Urdbean Leaf Crinkle Virus: A Mystery Waiting to Be Solved
Abstract
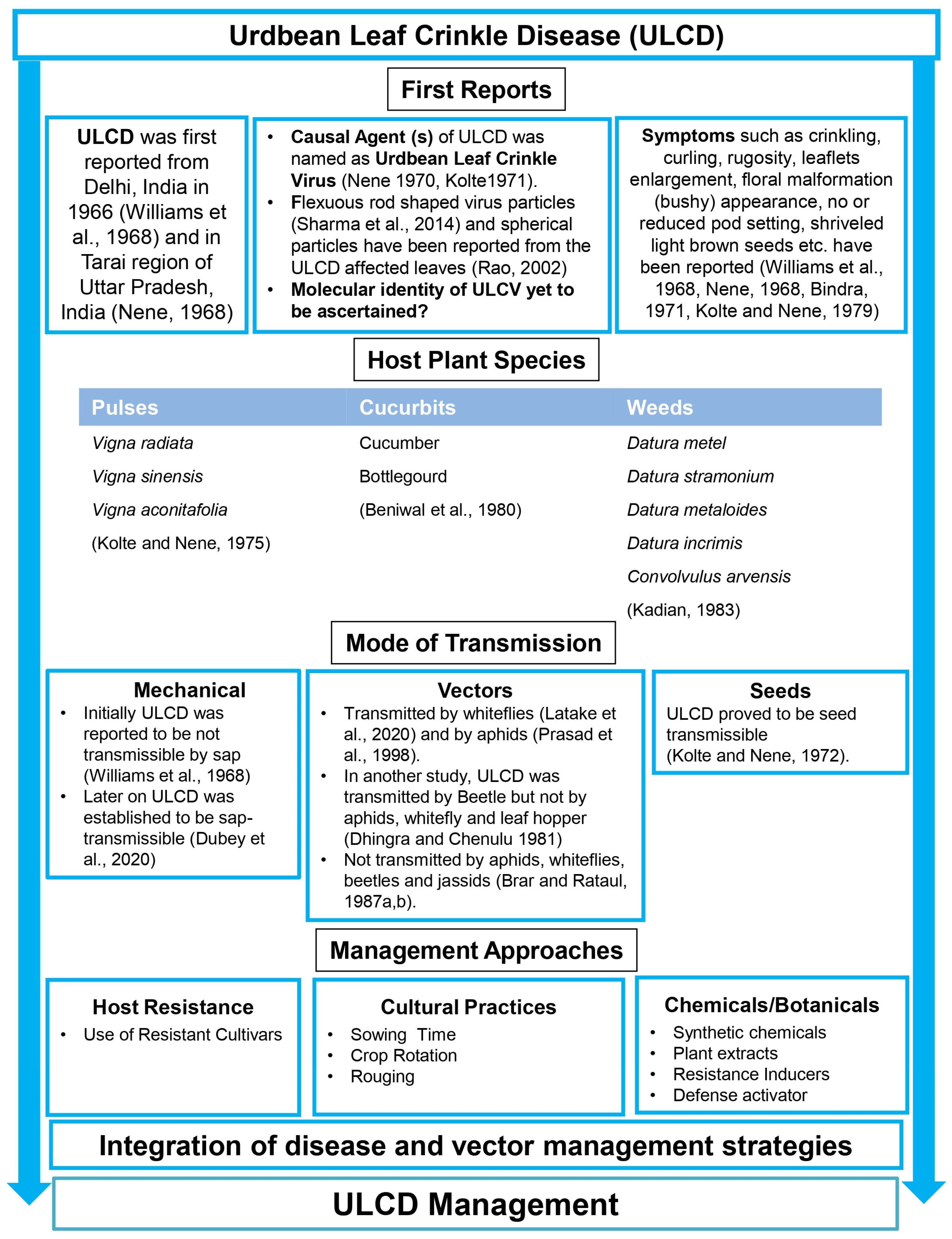
1. Introduction
2. Disease Symptoms
3. Yield Losses Due to ULCD
4. Epidemiology of ULCD
5. ULCD Transmission
5.1. Mechanical Sap Inoculation
5.2. Seed Transmission
5.3. Vector Transmission
6. Host Range
7. Resistance and Physiochemical Changes in Host Plant
8. Causal Organism—Urdbean Leaf Crinkle Virus
9. Management Options
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ICAR—Indian Institute of Pulses Research. All India Coordinated Research Project on Kharif Pulses; Project Coordinator Report; ICAR—Indian Institute of Pulses Research: Kanpur, India, 2022; 115p. [Google Scholar]
- Gupta, S.; Das, A.; Pratap, A.; Gupta, D.S. Urdbean. In The Beans and the Peas; Pratap, A., Gupta, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 33–54. ISBN 978-0-12-821450-3. [Google Scholar]
- Dasgupta, S.; Roy, I. Proceedings of the Regional Consultation on the Promotion of Pulses in Asia for Multiple Health Benefits, Bangkok, Thailand, 29–30 June 2015; RAP Publication (FAO) Eng No. 2015/05; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2016. [Google Scholar]
- Malathi, V.; John, P. Geminiviruses Infecting Legumes. In Characterization, Diagnosis & Management of Plant Viruses. Volume 3: Vegetable and Pulse Crops; Studium Press LLC: Huston, TX, USA, 2008; pp. 97–123. [Google Scholar]
- Ravinder Reddy, C.; Tonapi, V.A.; Varanavasiappan, S.; Navi, S.; Jayarajan, R. Influence of Plant Age on Infection and Symptomatological Studies on Urd Bean Leaf Crinkle Virus in Urd Bean (Vigna mungo). Int. J. Agric. Sci. 2005, 1, 1–6. [Google Scholar]
- Biswas, K.K.; Biswas, K.; Malathi, V.G.; Chattopadhyay, C. Evaluation of Urdbean Cultivars for Identification of Resistance to Leaf Crinkle Disease by Mechanical Sap Inoculation. Indian Phytopathol. 2012, 65, 416–417. [Google Scholar]
- Williams, F.; Grewal, J.; Amin, K. Serious and New Diseases of Pulse Crops in India in 1966. Plant Dis. Rep. 1968, 52, 300–304. [Google Scholar]
- Nene, Y.L. A Survey of Viral Disease of Pulse Crops in Uttar Pradesh; U.P. Agricultural University: Pantnagar, India, 1968; pp. 1–125. [Google Scholar]
- Kolte, S.; Nene, Y. Know the Leaf Crinkle Disease of Urd. Indian Farmers Dig. 1970, 3, 6–7. [Google Scholar]
- Nene, Y.L. A Survey of Viral Disease of Pulse Crops in Uttar Pradesh; U.P. Agricultural University: Pantnagar, India, 1970; pp. 1–39. [Google Scholar]
- Karthikeyan, A.; Akilan, M.; Samyuktha, S.M.; Ariharasutharsan, G.; Shobhana, V.G.; Veni, K.; Tamilzharasi, M.; Keerthivarman, K.; Sudha, M.; Pandiyan, M.; et al. Untangling the Physio-Chemical and Transcriptional Changes of Black Gram Cultivars After Infection with Urdbean Leaf Crinkle Virus. Front. Sustain. Food Syst. 2022, 6, 916795. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Curtis Hendrickson, R.; et al. Recent Changes to Virus Taxonomy Ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, S.; Chaubey, S.; Bharathan, N. Presence of Urdbean Leaf Crinkle Virus in Seeds of Mungbean Germplasm. Indian Phytopathol. 1980, 33, 360–361. [Google Scholar]
- Bindra, O. Studies on Arthropods in Relation to Plant Disease in Punjab. In Proceedings of the International Symposium Plant Pathology, IARI, New Delhi, India, 27 January–3 February 1971; pp. 20–22. [Google Scholar]
- Brar, J.S.; Rataul, H.S. Evidence against the Transmission of Urd Bean Leaf Crinkle Virus (ULCV) in Mash Bean, Vigna mungo (L) through Insects—Laboratory Studies. Indian J. Entomol. 1987, 49, 69–72. [Google Scholar]
- Brar, J.S.; Rataul, H.S. Evidence against the Transmission of Urd Bean Leaf Crinkle Virus (ULCV) in Mash, Vigna mungo (L) through Insects—A Field Approach. Indian J. Entomol. 1987, 49, 57–63. [Google Scholar]
- Dhingra, K.L.; Chenulu, V.V. Studies on the Transmission of Urdbean Leaf Crinkle and Chickpea Leaf Reduction Virus by Aphis Craccivora Koch. Indian Phytopathol. 1981, 34, 38–42. [Google Scholar]
- Dubey, A.K.; Singhal, P.; Dubey, S.K.; Nabi, S.U.; Yadav, M.K.; Saritha, R.K.; Baranwal, V.K. Effect of Stage of Mechanical Inoculation on Leaf Crinkle Disease Development in Urdbean (Vigna mungo L.) under Controlled Conditions. J. Pharmacogn. Phytochem. 2020, 9, 1136–1139. [Google Scholar]
- Kadian, O. Studies on Weed Plants as Host Range of Urdbean Leaf Crinkle Virus. Haryana Agric. Univ. J. Res. 1983, 13, 602–603. [Google Scholar]
- Kolte, S. Studies on the Leaf Crinkle Disease of Urdbean (Phaseolus mungo L.); U.P. Agricultural University: Pantnagar, India, 1971. [Google Scholar]
- Kolte, S.; Nene, Y. Urdbean (Vigna mungo) Leaf Crinkle Virus: Noteworthy Symptoms on Host and Influence of Growth Stages on Host Susceptibility. Trop. Grain Legume Bull. 1979, 15, 5–8. [Google Scholar]
- Kolte, S.; Nene, Y. Studies on Symptoms and Mode of Transmission of the Leaf Crinkle Virus of Urd Bean (Phaseolus mungo). Indian Phytopathol. 1972, 25, 401–404. [Google Scholar]
- Kolte, S.; Nene, Y. Host Range and Properties of Urd Bean Leaf Crinkle Virus. Indian Phytopathol. 1975, 28, 430–431. [Google Scholar]
- Latake, S.; Ranjale, S.; DA, T. Seed Transmission of Leaf Crinkle Virus in Urdbean and Identification of Resistant Genotypes to the Virus. Int. J. Recent Sci. Res. 2020, 11, 38002–38004. [Google Scholar]
- Prasad, M.; Sharma, B.; Kumar, S.; Prasad, M.; Kumar, S. Transmission Tests and Variety Screening for Urdbean Leaf Crinkle Virus in Black Gram (Vigna mungo L. Hepper). Ann. Plant Prot. Sci. 1998, 6, 205–207. [Google Scholar]
- Rao, N. Studies on Leaf Crinkle Disease of Blackgram (Vigna mungo (L.) Hepper); Acharya N. G. Ranga Agricultural University: Hyderabad, India, 2002. [Google Scholar]
- Sharma, P.; Sharma, A.; Sharma, O.; Sharma, S.; Garg, I. Association of an Unusual Filamentous Virus with Leaf Crinkle Disease of Urdbean in Himachal Pradesh. J. Mycol. Plant Pathol. 2014, 44, 257–263. [Google Scholar]
- Nene, Y. Viral Diseases of Some Warm Weather Pulse Crops in India. Plant Dis. Rep. 1973, 57, 463–467. [Google Scholar]
- Narayanasamy, P.; Jaganathan, T. Seed Transmission of Black Gram Leaf Crinkle Virus. Phytopathol. Z. 1975, 82, 107–110. [Google Scholar] [CrossRef]
- Khatri, H.; Bhatia, D.; Chohan, J. Brief Account of Work Done on Diseases of Kharif Pulse Crops at the Department of Botany and Plant Pathology, PAU Ludhiana during 1970–1971; Indian Council of Agricultural Research: New Delhi, India, 1971. [Google Scholar]
- Chowdhury, A.; Nath, P. Effect of Leaf Crinkle Virus on Nodule Characteristics of Urd Bean (Vigna mungo (L) Hepper). Indian J. Microbiol. 1983, 23, 224–225. [Google Scholar]
- Beniwal, S.; Chaubey, S. Urdbean Leaf Crinkle Virus: Effect on Yield Contributing Factors, Total Yield and Seed Characters of Urdbean (Vigna mungo). Seed Res. 1979, 7, 175–181. [Google Scholar]
- Binyamin, R.; Khan, M.A.; Ahmad, N.; Safdar, A. Relationship of Epidemiological Factors with Urdbean Leaf Crinkle Virus Disease and Its Management Using Plant Extracts. Int. J. Agric. Biol. 2011, 13, 411–414. [Google Scholar]
- Dubey, A.; Sinha, P.; Baranwal, V.; Mishra, S.; Saritha, R. Temperature Influence on Leaf Crinkle Disease Expression in Urdbean (Vigna mungo (L.) Hepper) and Potential Distribution of the Disease in India. Crop Prot. 2019, 120, 84–90. [Google Scholar] [CrossRef]
- Dubey, A.K.; Saritha, R.; Nabi, S.U.; Yadav, M.K.; Baranwal, V. Seed Transmission and Effect of Leaf Crinkle Disease on Seed Quality in Urdbean (Vigna mungo L. Hepper) under Controlled Environment. Indian Phytopathol. 2021, 74, 277–281. [Google Scholar] [CrossRef]
- Kanimozhi, S.; Ganapathy, T.; Rajinimala, N. Seed Transmission of ULCV in Mungbean and Urdbean Plants Infected with Both MYMV and ULCV. Arch. Phytopathol. Plant Prot. 2009, 42, 401–408. [Google Scholar] [CrossRef]
- Mandhare, V.; Suryawanshi, A.; Jamadagni, B. Leaf Crinkle Virus on Urdbean Seed Yield and Its Quality. Madras Agric. J. 2007, 94, 139–141. [Google Scholar]
- Priyanga, T.; Latha, T.; Teja, T.R.; Karthikeyan, G.; Prabakar, K. Urdbean Leaf Crinkle Diseaseassessment of Seed Transmissibility and Its Effect on Yield and Seed Quality in Urdbean [Vigna mungo (L.) Hepper]. Legume Res. Int. J. 2021, 1, 6. [Google Scholar]
- Sharma, P.; Sharma, A.; Singh, M. Effect of Leaf Crinkle Disease on Yield and Quality of Urdbean (Vigna mungo L. Hepper) in Himachal Pradesh. Himachal J. Agric. Res. 2015, 41, 80–82. [Google Scholar]
- Sravika, A.; Kennedy, J.; Rajabaskar, D.; Rajeswari, E. Transmission Studies of Leaf Crinkle Virus in Blackgram (Vigna mungo L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2514–2523. [Google Scholar] [CrossRef]
- Sivaprakasam, K.; Jagadeesan, M.; Kannapiran, C.; Pillayarsamy, K. Changes in the Mineral Content of Black Gram Leaves Due to Leaf Crinkle Virus. Madras Agric. J. 1976, 63, 123–125. [Google Scholar]
- Bhaktavatsalam, G.; Nene, Y.; Beniwal, S. Hyperauxiny in Urdbean Leaves Infected by Urdbean Leaf Crinkle Virus. Indian Phytopathol. 1982, 35, 683–685. [Google Scholar]
- Brar, J.; Rataul, H. Incidence and Extent of Losses Due to Leaf Crinkle Virus in Urdbean (Vigna mungo L.) Hepper. J. Res. Punjab Agric. Univ. 1989, 26, 65–70. [Google Scholar]
- Sharma, R.; Prasad, S.; Kudada, N. Leaf Crinkle Virus Disease in Urdbean (Vigna mungo Linn.). J. Res. Birsa Agric. Univ. 2007, 19, 73. [Google Scholar]
- Kadian, O. Yield Loss in Mung Bean and Urd Bean Due to Leaf Crinkle Disease. Indian Phytopathol. 1982, 35, 642–644. [Google Scholar]
- Subbarao, K. Studies on Leaf Crinkle Disease of Blackgram. Master’s Thesis, Acharya N. G. Ranga Agricultural University, Hyderabad, India, 1984. [Google Scholar]
- Bhagavan, V.B. Studies on the Effect of Leaf Crinkle Virus Infection on Growth and Physiology of Blackgram (Vigna mungo (L.) Hepper). Master’s Thesis, Acharya N. G. Ranga Agricultural University, Hyderabad, India, 1985. [Google Scholar]
- Singh, J. Effect of Virus Diseases on Growth Components and Yield of Mung Bean (Vigna radiata) and Urd Bean (Vigna mungo). Indian Phytopathol. 1980, 33, 405–408. [Google Scholar]
- Bashir, M.; Mughal, S.M.; Malik, B.A. Assessment of Yield Losses Due to Leaf Crinkle Virus in Urdbean, Vigna mungo (L.) Hepper. Pak. J. Bot. 1991, 23, 140–142. [Google Scholar]
- Kadian, O. Effect of Environment on Incidence and Development of Leaf Crinkle Disease in Urdbean. Indian Phytopathol 1989, 42, 272. [Google Scholar]
- Ashfaq, M.; Khan, M.A.; Javed, N. Characterization of Environmental Factors Conducive for Urdbean Leaf Crinkle (ULCV) Disease Development. Pak. J. Bot. 2008, 40, 2645–2653. [Google Scholar]
- Nene, Y.L. A Survey of Viral Disease of Pulse Crops in Uttar Pradesh; G.B. Pant University of Agriculture and Technology: Pantnagar, India, 1972; p. 191. [Google Scholar]
- Gupta, V. Leaf Crinkle, a Virus Disease of Phaseolus mungo L. in Himachal Pradesh. Indian J. Exp. Biol. 1974, 12, 477–478. [Google Scholar]
- Kadian, O. Mechanical and Seed Transmission of Urdbean Leaf Crinkle Virus (ULCV) in Haryana. Crop Res. 1994, 8, 565–569. [Google Scholar]
- Ahmad, Z.; Bashir, M.; Mtsueda, T. Evaluation of Legume Germplasm for Seed Borne Viruses in Harmonizing Agricultural Productivity and Conservation of Biodiversity. In Proceedings of the 8th SABRAO General Congress and the Annual Meeting of the Korean Breeding Society, Seoul, Republic of Korea, 24–28 September 1997; pp. 117–120. [Google Scholar]
- Sushma, M.; Reddy, K.B.; Madhavi, G.B.; Raghavendra, M.; Reddy, B.R.K. Mechanical Sap Transmission Studies of Urdbean Leaf Crinkle Disease Affected Genotypes. Int. J. Curr. Sci. 2023, 13, 358–363. [Google Scholar]
- Negi, H.; Vishunavat, K. Urdbean Leaf Crinkle Infection in Relation to Plant Age, Seed Quality, Seed Transmission and Yield in Urdbean. Ann. Plant Prot. Sci. 2006, 14, 169–172. [Google Scholar]
- Bhardwaj, S.; Dubey, G.; Sharma, I. Effect of Benlate on Infection and Transmission of Urdbean (Vigna radiata var. mungo) Leaf Crinkle Virus. J. Phytopathol. 1982, 105, 87–91. [Google Scholar]
- Beniwal, S.; Bharathan, N.; Chaubey, S. Two Cucurbitous Hosts of Urdbean Leaf Crinkle Virus. Indian Phytopathol. 1983, 36, 577–579. [Google Scholar]
- Narayanasamy, P.; Jaganathan, T. Vector Transmission of Black Gram Leaf Crinkle Virus. Madras Agric. J. 1973, 60, 651–652. [Google Scholar]
- Dhingra, K. Transmission of Urid Bean Leaf Crinkle Virus by Two Aphid Species. Indian Phytopathol. 1975, 28, 80–82. [Google Scholar]
- Dubey, G.; Sharma, I.; Prakash, N. Some Properties of Urdbean Leaf Crinkle Virus [Vigna mungo]. Indian Phytopathol. 1983, 36, 762–764. [Google Scholar]
- Bhardwaj, S.; Dubey, G. Studies on the Relationship of Urdbean Leaf Crinkle Virus and Its Vectors, Aphis Craccivora and Acyrthosiphon Pisum. J. Phytopathol. 1986, 115, 83–88. [Google Scholar] [CrossRef]
- Golluru, B.; Kumar, V.M. Incidence of Urdbean Leaf Crinkle with Other Viral Diseases of Urdbean in Guntur District of Andhra Pradesh. Trends Biosci. 2017, 10, 9087–9092. [Google Scholar]
- Beniwal, S.; Bharathan, N. Beetle Transmission of Urdbean Leaf Crinkle Virus. Indian Phytopathol. 1980, 33, 600–601. [Google Scholar]
- Sravika, A.; Kennedy, J.S.; Rajabaskar, D.; Rajeswari, E. Field Screening of Greengram (Vigna radiata L.) Genotypes for Resistance against Urdbean Leaf Crinkle Virus. Indian J. Agric. Res. 2019, 53, 458–462. [Google Scholar] [CrossRef]
- Biswas, K.; Tarafdar, A.; Kumar, A. Multiple Infection in Urdbean (Vigna mungo) in Natural Condition by Begomovirus, Tospovirus and Urdbean Leaf Crinkle Virus Complex. Indian Phytopathol. 2009, 62, 75–82. [Google Scholar]
- Godara, S.; Saha, B.; BHATTACHARYA, U.K.; Chattopadhyay, C.; Biswas, K. Lack of Resistance in Mungbean Genotypes against Urdbean Leaf Crinkle Disease Complex. Indian Phytopathol. 2014, 67, 426–427. [Google Scholar]
- Saha, P.; Singh, J.; Mohanty, T. Resistance in Urdbean against Yellow Mosaic, Leaf Crinkle and Cercospora Leaf Spot Diseases under Natural Epiphytotic Condition in Keonjhar District of Odisha. Indian Phytopathol. 2017, 70, 122–126. [Google Scholar] [CrossRef][Green Version]
- Kumar, B.; Shukla, A.; Singh, Y. Evaluation and Characterization of Germplasm Accessions of Urdbean (Vigna mungo L. Hepper). Trends Biosci. 2013, 6, 858–860. [Google Scholar]
- Chaudhry, M.; Ilyas, M.; Ghazanfar, M. Screening of Urdbean Germplasm for the Sources of Resistance against Urdbean Leaf Crinkle Virus. Mycopath 2007, 5, 1–4. [Google Scholar]
- Ashfaq, M.; Khan, M.A.; Mughal, S.; Javed, N.; Mukhtar, T.; Bashir, M. Evaluation of Urdbean Germplasm for Resistance against Urdbean Leaf Crinkle Virus. Pak. J. Bot 2007, 39, 2103–2111. [Google Scholar]
- Bashir, M.; Ahmad, Z.; Ghafoor, A. Sources of Genetic Resistance in Mungbean and Blackgram against Urdbean Leaf Crinkle Virus (ULCV). Pak. J. Bot 2005, 37, 47–51. [Google Scholar]
- Malik, S.; Kumar, P.; Panwar, J.; Rathi, Y. Physiological and Biochemical Alterations Induced by Urdbean Leaf Crinkle Virus in Vigna mungo (L.) Hepper. Ann. Plant Prot. Sci. 2002, 10, 91–94. [Google Scholar]
- Mahalingam, A. A New High Yielding MYMV Disease Resistant Blackgram Variety VBN 8. Electron. J. Plant Breed. 2018, 9, 1272–1279. [Google Scholar]
- Shanthi, P.; Iyanar, K.; Sassikumar, D.; Suresh, R.; Manimaran, R.; Pushpa, R.; Ravi, V.; Subrahmaniyan, K.; Suresh, S.; Rajappan, K. ADT 6-A High Yielding Blackgram Variety Suitable for Rice Fallow Condition of Cauvery Delta Zone. Electron. J. Plant Breed. 2019, 10, 1250–1254. [Google Scholar] [CrossRef]
- Sampol, B.; Bota, J.; Riera, D.; Medrano, H.; Flexas, J. Analysis of the Virus-induced Inhibition of Photosynthesis in Malmsey Grapevines. New Phytol. 2003, 160, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Y.; Lei, J.; Zou, L.; Zheng, J.; Yu, J. Effects of Potato Virus Y NTN Infection on Gas Exchange and Photosystem 2 Function in Leaves of Solanum tuberosum L. Photosynthetica 2004, 42, 417–423. [Google Scholar] [CrossRef]
- Funayama-Noguchi, S.; Terashima, I. Effects of Eupatorium Yellow Vein Virus Infection on Photosynthetic Rate, Chlorophyll Content and Chloroplast Structure in Leaves of Eupatorium Makinoi during Leaf Development. Funct. Plant Biol. 2006, 33, 165–175. [Google Scholar] [CrossRef]
- Ashfaq, M.; Khan, M.A.; Javed, N.; Mughal, S.; Shahid, M.; Sahi, S. Effect of Urdbean Leaf Crinkle Virus Infection on Total Soluble Protein and Antioxidant Enzymes in Blackgram Plants. Pak. J. Bot 2010, 42, 447–454. [Google Scholar]
- Srivastava, S.; Singh, A.K. Changes in Catalase Activity and Total Protein Content in Urdbean [Vigna mungo (L.) Hepper] Plants as a Result of UL CV Infection. Indian J. Sci. Res. 2010, 1, 67–69. [Google Scholar]
- Siddique, Z.; Akhtar, K.P.; Hameed, A.; Sarwar, N.; Imran-Ul-Haq; Khan, S.A. Biochemical Alterations in Leaves of Resistant and Susceptible Cotton Genotypes Infected Systemically by Cotton Leaf Curl Burewala Virus. J. Plant Interact. 2014, 9, 702–711. [Google Scholar] [CrossRef]
- Madhumitha, B.; Karthikeyan, A.; Devi, G.P.; Aiyanathan, K.E.A.; Sudha, M. Comparative Evaluation of Biochemical Changes in the Leaves of Resistant and Susceptible Mungbean Plants Infected by Mungbean Yellow Mosaic Virus. Res. J. Biotechnol. 2020, 15, 2. [Google Scholar]
- Brar, J.; Rataul, H. Leaf Crinkle Virus Induced Biochemical Changes in Mash Bean (Vigna mungo) and Its Effect on Aphis Craccivora Koch. J. Insect Sci. 1990, 3, 62–66. [Google Scholar]
- Thind, S.; Monga, P.; Kaur, N.; Cheema, S. Analysis of Some Biochemical and Micro-Nutrient Constituents of Yellow Mosaic Virus Infected Moong. Indian J. Virol. 1996, 12, 157–159. [Google Scholar]
- Taiwo, M.; Akinjogunla, O. Cowpea Viruses: Quantitative and Qualitative Effects of Single and Mixed Viral Infections. Afr. J. Biotechnol. 2006, 5, 1749–1756. [Google Scholar]
- Ngadze, E.; Icishahayo, D.; Coutinho, T.A.; Van der Waals, J.E. Role of Polyphenol Oxidase, Peroxidase, Phenylalanine Ammonia Lyase, Chlorogenic Acid, and Total Soluble Phenols in Resistance of Potatoes to Soft Rot. Plant Dis. 2012, 96, 186–192. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Ferulic Acid Impairs Rhizogenesis and Root Growth, and Alters Associated Biochemical Changes in Mung Bean (Vigna radiata) Hypocotyls. J. Plant Interact. 2014, 9, 267–274. [Google Scholar] [CrossRef]
- Gogoi, R.; Singh, D.; Srivastava, K. Phenols as a Biochemical Basis of Resistance in Wheat against Karnal Bunt. Plant Pathol. 2001, 50, 470–476. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Doraisamy, S.; Rabindran, R. Induction of Systemic Resistance in Blackgram (Vigna mungo) against Urdbean Leaf Crinkle Virus by Chemicals. Arch. Phytopathol. Plant Prot. 2009, 42, 1–15. [Google Scholar] [CrossRef]
- Ashfaq, M.; Khan, M.A.; Mukhtar, T.; Sahi, S.T. Role of Mineral Metabolism and Some Physiological Factors in Resistance against Urdbean Leaf Crinkle Virus in Blackgram Genotypes. Int. J. Agric. Biol. 2014, 16, 189–194. [Google Scholar]
- Chakraborty, N.; Basak, J. Molecular and Biochemical Characterization of Mungbean Yellow Mosaic India Virus Resistance in Leguminous Host Vigna mungo. J. Plant Biochem. Biotechnol. 2018, 27, 318–330. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Wang, X.-R.; Zhang, L.; Ahammed, G.J.; Li, Q.-Y.; Li, X. Epigallocatechin-3-Gallate Enhances Tomato Resistance to Tobacco Mosaic Virus by Modulating RBOH1-Dependent H2O2 Signaling. Plant Physiol. Biochem. 2020, 150, 263–269. [Google Scholar] [CrossRef]
- Jahan, A.A.; Anis, M.; Aref, I.M. Relative Examination of Antioxidative Enzymatic Activities in Plantlets of Cardiospermum halicacabum L. Differentiated from Hypocotyls in In Vivo and Ex Vitro Environment. Biotechnol. Rep. 2014, 4, 66–72. [Google Scholar] [CrossRef]
- Anjum, N.A. Book Review: Oxidative Damage to Plants-Antioxidant Networks and Signaling. Front. Plant Sci. 2015, 6, 452. [Google Scholar] [CrossRef]
- Van Wees, S.C.; De Swart, E.A.; Van Pelt, J.A.; Van Loon, L.C.; Pieterse, C.M. Enhancement of Induced Disease Resistance by Simultaneous Activation of Salicylate-and Jasmonate-Dependent Defense Pathways in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2000, 97, 8711–8716. [Google Scholar] [CrossRef]
- Irkitbay, A.; Madenova, A.; Sapakhova, Z. The Role of Salicylic Acid in the Plant Defense Mechanism. Bull. L.N. Gumilyov Eurasian Natl. Univ. Biosci Ser. 2022, 140, 83–96. [Google Scholar] [CrossRef]
- Nachappa, P.; Challacombe, J.; Margolies, D.C.; Nechols, J.R.; Whitfield, A.E.; Rotenberg, D. Tomato Spotted Wilt Virus Benefits Its Thrips Vector by Modulating Metabolic and Plant Defense Pathways in Tomato. Front. Plant Sci. 2020, 11, 575564. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N. Roles of Plant Hormones in the Regulation of Host–Virus Interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Shirasu, K.; Nakajima, H.; Rajasekhar, V.K.; Dixon, R.A.; Lamb, C. Salicylic Acid Potentiates an Agonist-Dependent Gain Control That Amplifies Pathogen Signals in the Activation of Defense Mechanisms. Plant Cell 1997, 9, 261–270. [Google Scholar] [PubMed]
- Love, A.J.; Laval, V.; Geri, C.; Laird, J.; Tomos, A.D.; Hooks, M.A.; Milner, J.J. Components of Arabidopsis Defense-and Ethylene-Signaling Pathways Regulate Susceptibility to Cauliflower Mosaic Virus by Restricting Long-Distance Movement. Mol. Plant-Microbe Interact. 2007, 20, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Zhu, F.; Xi, D.-H.; Yuan, S.; Xu, F.; Zhang, D.-W.; Lin, H.-H. Salicylic Acid and Jasmonic Acid Are Essential for Systemic Resistance against Tobacco Mosaic Virus in Nicotiana Benthamiana. Mol. Plant-Microbe Interact. 2014, 27, 567–577. [Google Scholar] [CrossRef]
- Knoester, M.; Linthorst, H.J.M.; Bol, J.F.; Van Loon, L.C. Involvement of Ethylene in Lesion Development and Systemic Acquired Resistance in Tobacco during the Hypersensitive Reaction to Tobacco Mosaic Virus. Physiol. Mol. Plant Pathol. 2001, 59, 45–57. [Google Scholar] [CrossRef]
- Bhaktavatsalam, G.; Nene, Y.; Beniwal, S. Influence of Certain Physico-Chemical Factors on the Infectivity and Stability of Urdbean Leaf Crinkle Virus. Indian Phytopathol. 1983, 36, 489–493. [Google Scholar]
- Patel, A.; Mishra, A.; Valand, G. Characterization of Leaf Crinkle Virus Disease of Urdbean (Vigna mungo L.). Indian J. Virol. 1999, 15, 101–105. [Google Scholar]
- Baranwal, V.; Jain, P.; Saritha, R.; Jain, R.; Gautam, N. Detection and Partial Characterization of Cowpea Mild Mottle Virus in Mungbean and Urdbean by Deep Sequencing and RT-PCR. Crop Prot. 2015, 75, 77–79. [Google Scholar] [CrossRef]
- Teja, T.R.; Latha, T.K.S.; Priyanga, T.; Prabakar, K.; Karthikeyan, G. Optimization of Sprout Seed Abrasion Method for Efficient Inoculation of Leaf Crinkle Disease in Urdbean [Vigna mungo (L.) Hepper]. Legume Res. 2022, 1, 5. [Google Scholar] [CrossRef]
- Kadian, O.P. Studies on Leaf Crinkle Disease of Urd Bean (Vigna mungo (L.) Hepper)/Mung Bean (Vigna radiata (L.) Wilczek) and Its Control. Ph.D. Thesis, Hissar College of Agriculture India, Hisar, India, 1980. [Google Scholar]
- Dubey, S.; Singh, B.; Tripathi, A. Integrated Management of Wet Root Rot, Yellow Mosaic, and Leaf Crinkle Diseases of Urdbean by Seed Treatment and Foliar Spray of Insecticide, Fungicide, and Biocontrol Agent. Crop Prot. 2018, 112, 269–273. [Google Scholar] [CrossRef]
- Haller, H.; Byadgi, A.; Nargund, V.; Balikai, R. Management of Leaf Crinkle Disease in Greengram. J. Exp. Zool. India 2020, 23, 695–700. [Google Scholar]
- Karthikeyan, G.; Doraisamy, S.; Rabindran, R.; Ganapathy, T. Evaluation of Antiviral Principles for the Induction of Systemic Resistance in Blackgram (Vigna mungo) against Urdbean Leaf Crinkle Virus. Arch. Phytopathol. Plant Prot. 2009, 42, 1172–1186. [Google Scholar] [CrossRef]
- Thirumalaisamy, P.; Rathi, Y.; Tripathi, H. Screening of Some Plant Extracts Inhibitory to Urdbean Leaf Crinkle Virus. Indian Phytopathol. 2003, 56, 233–235. [Google Scholar]
- Saleem, T.; Khan, M.A.; Rehman, A.; Mustafa, A. Evaluation of Different Plant Extracts in Reducing Bemisia Tabaci and Urdbean Leaf Crinkle Virus (Ulcv) Disease Incidence on Greengram. Pak. J. Phytopathol. 2018, 30, 7–10. [Google Scholar] [CrossRef]
- Shakeel, Q.; Mubeen, M.; Iftikhar, Y.; Arooj, S.; Basheer, S.; Iqbal, S.; Abbas, A. Management of Urdbean Leaf Crinkle Disease Using Micro-Nutrients and Plant Extracts in Relation to Environmental Factors. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 43–56. [Google Scholar]
- Karthikeyan, G.; Doraisamy, S.; Rabindran, R. Pseudomonas Fluorescens Mediated Systemic Resistance against Urdbean Leaf Crinkle Virus in Blackgram (Vigna mungo). Arch. Phytopathol. Plant Prot. 2009, 42, 201–212. [Google Scholar] [CrossRef]
- Zeshan, M.A.; Ali, S.; Khan, M.; Sahi, S. Role of Nutrients and Naphthalene Acetic Acid in the Management of Urdbean Leaf Crinkle Virus. Pak. J. Phytopathol. 2012, 24, 79–81. [Google Scholar]
- Islam, M.R.; Ali, M.A.; Islam, M.S.; Golam, A.; Hossain, G. Effect of Nutrients and Weeding on the Incidence of Mungbean Mosaic. Plant Pathol. J. 2002, 1, 48–50. [Google Scholar] [CrossRef]
- Shukla, A.; López-González, S.; Hoffmann, G.; Hafrén, A. Diverse Plant Viruses: A Toolbox for Dissection of Cellular Pathways. J. Exp. Bot. 2019, 70, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Noordam, D. Identification of Plant Viruses; Methods & Experiments; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1973. [Google Scholar]
- Radford, A.D.; Chapman, D.; Dixon, L.; Chantrey, J.; Darby, A.C.; Hall, N. Application of Next-Generation Sequencing Technologies in Virology. J. Gen. Virol. 2012, 93, 1853–1868. [Google Scholar] [CrossRef]
- Visser, M.; Bester, R.; Burger, J.T.; Maree, H.J. Next-Generation Sequencing for Virus Detection: Covering All the Bases. Virol. J. 2016, 13, 85. [Google Scholar] [CrossRef]
- Pecman, A.; Kutnjak, D.; Gutiérrez-Aguirre, I.; Adams, I.; Fox, A.; Boonham, N.; Ravnikar, M. Next Generation Sequencing for Detection and Discovery of Plant Viruses and Viroids: Comparison of Two Approaches. Front. Microbiol. 2017, 8, 1998. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaal, N.; Akram, M.; Pratap, A.; Kumar, D.; Nair, R.M. Urdbean Leaf Crinkle Virus: A Mystery Waiting to Be Solved. Viruses 2023, 15, 2120. https://doi.org/10.3390/v15102120
Kamaal N, Akram M, Pratap A, Kumar D, Nair RM. Urdbean Leaf Crinkle Virus: A Mystery Waiting to Be Solved. Viruses. 2023; 15(10):2120. https://doi.org/10.3390/v15102120
Chicago/Turabian StyleKamaal, Naimuddin, Mohammad Akram, Aditya Pratap, Deepender Kumar, and Ramakrishnan M. Nair. 2023. "Urdbean Leaf Crinkle Virus: A Mystery Waiting to Be Solved" Viruses 15, no. 10: 2120. https://doi.org/10.3390/v15102120
APA StyleKamaal, N., Akram, M., Pratap, A., Kumar, D., & Nair, R. M. (2023). Urdbean Leaf Crinkle Virus: A Mystery Waiting to Be Solved. Viruses, 15(10), 2120. https://doi.org/10.3390/v15102120






