Abstract
The herpes simplex virus (HSV) is a double-stranded DNA human virus that causes persistent infections with recurrent outbreaks. HSV exists in two forms: HSV-1, responsible for oral herpes, and HSV-2, primarily causing genital herpes. Both types can lead to significant complications, including neurological issues. Conventional treatment, involving acyclovir and its derivatives, faces challenges due to drug resistance. This underscores the imperative for continual research and development of new drugs, with a particular emphasis on exploring the potential of natural antivirals. Flavonoids have demonstrated promise in combating various viruses, including those within the herpesvirus family. This review, delving into recent studies, reveals the intricate mechanisms by which flavonoids decode their antiviral capabilities against HSV. By disrupting key stages of the viral life cycle, such as attachment to host cells, entry, DNA replication, latency, and reactivation, flavonoids emerge as formidable contenders in the ongoing battle against HSV infections.
1. Introduction
The herpes simplex virus (HSV) is an infectious human pathogen categorized within human herpesviruses as an alpha-herpesvirus. As a double-stranded DNA virus, it establishes a persistent infection in humans throughout their lifetime. HSV comprises two distinct species, namely HSV-1 and HSV-2 [1,2]. HSV-1 primarily leads to oral herpes through skin-to-skin contact and can also result in genital herpes through oral–genital contact. Genital herpes is commonly caused by the sexually transmitted HSV-2 virus, which can also infect the oral region [3,4]. Genital sores, often associated with HSV-2, can heighten the risk of both transmitting and acquiring other sexually transmitted infections, including the human immunodeficiency virus (HIV) [5,6]. Both types of HSV pose a risk of herpes disease in newborns and infants, often resulting in severe outcomes and substantial rates of mortality and morbidity [7].
From an epidemiological standpoint, the World Health Organization (WHO) approximates that globally, 3.7 billion individuals are affected by HSV-1, while 491 million are afflicted with HSV-2 [8]. HSV infection is predominantly contracted during childhood and is commonly transmitted through direct contact with an infected individual. Symptoms often go unnoticed, manifesting as asymptomatic viral shedding. Following the initial outbreak, infected persons may experience prodromal signs such as skin tingling, itching, or burning before the emergence of blisters. Given the persistent nature of HSV as a lifelong virus, multiple outbreaks can occur, with symptoms surfacing particularly when the immune system is compromised [9,10,11,12].
HSV is commonly managed with FDA-approved drugs, such as acyclovir and its derivatives. These medications function by inhibiting HSV DNA polymerase, a key enzyme in the replication process [13,14]. While effective in alleviating symptoms and shortening outbreaks, their overuse has led to drug resistance, compromising the overall efficacy of treatment. Additionally, HSV’s ability to establish latent infections in host cells adds complexity to antiviral approaches [15,16]. In the realm of anti-HSV research, scientists are actively seeking effective and natural remedies to counter HSV diseases. This quest has propelled investigations into the potential of flavonoids, derived from plants, as promising candidates [17,18].
Incorporating insights from recent studies, this review critically examines these compounds, evaluating their effectiveness in targeting various stages of the HSV life cycle. The ensuing comprehensive analysis within this review sheds light on their promising role in the ongoing battle against HSV infections.
We conducted an intensive literature search utilizing major online databases, including Web of Science Core Collection, Scopus, PubMed, SciFinder, ScienceDirect, Google Scholar, and ClinicalTrials.gov. Our search strategy involved employing specific keywords related to flavonoids with documented anti-HSV properties and elucidated mechanisms that target the viral life cycle. The collected data were sourced from studies published during the period spanning 2018 to 2023. To facilitate a thorough and nuanced evaluation and analysis, we have integrated select studies published before 2018.
2. A Brief Overview of the HSV Life Cycle
The HSV life cycle is a complex and dynamic process involving multiple stages. It typically commences with viral attachment and entry into host cells, specifically epithelial cells near mucous membranes or the skin [19,20,21]. This process is facilitated via viral glycoproteins interacting with cell surface receptors. Once inside the host cell, the virus releases its genetic material, consisting of double-stranded DNA, into the nucleus [22,23,24]. Subsequently, viral genes are transcribed and translated to produce new viral particles. The assembly of these particles takes place in the host cell’s cytoplasm, and mature virions are then transported to the cell membrane for release [25,26,27]. The virus can establish both lytic and latent infections. In a lytic infection, new virions are produced, leading to cell lysis and the release of viral progeny [28,29]. In contrast, during latent infection, the virus establishes a presence in sensory neurons, where the viral genome persists without active replication. Periodically, the virus may reactivate, leading to recurrent infections and the shedding of infectious particles [30,31]. Reactivation of the virus often occurs due to immunosuppression induced by various physiological and environmental influences that adversely affect the immune system [32,33]. The HSV life cycle is tightly regulated, involving intricate interactions between the virus and the cellular machinery of the host [34,35]. The ability of HSV to switch between lytic (active replication) and latent phases contributes to its persistence in the host. This complex life cycle provides multiple potential targets for antiviral interventions aimed at disrupting viral entry, replication, assembly, or release [36,37].
4. Blocking HSV-1 Infection via Flavonoids
The promising advances in utilizing flavonoids against HSV-1 over the past five years have been substantiated through a multitude of laboratory and animal investigations. Among these studies, two methoxyflavones, namely 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (PMF) and 5-hydroxy-3,6,7,3′,4′-pentamethoxyflavone (PMF-OH), isolated from Marcetia taxifolia, were found to significantly inhibit HSV-1 activity by diminishing viral DNA replication [54].
Morusin, a prenylated flavone extracted from the young twig of Morus alba L. (Mori ramulus), inhibits HSV-1 DNA replication by targeting the synthesis of HSV-1 glycoprotein D (gD) and suppressing reactive oxygen species (ROS) induced by HSV-1. These dual mechanisms highlight its potential as a promising antiviral agent [55].
In a related experiment involving Morus alba L., Čulenová and coworkers [56] identified three prenylated flavonoids: kuwanon C, kuwanon T (flavones), and kuwanon U (flavanone). These compounds effectively impede HSV-1 multiplication, with molecular docking suggesting interference with HSV-1 DNA polymerase. A preliminary structure–activity relationship study features the potent efficacy of kuwanon T, attributed to its two prenyl units.
Wogonin, an active flavone from Scutellaria baicalensis Georgi, demonstrates a potent anti-HSV-1 effect in vitro. Its mechanisms include suppressing DNA replication, glycoprotein D (gD) mRNA transcription, and immediate-early (IE) gene expression [57].
In another research study using Scutellaria baicalensis Georgi, the flavone baicalein has proven effective in blocking HSV-1 replication, including acyclovir-resistant strains. Demonstrating efficacy in various models, it reduces viral loads, inflammation, and mortality in mice. Its dual mechanism, encompassing both viral particle inactivation and the inhibition of IκB kinase beta (IKK-β) phosphorylation, underscores its potential as a promising antiviral candidate against HSV-1 and its resistant forms [58].
Vitexin, a bioactive flavone from Erythrina speciosa, displays anti-HSV-1 activity by targeting the HSV-1 DNA polymerase, as revealed in laboratory tests and molecular docking analysis [59].
Luteolin, a natural flavone, efficiently combats drug-resistant strains and hinders early HSV-1 infection, showcasing potent antiviral properties. In both in vitro and in vivo experiments, it proved highly effective against herpes encephalitis (HSE). By activating the cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS)/stimulator of the interferon gene (STING) pathway, luteolin enhances interferon production, thereby impeding HSV-1 post-entry. Its dual impact involves blocking viral entry and bolstering the immune response [60].
Amentoflavone, a biflavonoid identified in Ginkgo biloba L., Biophytum sensitivum, and various Garcinia species, showcases robust antiviral efficacy against a spectrum of HSV-1 strains, including those exhibiting resistance. It adeptly disrupts the initial phases of HSV-1 infection through multifaceted mechanisms of action [61].
In a thorough investigation, myricetin, a dietary flavonol inherent in diverse vegetables and fruits, demonstrated significant antiviral capability against HSV-1 through multiple molecular mechanisms [62].
Yarmolinsky and colleagues [63] successfully isolated two flavonols, namely quercetin 3-O-rutinoside and quercetin 3-O-arabinoside, from Phlomis viscosa Poiret. Both compounds exhibit suppressive effects on HSV-1 infection, effectively restraining viral replication by diminishing the formation of viral plaques.
In another study, quercetin 3-O-rutinoside and kaempferol 3-O-rutinoside, both flavonols derived from Lespedeza bicolor, displayed their ability to combat HSV-1 through virucidal effects. Additionally, they induced efficacy in blocking viral infection by hampering the replication of viral DNA [64].
Another research team explored kaempferol-3-O-rhamnoside’s potential in treating HSE through a cell culture and mouse model. Their findings indicate that this flavonol reduces inflammation, inhibits viral-induced brain injury, and alleviates brain tissue damage in mice. It emerges as a promising therapeutic candidate for HSV-1-induced brain injury [65].
The flavonol isorhamnetin, present in Ginkgo biloba, significantly disrupted the initial infection of HSV-1 by hindering viral DNA replication [66].
Dihydromyricetin, alternatively recognized as ampelopsin, is a dihydroflavonol derived from Ampelopsis grossedentata. It effectively suppresses HSV-1 through diverse pathways, as supported by virological and biochemical analyses [67].
Biochanin A (BCA), an isoflavone extracted from Trifolium pratense L., displays significant anti-HSV-1 efficacy by inhibiting viral replication in vitro. In mouse models mimicking herpes simplex keratitis (HSK), the administration of BCA through eye drops not only reduces ocular lesions but also effectively suppresses HSV-1. These observations emphasize the potential therapeutic utility of BCA in the context of HSK treatment [68].
In an experiment exploring the impact of temperature on treating HSV-1 infection, the main catechin in Camellia sinensis, epigallocatechin gallate (EGCG), showed notable suppression of HSV-1 virions between 25 and 37 °C. While the study refrained from specifying the precise mechanism of action, the authors proposed that EGCG might disrupt various steps in the HSV-1 life cycle, drawing on prior research [69]. Meanwhile, a different research group studied EGCG’s effects on HSV-1 infection in oral epithelial cells, revealing confirmed mechanisms of action [70].
Wang et al. [71] illuminated the inhibitory potency of isoliquiritigenin, a chalcone-type compound, against HSV-1 replication. They revealed that the antiviral mechanism linked to isoliquiritigenin correlates with its agonistic impact on nuclear factor erythroid 2-related factor 2 (NRF2).
Vicente and colleagues [72] explored how cyanidin, an anthocyanin-type substance found in various berries, actively hinders HSV-1. They observed that this compound impedes viral adsorption and DNA replication, thereby blocking viral infection.
The anthocyanin delphinidin-3-glucoside chloride, also known as myrtillin, found in Ribes nigrum L. and Vaccinium myrtillus L., demonstrates anti-infectivity properties against HSV-1 by targeting viral DNA replication [73].
In a dual in vitro and in vivo study, the total flavonoids obtained from Robinia pseudoacacia cv. idaho impeded viral DNA replication in vitro, showing significant anti-HSV-1 activity. Additionally, researchers observed no adverse effects during the in vivo phase, affirming the safety of its potential practical applications [74].
In an in vivo experiment, the administration of total flavonoids from Ixeris sonchifolia (Bae.) Hance to mice with HSK significantly improved corneal lesions, reduced infection, and increased survival rates, underscoring its therapeutic potential [75].
To sum up, the examined flavonoids, as discussed above, play a crucial role in interacting with different phases of the HSV-1 life cycle. They obstruct viral attachment, entry into target cells, DNA replication, and the expression of various viral genes and proteins. Consequently, they impede the virus’s capacity to infect, concurrently influencing essential cellular pathways integral to the viral life cycle. Table 1 details the anti-HSV-1 activities and mechanisms of flavonoids, complemented by Figure 1, which elucidates their chemical structures.

Table 1.
Flavonoids and their mechanisms in combatting the HSV-1 life cycle.
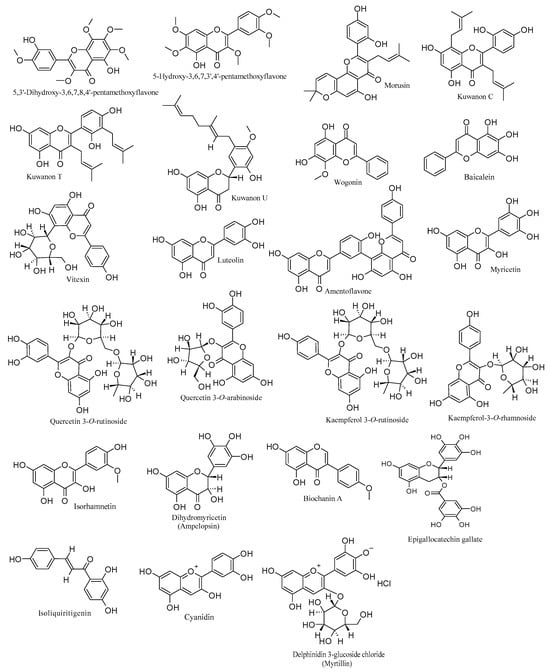
Figure 1.
Chemical structures of flavonoids with anti-HSV-1 properties.
5. Blocking HSV-2 Infection via Flavonoids
In the last five years, there has been limited progress in investigating flavonoids in the context of HSV-2. Nevertheless, there is a clear emphasis on conducting experiments to delve into the mechanisms of action, reflecting a concentrated effort to understand how flavonoids may interact with the HSV-2 life cycle. In a laboratory setting, the flavone wogonin from Scutellaria baicalensis demonstrated significant inhibition of HSV-2 entry into Vero cells during the post-entry stage, resulting in a notable reduction in viral replication. Its mode of action has been clarified as targeting IE genes and gD expressions, as well as regulating cellular NF-κB and JNK/p38 MAPK pathways [57].
Flavones, specifically apigenin and luteolin, extracted from Arisaema tortuosum, were identified as inhibitors of HSV-2 replication, showing a reduction in viral progeny production [76].
The anti-HSV-2 activity of the flavonol myricetin was revealed through mechanisms targeting virus adsorption, membrane fusion, and DNA replication. Additionally, it affects several cellular pathways [62].
Isorhamnetin, a flavonol derived from Ginkgo biloba, markedly inactivated the primary infection of HSV-2 by inhibiting the replication of viral DNA [66].
The flavanone kuwanon E, extracted from Morus alba, exhibits the capacity to suppress HSV-2 replication in infected Vero cells. This antiviral effect is attributed to its interaction with the HSV-2 protease, as predicted with a molecular docking approach. Furthermore, a structure–activity relationship study associates its activity with the presence of a hydroxyl group at C-4′ [56].
Stamos et al. [77] revealed the antiherpetic efficacy of EGCG, derived from Camellia sinensis, against HSV-2. This compound impressively inhibits viral replication (99.9% inhibition), effectively impeding HSV-2 attachment to Vero cells by hindering glycoprotein D expression.
To recapitulate, flavonoids such as wogonin, apigenin, luteolin, myricetin, isorhamnetin, and kuwanon E exert notable anti-HSV-2 effects by targeting multiple stages of the viral life cycle and different cellular pathways. Additionally, EGCG stands out for its striking inhibition of HSV-2 replication. These findings emphasize the diverse and promising potential of flavonoids as effective anti-HSV-2 agents, acting through multifaceted mechanisms that involve interference with viral processes and modulation of cellular pathways. Table 2 outlines the anti-HSV-2 mechanisms of flavonoids, while Figure 2 highlights their respective chemical structures.

Table 2.
Flavonoids and their mechanisms in targeting the HSV-2 life cycle.
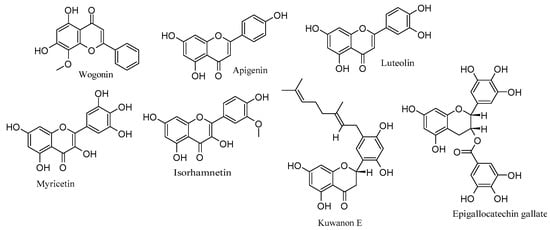
Figure 2.
Chemical structures of flavonoids with anti-HSV-2 properties.
6. Flavonoid-Enhanced Approaches to Elevate HSV Therapy
6.1. Nanoparticles and Gel-Formulation-Based Strategies
Incorporating flavonoids into nanoparticles enhances drug delivery to HSV-affected cells by improving stability and bioavailability [78,79]. The nanoparticles boost drug solubility, prolong circulation time, and target specific cells, ensuring a focused approach that minimizes potential side effects in treating HSV diseases [80,81]. Elste et al. [82] conducted a study illustrating the potent antiviral capacity of plant cell-engineered gold nanoparticles conjugated with quercetin (pAuNPsQ) against HSV-1. These formulated nanoparticles effectively inhibit HSV-1 entry and replication through various mechanisms. Remarkably, pAuNPsQ shows promising outcomes in both pre-treating target cells and inducing virus neutralization. These findings underscore the potential of modifying plant cell-based nanomaterials with quercetin to develop cutting-edge antiviral formulations.
Another investigation explores EGCG-modified silver nanoparticles (EGCG-AgNPs) as a potential therapeutic intervention for herpes infections. These modified nanoparticles exhibit enhanced inhibition of the attachment and entry of both HSV-1 and HSV-2 in human keratinocytes compared to EGCG alone. In mouse models, EGCG-AgNPs significantly reduce virus titers and elicit a robust immune response in mucosal tissues, characterized by increased cell infiltration and elevated expression of key immune markers. These outcomes suggest EGCG-AgNPs as a promising dual-functioning antiviral intervention for mucosal applications [83].
Caldas Dos Santos et al. [84] harnessed C-glycosylflavonoids from Cecropia glaziovii, encapsulating them in PLGA nanoparticles. This formulation achieved 100% inhibition of HSV-1 replication with an IC50 value of 8.2 µg/mL, exhibiting no cytotoxicity on Vero cells. The study indicates the promising application of this preparation for effective HSV-1 infection therapy.
Gel-formulated polyphenols, including flavonoids, offer enhanced stability and controlled release, optimizing the delivery of antiviral compounds for the effective and targeted treatment of HSV infectivity [85]. Building on these advantages, an investigation into quercetin-loaded gels for treating HSV-1 revealed encouraging results. The poloxamer-based gel showed promise by efficiently restraining quercetin diffusion, exhibiting higher stability, and demonstrating a prolonged virucidal impact against the virus. These findings feature the potential of quercetin in a poloxamer-based gel as a favorable and precise treatment option for HSV-1 infection [85].
In the research conducted by Dickinson et al. [86], the PTV80 hand gel prototype, featuring EGCG-palmitate (EC16), revealed an efficacy exceeding 99.9% in reducing HSV-1 infectivity within a 60 s timeframe. These results highlight a robust and rapid virucidal effect against the virus, supporting the potential application of the hand gel in hand hygiene products to mitigate and manage outbreaks associated with this virus. The formulation’s non-toxic properties, as identified in the study, further enhance its suitability for widespread use in promoting hand hygiene and preventing infection transmission.
6.2. Physical Properties Targeting Approach
The regulation of herpesvirus infection is intricately tied to its molecular and physical properties. HSV medications, including acyclovir and its analogs, primarily target the molecular aspects of the virus, specifically focusing on viral proteins such as DNA polymerase [87,88]. Researchers have unveiled an innovative treatment targeting the physical properties of the HSV. This cutting-edge biophysical strategy adeptly mitigates the virus’s genome pressure, ensuring efficacy without compromising host cells. Functioning at an internal pressure of 20 atmospheres, the herpesvirus rapidly delivers genetic materials into the host cell nucleus upon entry. By finely adjusting this viral pressure, scientists have successfully hindered the virus’s propagation to other cells, thereby amplifying the effectiveness of antiherpetic drugs [89]. Considering this breakthrough, the synergistic potential between this strategy and anti-HSV drugs, including flavonoids, not only opens new possibilities for addressing HSV infections but also paves the way for a more nuanced understanding of antiviral approaches.
6.3. Combination Therapies
In recent years, scientific inquiry into the antiviral potential of flavonoids has expanded, shedding light on their synergistic effects when combined with conventional antiviral drugs. These combination therapies hold significant promise for enhancing efficacy and addressing some of the challenges associated with treating HSV infections, including drug resistance [48,90]. Wu and colleagues [70] investigated the combined impact of EGCG at 25 µg/mL and acyclovir at 50 µg/mL on HSV-1 infection in oral epithelial cells. Their findings revealed a significant inhibitory effect on HSV-1 replication, leading to a reduction in intracellular viral DNA at 20 h post-infection. Furthermore, the combined treatment repressed the expression of viral proteins ICP5 and gD, highlighting its potent antiviral efficacy. In a related study, the combined effects of wogonin and acyclovir in combating HSV-2 were explored using an in-cell western assay. The results indicated a moderate synergism (combination index (CI) = 0.8), suggesting that the combination could offer enhanced therapeutic benefits for HSV-2 treatment [57].
7. Clinical Studies
Over the last five years, challenges in studying flavonoids in clinical trials have been acknowledged, primarily due to constraints associated with conducting comprehensive investigations. However, a recent trial involving 68 individuals with oral herpes revealed promising results for the herbal blend Gene-Eden-VIR/Novirin. Enriched with quercetin (100 mg), the blend was administered daily for 2 to 36 months alongside standard protocols, significantly reducing the frequency and duration of outbreaks. Notably, it proved safer and more effective than traditional acyclovir and valacyclovir approaches [91].
In a preceding clinical study, the utilization of Gene-Eden-VIR/Novirin, containing 100 mg of quercetin, effectively diminished genital herpes infections in 90.8% of 139 participants over 2–48 months. The product exhibited superior efficacy and safety compared to standard antiviral drugs (acyclovir, valacyclovir, and famciclovir). These findings affirm that the product presents a favorable and well-tolerated choice for addressing genital herpes outbreaks, outperforming conventional treatments in clinical efficacy [92]. Moreover, this herbal product consistently showcased efficacy in curing both severe and mild cases of genital herpes infections, validated by a clinical study with an 87% success rate among 137 participants over 2–48 months [93].
8. Conclusions, Challenges, and Future Prospects
In conclusion, this review points out the robust potential of flavonoids as anti-HSV agents, emphasizing their antiviral efficacy across various stages of the viral life cycle. A synergy of in vitro, in vivo, and computational studies adds depth to the evidence supporting flavonoids’ multifaceted anti-HSV mechanism. By disrupting key molecular processes crucial for HSV propagation, flavonoids exhibit versatility in interfering with viral attachment, penetration, and replication. Additionally, they engage with critical cellular pathways integral to the viral life cycle, simultaneously boosting the immune system. Furthermore, their diverse structures, combined with the selective targeting of specific viral genes and proteins, position them as a prospective foundation for the development of novel anti-HSV drugs. This review also features strategies involving flavonoids to enhance HSV treatment.
However, the journey from promising research to mainstream anti-HSV treatments encounters intricate challenges. Limited bioavailability, attributed to issues such as poor solubility and rapid metabolism, complicates their efficacy. Achieving specificity to target HSV strains without adversely affecting host cells requires careful molecular design. Formulating flavonoids into stable delivery systems remains challenging, demanding innovations for enhanced solubility and controlled release. The structural complexity of flavonoids poses hurdles in synthesis and large-scale production, accompanied by cost implications. Concerns about viral resistance and the evolving HSV strains necessitate ongoing research. To overcome these challenges, future research should focus on improving bioavailability through novel delivery systems, conducting comprehensive structure-activity relationship studies, exploring combination therapies, and advancing genomic and proteomic approaches. Additionally, rigorous clinical trials and translational research are essential for bridging the gap between preclinical promise and practical applications, establishing flavonoids as a viable antiviral therapeutic option.
Author Contributions
Conceptualization, S.T.S.H.; writing—original draft preparation, M.Š. and S.T.S.H.; writing—review and editing, M.Š. and S.T.S.H.; supervision, S.T.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the manuscript encompass all relevant information.
Acknowledgments
The authors acknowledge their institutions for supplying the essential materials and granting access to the subscribed databases needed for conducting the literature search.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tognarelli, E.I.; Palomino, T.F.; Corrales, N.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Herpes Simplex Virus Evasion of Early Host Antiviral Responses. Front. Cell. Infect. Microbiol. 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Omarova, S.; Cannon, A.; Weiss, W.; Bruccoleri, A.; Puccio, J. Genital Herpes Simplex Virus—An Updated Review. Adv. Pediatr. 2022, 69, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Petti, S.; Lodi, G. The Controversial Natural History of Oral Herpes Simplex Virus Type 1 Infection. Oral Dis. 2019, 25, 1850–1865. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, D.M.; Sousa, J.D.; Libin, P.J.K.; Delva, W.; Liesenborgs, J.; Hens, N.; Müller, V.; Vandamme, A.-M. Comparison of Two Simulators for Individual Based Models in HIV Epidemiology in a Population with HSV 2 in Yaoundé (Cameroon). Sci. Rep. 2021, 11, 14696. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.V.; Kulkarni, S.S. Herpes Simplex Virus: The Interplay Between HSV, Host, and HIV-1. Viral Immunol. 2015, 28, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Pinninti, S.G.; Kimberlin, D.W. Neonatal Herpes Simplex Virus Infections. Semin. Perinatol. 2018, 42, 168–175. [Google Scholar] [CrossRef]
- Herpes Simplex Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 5 November 2023).
- Alareeki, A.; Osman, A.M.M.; Khandakji, M.N.; Looker, K.J.; Harfouche, M.; Abu-Raddad, L.J. Epidemiology of Herpes Simplex Virus Type 2 in Europe: Systematic Review, Meta-Analyses, and Meta-Regressions. Lancet Reg. Health—Eur. 2023, 25, 100558. [Google Scholar] [CrossRef]
- Samies, N.L.; James, S.H. Prevention and Treatment of Neonatal Herpes Simplex Virus Infection. Antiviral Res. 2020, 176, 104721. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schwartz, R.A. Human Herpes Simplex Virus Infections: Epidemiology, Pathogenesis, Symptomatology, Diagnosis, and Management. J. Am. Acad. Dermatol. 2007, 57, 737–763, quiz 764–766. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Orzalli, M.H.; Knipe, D.M. Innate Immune Mechanisms and Herpes Simplex Virus Infection and Disease. Adv. Anat. Embryol. Cell Biol. 2017, 223, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.L.; James, S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018, 40, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir Resistance in Herpes Simplex Viruses: Prevalence and Therapeutic Alternatives. Biochem. Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules 2019, 24, 2912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-C.; Feng, H.; Lin, Y.-C.; Guo, X.-R. New Strategies against Drug Resistance to Herpes Simplex Virus. Int. J. Oral Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruchawapol, C.; Yuan, M.; Wang, S.-M.; Fu, W.-W.; Xu, H.-X. Natural Products and Their Derivatives against Human Herpesvirus Infection. Molecules 2021, 26, 6290. [Google Scholar] [CrossRef]
- Cairns, T.M.; Connolly, S.A. Entry of Alphaherpesviruses. Curr. Issues Mol. Biol. 2021, 41, 63–124. [Google Scholar] [CrossRef]
- Agelidis, A.M.; Shukla, D. Cell Entry Mechanisms of HSV: What We Have Learned in Recent Years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Azab, W.; Osterrieder, K. Initial Contact: The First Steps in Herpesvirus Entry. Adv. Anat. Embryol. Cell Biol. 2017, 223, 1–27. [Google Scholar] [CrossRef]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The Structural Basis of Herpesvirus Entry. Nat. Rev. Microbiol. 2021, 19, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Arii, J.; Kawaguchi, Y. The Role of HSV Glycoproteins in Mediating Cell Entry. Adv. Exp. Med. Biol. 2018, 1045, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Heming, J.D.; Conway, J.F.; Homa, F.L. Herpesvirus Capsid Assembly and DNA Packaging. Adv. Anat. Embryol. Cell Biol. 2017, 223, 119–142. [Google Scholar] [CrossRef]
- Adlakha, M.; Livingston, C.M.; Bezsonova, I.; Weller, S.K. The Herpes Simplex Virus 1 Immediate Early Protein ICP22 Is a Functional Mimic of a Cellular J Protein. J. Virol. 2020, 94, e01564-19. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; Sattler, C.; Adler, H. Herpesviruses and Their Host Cells: A Successful Liaison. Trends Microbiol. 2017, 25, 229–241. [Google Scholar] [CrossRef]
- Krawczyk, E.; Kangas, C.; He, B. HSV Replication: Triggering and Repressing STING Functionality. Viruses 2023, 15, 226. [Google Scholar] [CrossRef]
- Rice, S.A. Release of HSV-1 Cell-Free Virions: Mechanisms, Regulation, and Likely Role in Human-Human Transmission. Viruses 2021, 13, 2395. [Google Scholar] [CrossRef]
- Cohen, J.I. Herpesvirus Latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef]
- Lomonte, P. Herpesvirus Latency: On the Importance of Positioning Oneself. Adv. Anat. Embryol. Cell Biol. 2017, 223, 95–117. [Google Scholar] [CrossRef]
- Ostler, J.B.; Sawant, L.; Harrison, K.; Jones, C. Regulation of Neurotropic Herpesvirus Productive Infection and Latency-Reactivation Cycle by Glucocorticoid Receptor and Stress-Induced Transcription Factors. Vitam. Horm. 2021, 117, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Reese, T.A. Coinfections: Another Variable in the Herpesvirus Latency-Reactivation Dynamic. J. Virol. 2016, 90, 5534–5537. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.S.; Jones, C. Regulation of Herpes Simplex Virus Type 1 Latency-Reactivation Cycle and Ocular Disease by Cellular Signaling Pathways. Exp. Eye Res. 2022, 218, 109017. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Sharma-Walia, N. Targeting Host Cellular Factors as a Strategy of Therapeutic Intervention for Herpesvirus Infections. Front. Cell. Infect. Microbiol. 2021, 11, 603309. [Google Scholar] [CrossRef] [PubMed]
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human Herpes Simplex Virus: Life Cycle and Development of Inhibitors. Biochemistry 2014, 79, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Packard, J.E.; Dembowski, J.A. HSV-1 DNA Replication-Coordinated Regulation by Viral and Cellular Factors. Viruses 2021, 13, 2015. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, Bioactivity, and Synthesis of Methylated Flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, Metabolism and Bioavailability of Flavonoids: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-Function and Mechanisms of Action and Opportunities for Drug Development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids--Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and Bioavailability: An Update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M. Molecular Mechanisms of Flavonoids against Tumor Gamma-Herpesviruses and Their Correlated Cancers—A Focus on EBV and KSHV Life Cycles and Carcinogenesis. Int. J. Mol. Sci. 2022, 24, 247. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A.; Malik, A.K. Flavonoids Biosynthesis in Plants and Its Further Analysis by Capillary Electrophoresis. Electrophoresis 2017, 38, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and Their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Berchová-Bímová, K.; Mazurakova, A.; Šamec, D.; Kubatka, P.; Hassan, S.T.S. Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights. Viruses 2022, 14, 592. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of Flavonoids against Coronavirus Infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Sharma, V.; Sehrawat, N.; Sharma, A.; Yadav, M.; Verma, P.; Sharma, A.K. Multifaceted Antiviral Therapeutic Potential of Dietary Flavonoids: Emerging Trends and Future Perspectives. Biotechnol. Appl. Biochem. 2021, 69, 2028–2045. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Masarčíková, R.; Berchová, K. Bioactive Natural Products with Anti-Herpes Simplex Virus Properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising Natural Compounds against Viral Infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.T.; Serrano, M.L.; Suárez, A.I.; Baptista, J.; Pujol, F.H.; Cavallaro, L.V.; Campos, H.R.; Rangel, H.R. Antiviral Activity of Flavonoids Present in Aerial Parts of Marcetia Taxifolia against Hepatitis B Virus, Poliovirus, and Herpes Simplex Virus in Vitro. EXCLI J. 2019, 18, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Kwon, E.-B.; Oh, Y.-C.; Go, Y.; Choi, J.-G. Mori Ramulus and Its Major Component Morusin Inhibit Herpes Simplex Virus Type 1 Replication and the Virus-Induced Reactive Oxygen Species. Am. J. Chin. Med. 2021, 49, 163–179. [Google Scholar] [CrossRef]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In Vitro Biological Effects of Phenolic Compounds from Morus alba Root Bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef]
- Chu, Y.; Lv, X.; Zhang, L.; Fu, X.; Song, S.; Su, A.; Chen, D.; Xu, L.; Wang, Y.; Wu, Z.; et al. Wogonin Inhibits in Vitro Herpes Simplex Virus Type 1 and 2 Infection by Modulating Cellular NF-κB and MAPK Pathways. BMC Microbiol. 2020, 20, 227. [Google Scholar] [CrossRef]
- Luo, Z.; Kuang, X.-P.; Zhou, Q.-Q.; Yan, C.-Y.; Li, W.; Gong, H.-B.; Kurihara, H.; Li, W.-X.; Li, Y.-F.; He, R.-R. Inhibitory Effects of Baicalein against Herpes Simplex Virus Type 1. Acta Pharm. Sin. B 2020, 10, 2323–2338. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; Moghannem, S.; Azam, F.; El-Shazly, M.; Singab, A.N. Breaking Down the Barriers to a Natural Antiviral Agent: Antiviral Activity and Molecular Docking of Erythrina Speciosa Extract, Fractions, and the Major Compound. Chem. Biodivers. 2020, 17, e1900511. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Wang, Z.; Song, X.; Ren, Z.; Wang, X.; Wang, Y.; Zheng, K. Luteolin Inhibits Herpes Simplex Virus 1 Infection by Activating Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Mediated Antiviral Innate Immunity. Phytomedicine 2023, 120, 155020. [Google Scholar] [CrossRef]
- Li, F.; Song, X.; Su, G.; Wang, Y.; Wang, Z.; Jia, J.; Qing, S.; Huang, L.; Wang, Y.; Zheng, K.; et al. Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection. Viruses 2019, 11, 466. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Hao, C.; Zhang, Y.; Wang, Z.; Wang, S.; Wang, W. Inhibition of Herpes Simplex Virus by Myricetin through Targeting Viral gD Protein and Cellular EGFR/PI3K/Akt Pathway. Antivir. Res. 2020, 177, 104714. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Nakonechny, F.; Budovsky, A.; Zeigerman, H.; Khalfin, B.; Sharon, E.; Yarmolinsky, L.; Ben-Shabat, S.; Nisnevitch, M. Antimicrobial and Antiviral Compounds of Phlomis Viscosa Poiret. Biomedicines 2023, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Tarbeeva, D.V.; Krylova, N.V.; Iunikhina, O.V.; Likhatskaya, G.N.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Shchelkanov, M.Y.; Fedoreyev, S.A. Biologically Active Polyphenolic Compounds from Lespedeza bicolor. Fitoterapia 2022, 157, 105121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, F.; Tang, B.; Han, J.; Li, X.; Lian, G.; Li, X.; Hao, S. Anti-Inflammatory Effects of Kaempferol-3-O-Rhamnoside on HSV-1 Encephalitis in Vivo and in Vitro. Neurosci. Lett. 2021, 765, 136172. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Sobczyński, M.; Ochnik, M.; Zwolińska, K.; Leszek, J. Hampering Herpesviruses HHV-1 and HHV-2 Infection by Extract of Ginkgo Biloba (EGb) and Its Phytochemical Constituents. Front. Microbiol. 2019, 10, 2367. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Gao, S.-Q.; Gong, Y.-S.; Lin, T.; Tong, S.; Xiong, W.; Shi, C.-Y.; Wang, W.-Q.; Fang, J.-G. Anti-HSV-1 Effect of Dihydromyricetin from Ampelopsis grossedentata via the TLR9-Dependent Anti-Inflammatory Pathway. J. Glob. Antimicrob. Resist. 2020, 23, 370–376. [Google Scholar] [CrossRef]
- Zhou, N.; Zheng, D.; You, Q.; Chen, T.; Jiang, J.; Shen, W.; Zhang, D.; Liu, J.; Chen, D.; Hu, K. Therapeutic Potential of Biochanin A in Herpes Simplex Keratitis. Pharmaceuticals 2023, 16, 1240. [Google Scholar] [CrossRef]
- Pradhan, P.; Nguyen, M.L. Herpes Simplex Virus Virucidal Activity of MST-312 and Epigallocatechin Gallate. Virus Res. 2018, 249, 93–98. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yu, Z.-Y.; Chen, Y.-C.; Hung, S.-L. Effects of Epigallocatechin-3-Gallate and Acyclovir on Herpes Simplex Virus Type 1 Infection in Oral Epithelial Cells. J. Formos. Med. Assoc. 2021, 120, 2136–2143. [Google Scholar] [CrossRef]
- Wang, H.; Jia, X.; Zhang, M.; Cheng, C.; Liang, X.; Wang, X.; Xie, F.; Wang, J.; Yu, Y.; He, Y.; et al. Isoliquiritigenin Inhibits Virus Replication and Virus-Mediated Inflammation via NRF2 Signaling. Phytomedicine 2023, 114, 154786. [Google Scholar] [CrossRef]
- Vicente, J.; Benedetti, M.; Martelliti, P.; Vázquez, L.; Gentilini, M.V.; Peñaranda Figueredo, F.A.; Nabaes Jodar, M.S.; Viegas, M.; Barquero, A.A.; Bueno, C.A. The Flavonoid Cyanidin Shows Immunomodulatory and Broad-Spectrum Antiviral Properties, Including SARS-CoV-2. Viruses 2023, 15, 989. [Google Scholar] [CrossRef] [PubMed]
- Sivarajan, R.; Oberwinkler, H.; Roll, V.; König, E.-M.; Steinke, M.; Bodem, J. A Defined Anthocyanin Mixture Sourced from Bilberry and Black Currant Inhibits Measles Virus and Various Herpesviruses. BMC Complement. Med. Ther. 2022, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wan, X.; Niu, F.; Sun, J.; Shi, C.; Ye, J.M.; Zhou, C. Evaluation of Antiviral Effect and Toxicity of Total Flavonoids Extracted from Robinia pseudoacacia Cv. Idaho. Biomed. Pharmacother. 2019, 118, 109335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Cai, L.; Zhang, N.; Zhang, J.; Wang, H.-H.; Zhu, W. Protective Effect of Total Flavonoids from Ixeris Sonchifolia on Herpes Simplex Virus Keratitis in Mice. BMC Complement. Med. Ther. 2020, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Rittà, M.; Marengo, A.; Civra, A.; Lembo, D.; Cagliero, C.; Kant, K.; Lal, U.R.; Rubiolo, P.; Ghosh, M.; Donalisio, M. Antiviral Activity of a Arisaema tortuosum Leaf Extract and Some of Its Constituents against Herpes Simplex Virus Type 2. Planta Med. 2020, 86, 267–275. [Google Scholar] [CrossRef]
- Stamos, J.D.; Lee, L.H.; Taylor, C.; Elias, T.; Adams, S.D. In Vitro and In Silico Analysis of the Inhibitory Activity of EGCG-Stearate against Herpes Simplex Virus-2. Microorganisms 2022, 10, 1462. [Google Scholar] [CrossRef]
- Obisesan, O.; Katata-Seru, L.; Mufamadi, S.; Mufhandu, H. Applications of Nanoparticles for Herpes Simplex Virus (HSV) and Human Immunodeficiency Virus (HIV) Treatment. J. Biomed. Nanotechnol. 2021, 17, 793–808. [Google Scholar] [CrossRef]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Ranoszek-Soliwoda, K.; Bednarczyk, K.; Lech, A.; Janicka, M.; Chodkowski, M.; Psarski, M.; Celichowski, G.; Krzyzowska, M.; Grobelny, J. Anti-HSV Activity of Metallic Nanoparticles Functionalized with Sulfonates vs. Polyphenols. Int. J. Mol. Sci. 2022, 23, 13104. [Google Scholar] [CrossRef]
- Paradowska, E.; Studzińska, M.; Jabłońska, A.; Lozovski, V.; Rusinchuk, N.; Mukha, I.; Vitiuk, N.; Leśnikowski, Z.J. Antiviral Effect of Nonfunctionalized Gold Nanoparticles against Herpes Simplex Virus Type-1 (HSV-1) and Possible Contribution of Near-Field Interaction Mechanism. Molecules 2021, 26, 5960. [Google Scholar] [CrossRef]
- Elste, J.; Kumari, S.; Sharma, N.; Razo, E.P.; Azhar, E.; Gao, F.; Nunez, M.C.; Anwar, W.; Mitchell, J.C.; Tiwari, V.; et al. Plant Cell-Engineered Gold Nanoparticles Conjugated to Quercetin Inhibit SARS-CoV-2 and HSV-1 Entry. Int. J. Mol. Sci. 2023, 24, 14792. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Janicka, M.; Chodkowski, M.; Patrycy, M.; Obuch-Woszczatyńska, O.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; Grobelny, J. Epigallocatechin Gallate-Modified Silver Nanoparticles Show Antiviral Activity against Herpes Simplex Type 1 and 2. Viruses 2023, 15, 2024. [Google Scholar] [CrossRef]
- Caldas Dos Santos, T.; Rescignano, N.; Boff, L.; Reginatto, F.H.; Simões, C.M.O.; de Campos, A.M.; Mijangos, C. In Vitro Antiherpes Effect of C-Glycosyl Flavonoid Enriched Fraction of Cecropia glaziovii Encapsulated in PLGA Nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Sicurella, M.; Sguizzato, M.; Mariani, P.; Pepe, A.; Baldisserotto, A.; Buzzi, R.; Huang, N.; Simelière, F.; Burholt, S.; Marconi, P.; et al. Natural Polyphenol-Containing Gels against HSV-1 Infection: A Comparative Study. Nanomaterials 2022, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.; Marsh, B.; Shao, X.; Liu, E.; Sampath, L.; Yao, B.; Jiang, X.; Hsu, S. Virucidal Activities of Novel Hand Hygiene and Surface Disinfectant Formulations Containing EGCG-Palmitates (EC16). Am. J. Infect. Control 2022, 50, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Brandariz-Nuñez, A.; Liu, T.; Du, T.; Evilevitch, A. Pressure-Driven Release of Viral Genome into a Host Nucleus Is a Mechanism Leading to Herpes Infection. Elife 2019, 8, e47212. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.W.; Huffman, J.B.; Homa, F.L.; Evilevitch, A. Herpes Virus Genome, the Pressure Is on. J. Am. Chem. Soc. 2013, 135, 11216–11221. [Google Scholar] [CrossRef] [PubMed]
- Brandariz-Nuñez, A.; Robinson, S.J.; Evilevitch, A. Pressurized DNA State inside Herpes Capsids—A Novel Antiviral Target. PLoS Pathog. 2020, 16, e1008604. [Google Scholar] [CrossRef]
- Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. [Google Scholar] [CrossRef]
- Polansky, H.; Javaherian, A.; Itzkovitz, E. Clinical Trial of Herbal Treatment Gene-Eden-VIR/Novirin in Oral Herpes. J. Evid. Based Integr. Med. 2018, 23, 2515690X18806269. [Google Scholar] [CrossRef]
- Polansky, H.; Javaherian, A.; Itzkovitz, E. Clinical Study in Genital Herpes: Natural Gene-Eden-VIR/Novirin versus Acyclovir, Valacyclovir, and Famciclovir. Drug Des. Devel Ther. 2016, 10, 2713–2722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polansky, H.; Itzkovitz, E.; Javaherian, A. Clinical Study of Gene-Eden-VIR/Novirin in Genital Herpes: Suppressive Treatment Safely Decreases the Duration of Outbreaks in Both Severe and Mild Cases. Clin. Transl. Med. 2016, 5, 40. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).