Treatment with Tenofovir Alafenamide for Chronic Hepatitis B Results in High Rates of Viral Suppression and Favorable Renal and Bone Safety. Am. J. Gastroenterol. 2023, 10, 14309. [Google Scholar] [CrossRef]
- Wang, F.-D.; Zhou, J.; Li, L.-Q.; Li, Y.-J.; Wang, M.-L.; Tao, Y.-C.; Zhang, D.-M.; Wang, Y.-H.; Chen, E.-Q. Improved bone and renal safety in younger tenofovir disoproxil fumarate experienced chronic hepatitis B patients after switching to tenofovir alafenamide or entecavir. Ann. Hepatol. 2023, 28, 101119. [Google Scholar] [CrossRef]
- Baranek, B.; Wang, S.; Cheung, A.M.; Mishra, S.; Tan, D.H. The effect of tenofovir disoproxil fumarate on bone mineral density: A systematic review and meta-analysis. Antivir. Ther. 2020, 25, 21–32. [Google Scholar] [CrossRef]
- Teichmann, J.; Stephan, E.; Lange, U.; Discher, T.; Friese, G.; Lohmeyer, J.; Stracke, H.; Bretzel, R. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J. Infect. 2003, 46, 221–227. [Google Scholar] [CrossRef]
- Madeddu, G.; Spanu, A.; Solinas, P.; Calia, G.M.; Lovigu, C.; Chessa, F.; Mannazzu, M.; Falchi, A.; Mura, M.S.; Madeddu, G. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q. J. Nucl. Med. Mol. Imaging 2004, 48, 39–48. [Google Scholar]
- Yin, M.T.; Modarresi, R.; Shane, E.; Santiago, F.; Ferris, D.C.; McMahon, D.J.; Zhang, C.A.; Cremers, S.; Laurence, J. Effects of HIV infection and antiretroviral therapy with ritonavir on induction of osteoclast-like cells in postmenopausal women. Osteoporos. Int. 2011, 22, 1459–1468. [Google Scholar] [CrossRef]
- Kinai, E.; Nishijima, T.; Mizushima, D.; Watanabe, K.; Aoki, T.; Honda, H.; Yazaki, H.; Genka, I.; Tanuma, J.; Teruya, K.; et al. Long-term use of protease inhibitors is associated with bone mineral density loss. AIDS Res. Hum. Retroviruses 2014, 30, 553–559. [Google Scholar] [CrossRef]
- Braithwaite, K.; McPherson, T.D.; Shen, Y.; Arpadi, S.; Shiau, S.; Sorour, G.; Technau, K.-G.; Yin, M.T. Bone outcomes in virally suppressed youth with HIV switching to tenofovir disoproxil fumarate. S. Afr. J. HIV Med. 2021, 22, 1243. [Google Scholar] [CrossRef]
- Macdonald, H.M.; Maan, E.J.; Berger, C.; Côte, H.C.F.; Murray, M.C.M.; Pick, N.; Prior, J.C. Long-Term Change in Bone Mineral Density in Women Living with HIV: A 10-Year Prospective Controlled Cohort Study. JBMR Plus 2023, 7, e10761. [Google Scholar] [CrossRef]
- McGinty, T.; Mirmonsef, P.; Mallon, P.W.G.; Landay, A.L. Does systemic inflammation and immune activation contribute to fracture risk in HIV? Curr. Opin. HIV AIDS 2016, 11, 253–260. [Google Scholar] [CrossRef]
- Yin, M.T.; Falutz, J. How to predict the risk of fracture in HIV? Curr. Opin. HIV AIDS 2016, 11, 261–267. [Google Scholar] [CrossRef]
- European AIDS Clinical Society, 11th Version Guidelines, October 2021. Available online: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf (accessed on 17 May 2022).
- Pothuaud, L.; Carceller, P.; Hans, D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: Applications in the study of human trabecular bone microarchitecture. Bone 2008, 42, 775–787. [Google Scholar] [CrossRef]
- Sharma, A.; Ma, Y.; Tien, P.C.; Scherzer, R.; Anastos, K.; Cohen, M.H.; Hans, D.; Yin, M.T. HIV Infection Is Associated with Abnormal Bone Microarchitecture: Measurement of Trabecular Bone Score in the Women’s Interagency HIV Study. J. Acquir. Immune Defic. Syndr. 2018, 78, 441–449. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; Villiers, T.J.d.; STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10, addressing the unfinished agenda of staging reproductive aging. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef]
- Battegary, M.; Ryom, L.; Switzerland, B. European AIDS Clinical Society (EACS). Guideliness 9.1, p. 1–103. 2018. Available online: http://www.eacsociety.org (accessed on 17 May 2022).
- De Vincentis, S.; Decaroli, M.C.; Fanelli, F.; Diazzi, C.; Mezzullo, M.; Morini, F.; Bertani, D.; Milic, J.; Carli, F.; Cuomo, G.; et al. Health status is related to testosterone, estrone and body fat: Moving to functional hypogonadism in adult men with HIV. Eur. J. Endocrinol. 2021, 184, 107–122. [Google Scholar] [CrossRef]
- World Health Organization. Who Scientific Group on the Assessment of Osteoporosis at Primary Health; World Health Organization: Geneva, Switzerland, 2004; Volume 5, pp. 5–7. [Google Scholar]
- Winzenrieth, R.; Michelet, F.; Hans, D. Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: Effects of resolution and noise. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2013, 16, 287–296. [Google Scholar] [CrossRef]
- Roux, J.P.; Wegrzyn, J.; Boutroy, S.; Bouxsein, M.L.; Hans, D.; Chapurlat, R. The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: An ex vivo study. Osteoporos. Int. 2013, 24, 2455–2460. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Cooper, C.; Johansson, H.; Oden, A.; McCloskey, E.V. A systematic review of intervention thresholds based on FRAX: A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch. Osteoporos. 2016, 11, 25. [Google Scholar] [CrossRef]
- Brown, T.T.; Hoy, J.; Borderi, M.; Guaraldi, G.; Renjifo, B.; Vescini, F.; Yin, M.T.; Powderly, W.G. Recommendations for evaluation and management of bone disease in HIV. Clin. Infect. Dis. 2015, 60, 1242–1251. [Google Scholar] [CrossRef]
- Yin, M.; Dobkin, J.; Brudney, K.; Becker, C.; Zadel, J.L.; Manandhar, M.; Addesso, V.; Shane, E. Bone mass and mineral metabolism in HIV+ postmenopausal women. Osteoporos. Int. 2005, 16, 1345–1352. [Google Scholar] [CrossRef]
- Cezarino, P.Y.A.; Simões, R.d.S.; Baracat, E.C.; Soares Junior, J.M. Are women living with HIV prone to osteoporosis in postmenopause? A systematic review. Rev. Assoc. Med. Bras. 2018, 64, 469–473. [Google Scholar] [CrossRef]
- Fantry, L.E.; Zhan, M.; Taylor, G.H.; Sill, A.M.; Flaws, J.A. Age of Menopause and Menopausal Symptoms in HIV-Infected Women. AIDS Patient Care STDS 2005, 19, 703–711. [Google Scholar] [CrossRef]
- Massad, L.S.; Evans, C.T.; Minkoff, H.; Watts, D.H.; Greenblatt, R.M.; Levine, A.M.; Anastos, K.; Young, M.; Seifer, D.B.; Golub, E.; et al. Effects of HIV Infection and Its Treatment on Self-Reported Menstrual Abnormalities in Women. J. Women’s Health 2006, 15, 591–598. [Google Scholar] [CrossRef]
- Schoenbaum, E.E.; Hartel, D.; Lo, Y.; Howard, A.A.; Floris-Moore, M.; Arnsten, J.H.; Santoro, N. HIV Infection, Drug Use, and Onset of Natural Menopause. Clin. Infect. Dis. 2005, 41, 1517–1524. [Google Scholar] [CrossRef]
- De Pommerol, M.; Hessamfar, M.; Lawson-Ayayi, S.; Neau, D.; Geffard, S.; Farbos, S.; Uwamaliya, B.; Vandenhende, M.-A.; Pellegrin, J.-L.; Blancpain, S.; et al. Menopause and HIV infection: Age at onset and associated factors, ANRS CO3 Aquitaine cohort. Int. J. STD AIDS 2011, 22, 67–72. [Google Scholar] [CrossRef]
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Harrington, L.M.; Breggia, A.; Rosen, C.J.; Miller, K.K. Determinants of bone mineral density in obese premenopausal women. Bone 2011, 48, 748–754. [Google Scholar] [CrossRef]
- Douchi, T.; Oki, T.; Nakamura, S.; Ijuin, H.; Yamamoto, S.; Nagata, Y. The effect of body composition on bone density in pre- and postmenopausal women. Maturitas 1997, 27, 55–60. [Google Scholar] [CrossRef]
- Russell, M.; Mendes, N.; Miller, K.K.; Rosen, C.J.; Lee, H.; Klibanski, A.; Misra, M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J. Clin. Endocrinol. Metab. 2010, 95, 1247–1255. [Google Scholar] [CrossRef]
- Looker, A.C.; Sarafrazi Isfahani, N.; Fan, B.; Shepherd, J.A. Trabecular bone scores and lumbar spine bone mineral density of US adults: Comparison of relationships with demographic and body size variables. Osteoporos. Int. 2016, 27, 2467–2475. [Google Scholar] [CrossRef]
- González, J.; Rodríguez-Fraile, M.; Rivera, P.; Restituto, P.; Colina, I.; Calleja, M.d.L.D.; Alcaide, A.B.; Campo, A.; Bertó, J.; Seijo, L.M.; et al. Trabecular bone score in active or former smokers with and without COPD. PLoS ONE 2019, 14, e0209777. [Google Scholar] [CrossRef]
- Whitcomb, B.W.; Purdue-Smithe, A.C.; Szegda, K.L.; Boutot, M.E.; Hankinson, S.E.; Manson, J.E.; Rosner, B.; Willett, W.C.; Eliassen, A.H.; Bertone-Johnson, E.R. Cigarette Smoking and Risk of Early Natural Menopause. Am. J. Epidemiol. 2018, 187, 696–704. [Google Scholar] [CrossRef]
- Trevisan, C.; Alessi, A.; Girotti, G.; Zanforlini, B.M.; Bertocco, A.; Mazzochin, M.; Zoccarato, F.; Piovesan, F.; Dianin, M.; Giannini, S.; et al. The Impact of Smoking on Bone Metabolism, Bone Mineral Density and Vertebral Fractures in Postmenopausal Women. J. Clin. Densitom. 2020, 23, 381–389. Available online: https://www.sciencedirect.com/science/article/pii/S1094695019301088 (accessed on 22 May 2022). [CrossRef]
- Shin, M.-S.; Cho, E.-H.; Kim, H.Y. Longitudinal Change in Trabecular Bone Score during and after Treatment of Osteoporosis in Postmenopausal Korean Women. J. Bone Metab. 2017, 24, 117–124. [Google Scholar] [CrossRef]
- Leslie, W.D.; Goel, H.; Binkley, N.; McCloskey, E.V.; Hans, D. Contributions of Clinical and Technical Factors to Longitudinal Change in Trabecular Bone Score and Bone Density: A Registry-Based Individual-Level Analysis. J. Bone Miner. Res. 2023, 38, 512–521. [Google Scholar] [CrossRef]
- Guaraldi, G.; Zona, S.; Silva, A.R.; Menozzi, M.; Dolci, G.; Milic, J.; Carli, F.; Mussini, C. The dynamic association between Frailty, CD4 and CD4/CD8 ratio in people aging with HIV. PLoS ONE 2019, 14, e0212283. [Google Scholar] [CrossRef]
- Bagri, P.; Ghasemi, R.; McGrath, J.J.C.; Thayaparan, D.; Yu, E.; Brooks, A.G.; Stämpfli, M.R.; Kaushic, C. Estradiol Enhances Antiviral CD4+ Tissue-Resident Memory T Cell Responses following Mucosal Herpes Simplex Virus 2 Vaccination through an IL-17-Mediated Pathway. J. Virol. 2020, 95, 10–1128. [Google Scholar] [CrossRef]
- Shao, B.-Y.; Wang, L.; Yu, Y.; Chen, L.; Gan, N.; Huang, W.-M. Effects of CD4+ T lymphocytes from ovariectomized mice on bone marrow mesenchymal stem cell proliferation and osteogenic differentiation. Exp. Ther. Med. 2020, 20, 84. [Google Scholar] [CrossRef]
- Guan, W.-M.; Pan, W.; Yu, W.; Cao, W.; Lin, Q.; Zhang, Z.-Z.; Song, X.-J.; Li, Y.-L.; Tian, J.-P.; Xu, Y.; et al. Changes in trabecular bone score and bone mineral density in Chinese HIV-Infected individuals after one year of antiretroviral therapy. J. Orthop. Transl. 2021, 29, 72–77. [Google Scholar] [CrossRef]
- Yang, J.; Sharma, A.; Shi, Q.; Anastos, K.; Cohen, M.H.; Golub, E.T.; Gustafson, D.; Merenstein, D.; Mack, W.J.; Tien, P.C.; et al. Improved fracture prediction using different fracture risk assessment tool adjustments in HIV-infected women. AIDS 2018, 32, 1699–1706. [Google Scholar] [CrossRef]
| Characteristic | BMD | TBS | ||||
|---|---|---|---|---|---|---|
| Pre-Menopause, N = 199 | Post-Menopause, N = 199 | p | Pre-Menopause, N = 48 | Post-Menopause, N = 48 | p | |
| Demographic, anthropometric, and lifestyle characteristics | ||||||
| Age, years, median (Q1, Q3) | 46.2 (44.2, 48.4) | 53.1 (50.7, 55.9) | <0.001 * | 49.5 (47.2, 51.9) | 53.5 (51.6, 56.2) | <0.001 * |
| Body mass index, kg/m2, median (Q1, Q3) | 22.1 (20.2, 24.3) | 22.5 (20.4, 25.2) | <0.001 * | 22.4 (20.1, 25.7) | 22.9 (20.4, 25.6) | 0.001 * |
| Body mass index > 25 kg/m2, N (%) | 54 (27%) | 72 (36%) | 0.003 * | 14 (29%) | 18 (38%) | 0.20 |
| Waist circumference, cm, median (Q1, Q3) | 82 (77, 88) | 84 (77, 91) | <0.001 * | 80 (73, 90) | 85 (78, 90) | <0.001 * |
| Currently smoking, N (%) | 87 (44%) | 80 (40%) | 0.20 | 19 (40%) | 17 (35%) | 0.70 |
| Alcohol use, N (%) | 94 (47%) | 88 (44%) | 0.50 | 15 (31%) | 16 (33%) | 0.90 |
| HIV-related variables | ||||||
| Nadir CD4 cell count < 200 c/microL, N (%) | 112 (56%) | 115 (58%) | 0.20 | 22 (46%) | 22 (46%) | NA |
| Nadir CD4 cell count, c/microL, median (Q1, Q3) | 183 (100, 254) | 190 (108, 262) | NA | 225 (138, 300) | 225 (138, 300) | NA |
| Current CD4 cell count > 500 c/microL, N (%) | 158 (79%) | 177 (89%) | 0.001 * | 43 (90%) | 43 (90%) | 0.90 |
| Current CD4 cell count, c/microL, median (Q1, Q3) | 606 (433, 775) | 766 (598, 930) | <0.001 * | 712 (559, 889) | 787 (568, 1074) | 0.004 * |
| Years since HIV diagnosis, years, mean (SD) | 18 (6) | 25 (6) | <0.001 * | 24 (5) | 28 (6) | <0.001 * |
| Undetectable HIV RNA viral load, N (%) | 173 (87%) | 198 (99%) | <0.001 * | 48 (100%) | 47 (98%) | NA |
| ARTs | ||||||
| Exposure to TAF, N (%) | 2 (1.0%) | 45 (23%) | <0.001 * | 1 (2.1%) | 12 (25%) | 0.003 * |
| Exposure to TDF, N (%) | 145 (73%) | 96 (48%) | <0.001 * | 25 (52%) | 11 (23%) | <0.001 * |
| Exposure to protease inhibitors, N (%) | 123 (62%) | 104 (52%) | 0.009 * | 28 (58%) | 19 (40%) | 0.02 * |
| DEXA variables | ||||||
| BMD, g/cm2, mean (SD) | 1.03 (0.14) | 0.92 (0.12) | <0.001 * | |||
| TBS, median (Q1, Q3) | 1.41 (1.35, 1.45) | 1.32 (1.28, 1.39) | <0.001 * | |||
| FRAX, %, median (Q1, Q3) | 2.96 (2.35, 4.21) | 3.97 (3.09, 5.04) | <0.001 * | 3.55 (2.95, 4.61) | 4.11 (3.13, 4.84) | 0.004 * |
| Endocrinological variables | ||||||
| FSH, mIU/mL, median (Q1, Q3) | 13 (8, 36) | 70 (50, 94) | 0.004 * | 19 (7, 34) | 77 (55, 97) | <0.001 * |
| LH, mIU/mL, median (Q1, Q3) | 15 (7, 25) | 35 (30, 40) | 0.03 * | 19 (6, 24) | 41 (31, 48) | 0.004 * |
| PTH, pg/mL, median (Q1, Q3) | 53 (37, 66) | 39 (29, 54) | <0.001 * | 36 (25, 53) | 33 (28, 48) | 0.30 |
| Phosphorus, mg/dL, mean (SD) | 3.31 (0.49) | 3.37 (0.38) | 0.11 | 2.98 (0.43) | 3.17 (0.43) | 0.02 |
| Estradiol, pg/mL, median (Q1, Q3) | 87 (36, 154) | 12 (7, 19) | 0.004 * | 40 (4, 15) | 12 (5, 15) | 0.90 |
| Osteopenia and osteoporosis | ||||||
| Osteopenia, N (%) | 126 (63%) | 151 (76%) | <0.001 * | 29 (60%) | 31 (65%) | 0.50 |
| Osteoporosis, N (%) | 31 (16%) | 71 (36%) | <0.001 * | 10 (21%) | 12 (25%) | 0.50 |
| Use of bisphosphonates, N (%) | 7 (3.5%) | 32 (16%) | <0.001 * | 4 (8.3%) | 5 (10%) | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milic, J.; Renzetti, S.; Morini, D.; Motta, F.; Carli, F.; Menozzi, M.; Cuomo, G.; Mancini, G.; Simion, M.; Romani, F.; et al. Bone Mineral Density and Trabecular Bone Score Changes throughout Menopause in Women with HIV. Viruses 2023, 15, 2375. https://doi.org/10.3390/v15122375
Milic J, Renzetti S, Morini D, Motta F, Carli F, Menozzi M, Cuomo G, Mancini G, Simion M, Romani F, et al. Bone Mineral Density and Trabecular Bone Score Changes throughout Menopause in Women with HIV. Viruses. 2023; 15(12):2375. https://doi.org/10.3390/v15122375
Chicago/Turabian StyleMilic, Jovana, Stefano Renzetti, Denise Morini, Federico Motta, Federica Carli, Marianna Menozzi, Gianluca Cuomo, Giuseppe Mancini, Mattia Simion, Federico Romani, and et al. 2023. "Bone Mineral Density and Trabecular Bone Score Changes throughout Menopause in Women with HIV" Viruses 15, no. 12: 2375. https://doi.org/10.3390/v15122375
APA StyleMilic, J., Renzetti, S., Morini, D., Motta, F., Carli, F., Menozzi, M., Cuomo, G., Mancini, G., Simion, M., Romani, F., Spadoni, A., Baldisserotto, I., Barp, N., Diazzi, C., Mussi, C., Mussini, C., Rochira, V., Calza, S., & Guaraldi, G. (2023). Bone Mineral Density and Trabecular Bone Score Changes throughout Menopause in Women with HIV. Viruses, 15(12), 2375. https://doi.org/10.3390/v15122375








