Assessment of Peste des Petits Ruminants Antibodies in Vaccinated Pregnant Ewes of Kazakh Breed Fine-Fleeced and Determination of the Decreasing Trend of Maternal Immunity in Their Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaccination
2.3. Blood Sample Collection
2.4. Serology Tests
2.4.1. VNT
2.4.2. Competitive ELISA (c-ELISA)
2.5. Molecular Test
Real-Time RT-PCR
2.6. Evaluation of the Protective Effectiveness of Passive Immunity
2.7. Challenge Study
2.8. Data Analysis
3. Results
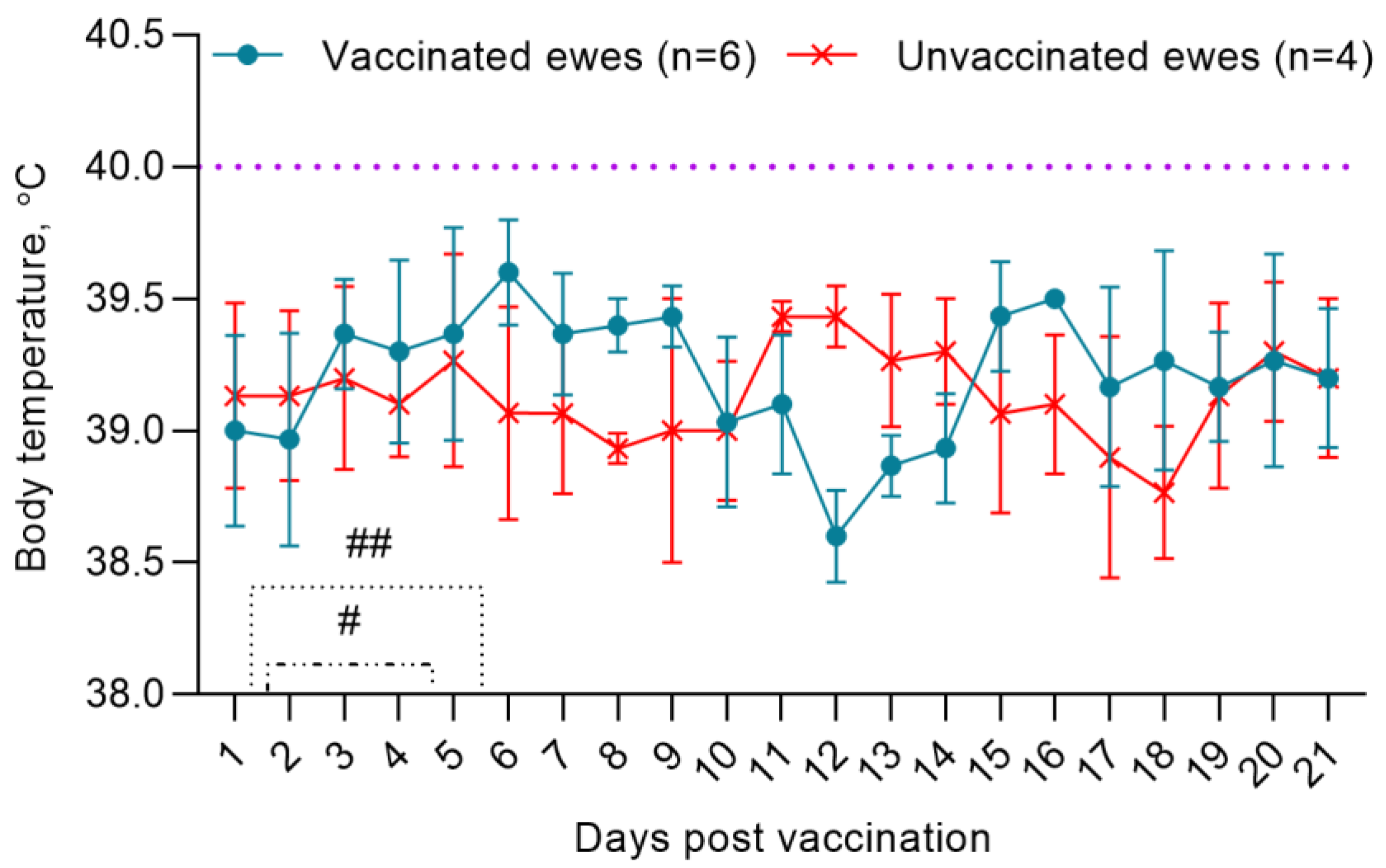
3.1. Adverse Reaction Monitoring
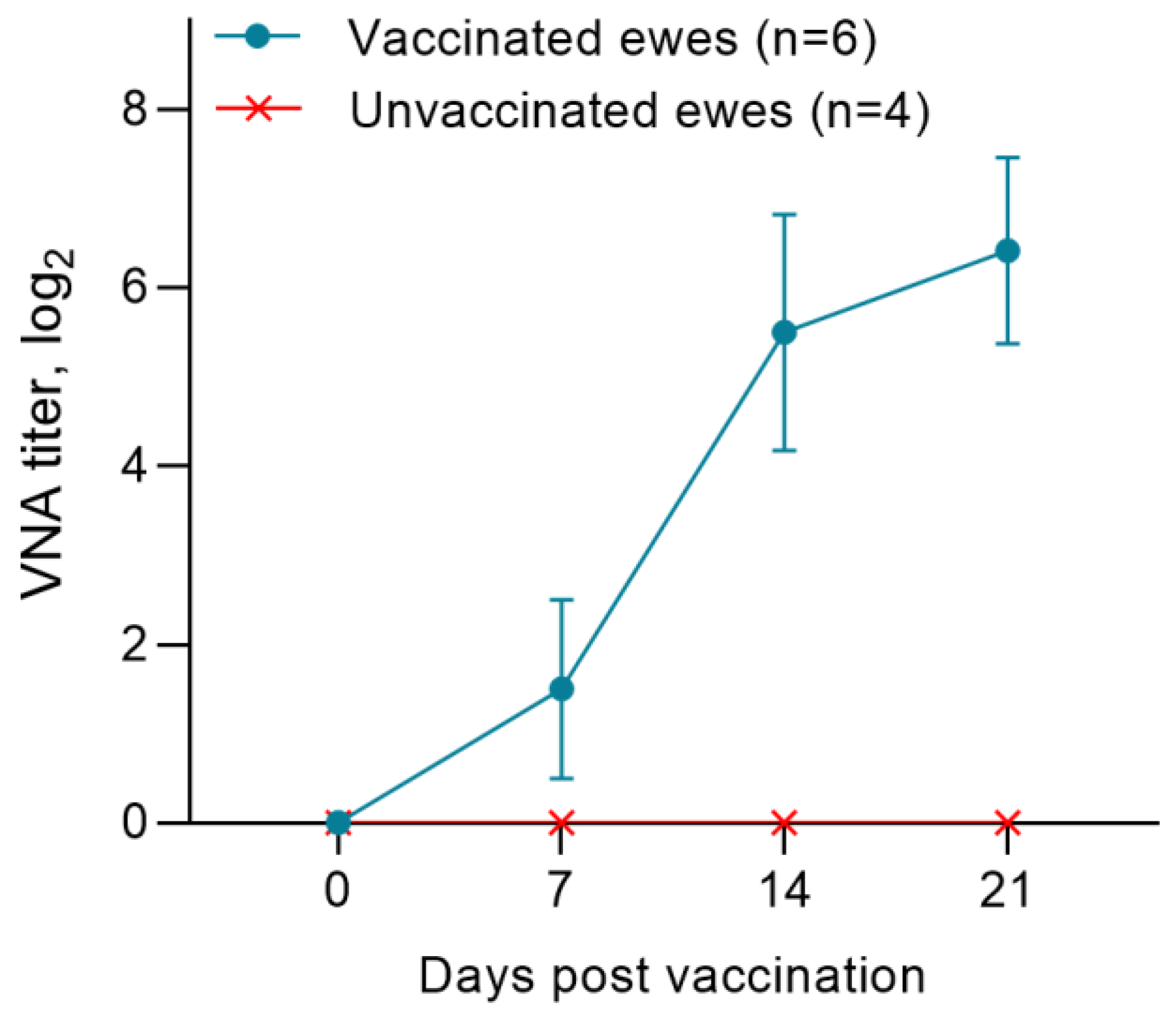
3.2. Post-Vaccination Titers of Neutralizing Antibodies to the PPRV in Pregnant Ewes
3.3. Post-Vaccination Titers of Antibodies to the PPRV in ELISA in Pregnant Ewes
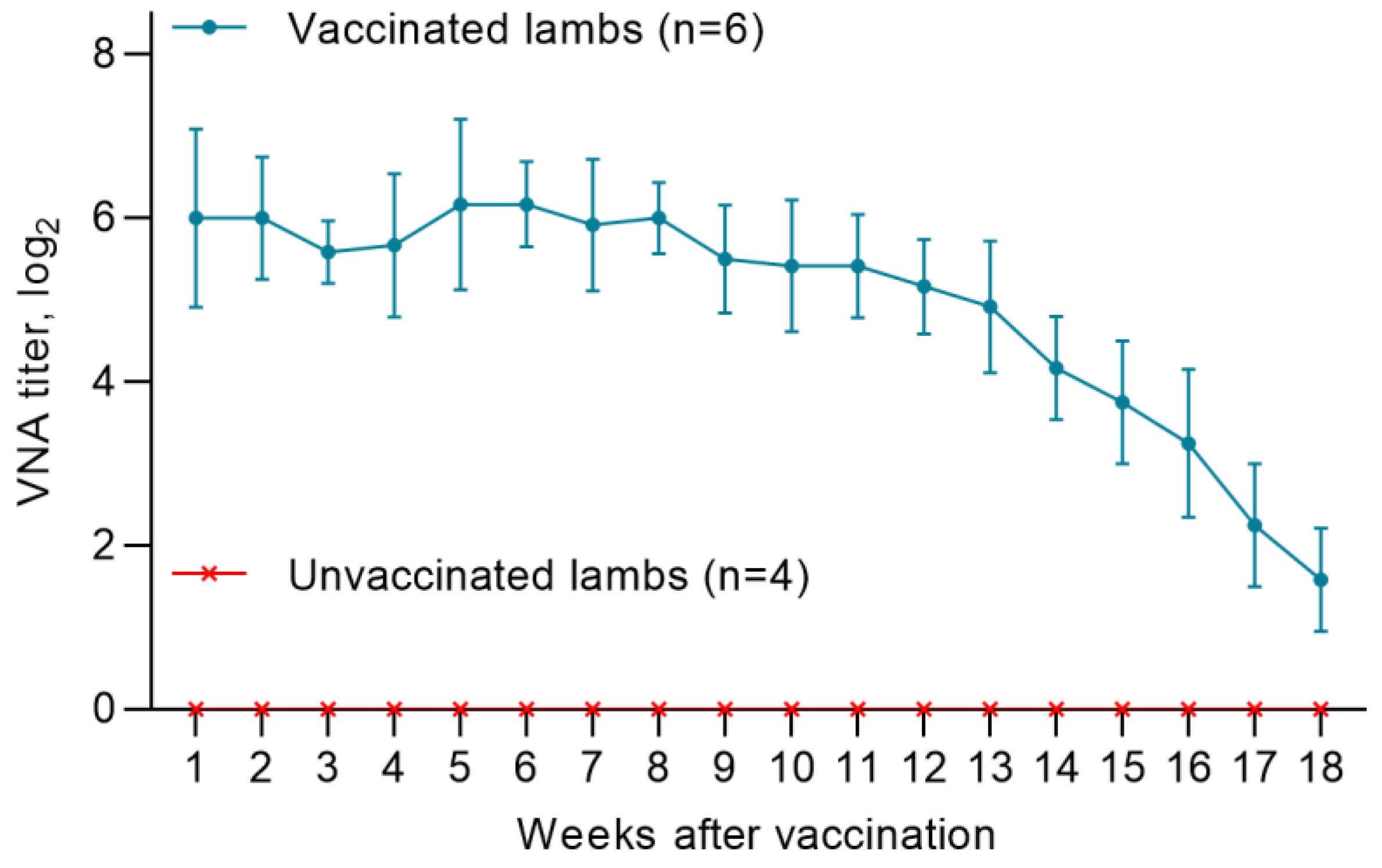
3.4. Titers of Maternal Antibodies in Lambs Born from Vaccinated Ewes
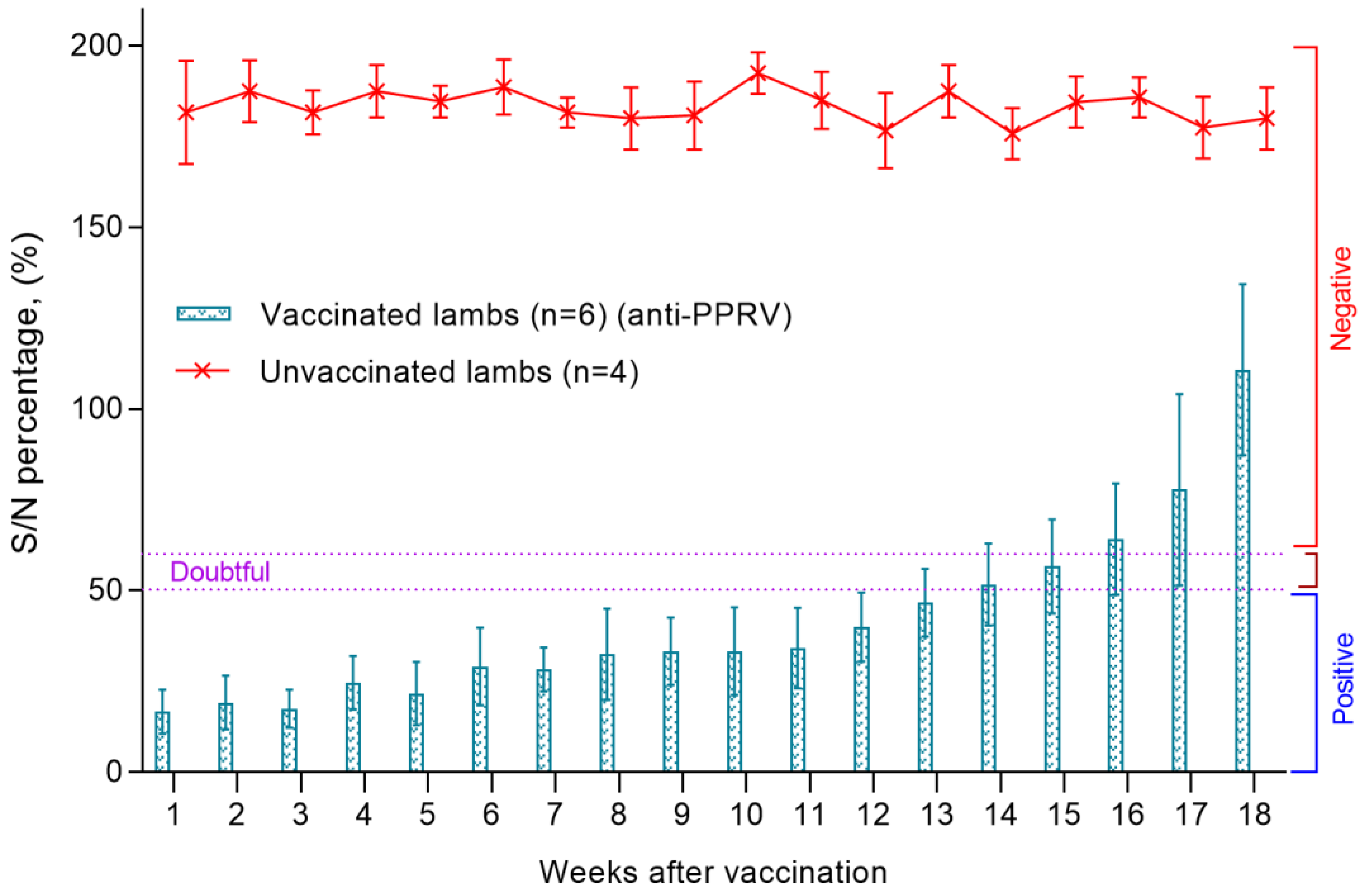
3.5. Titers of Antibodies to the PPRV in ELISA in Lambs Born from Vaccinated Ewes
3.6. Post-Vaccination Titer of Neutralizing Antibodies to the PPRV in a Lamb Born with MAs
3.7. Assessment of Viral Genomic Load in Blood and Swabs in Lambs
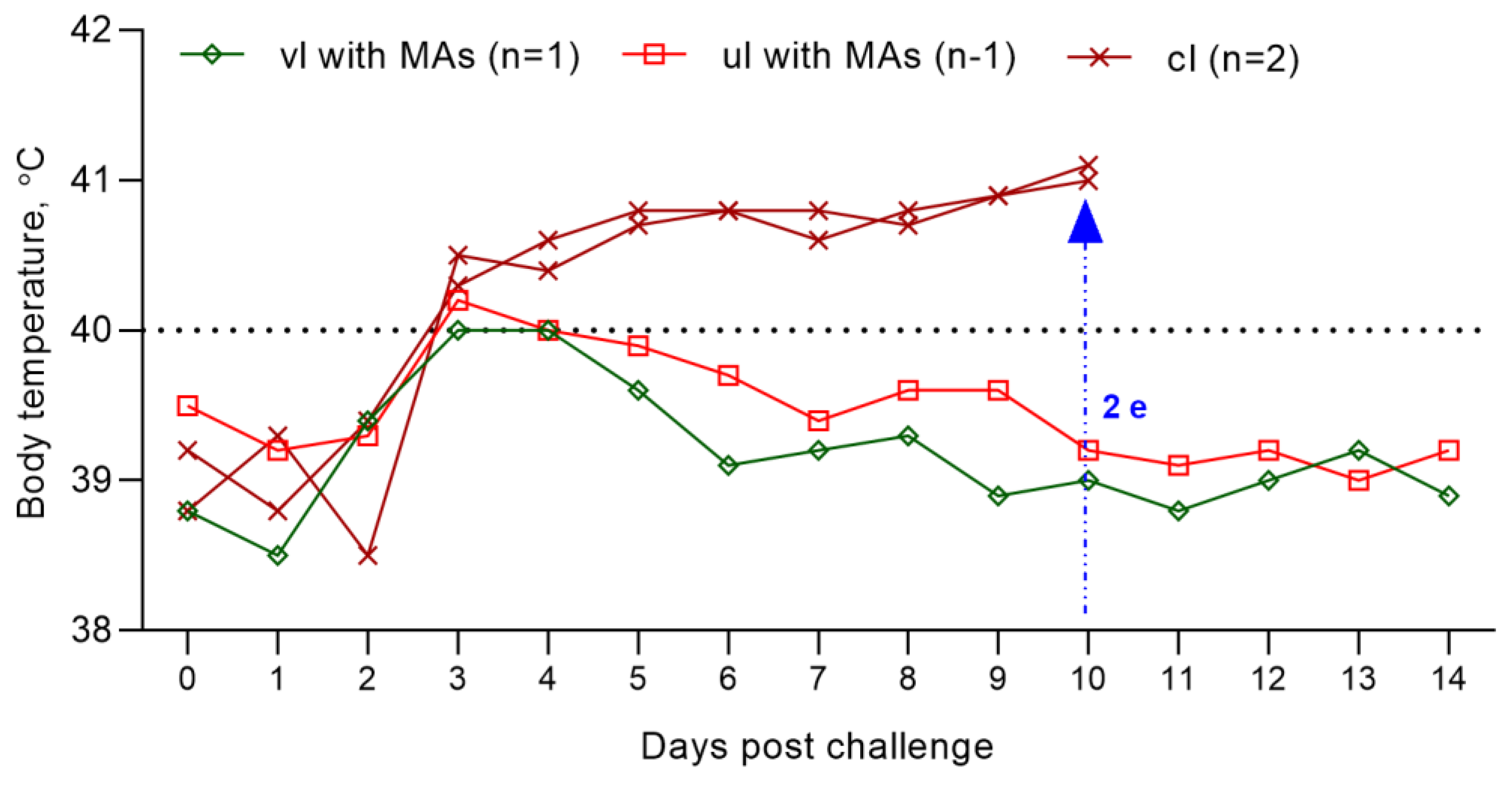
3.8. Evaluation of the Resistance of Experimental and Unvaccinated Lambs against Inoculation with the Field PPRV
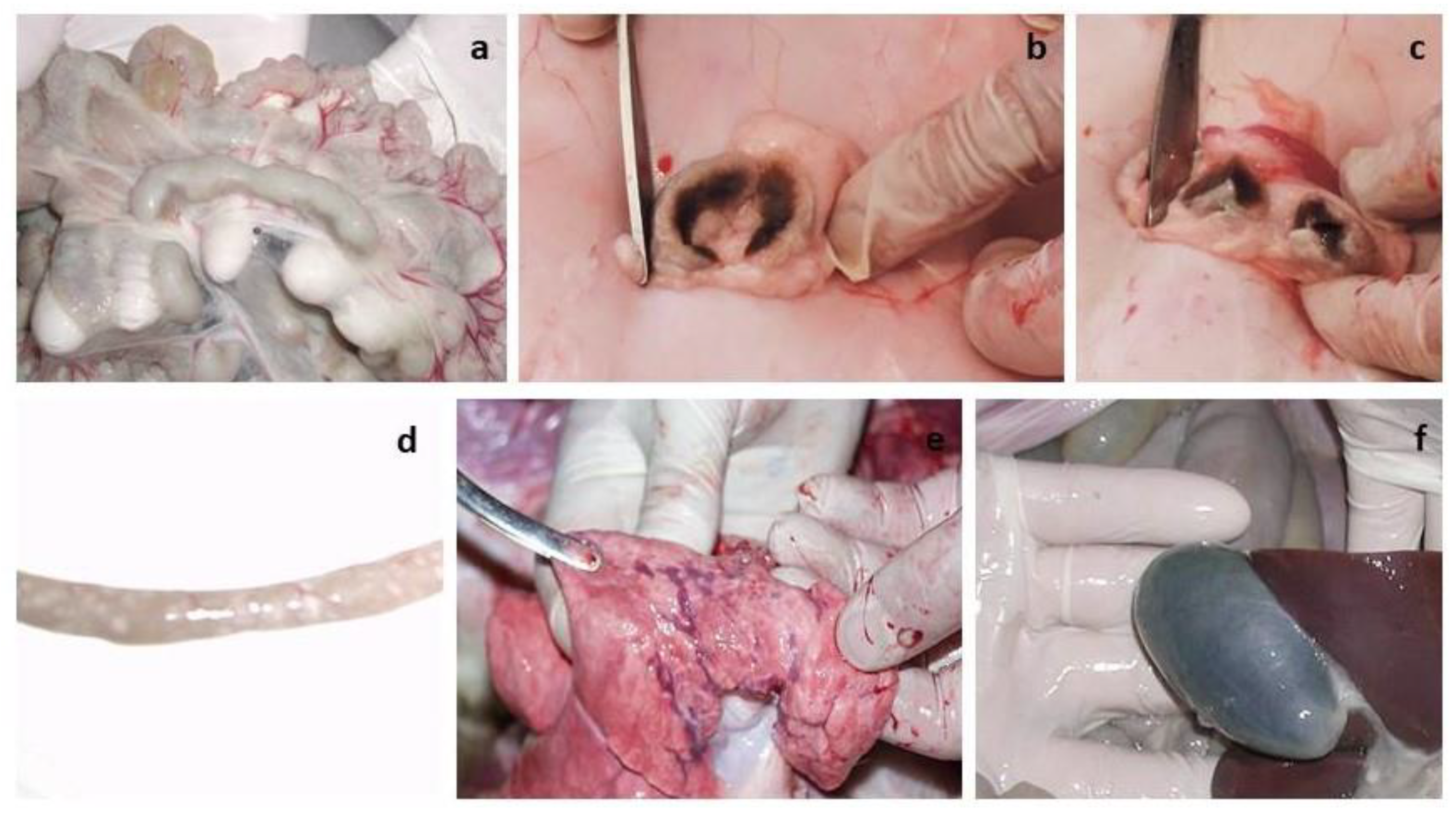
3.9. Necropsy of Euthanized Lambs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantip, S.E.; Shamaki, D.; Farougou, S. Peste des petits ruminants in Africa: Meta-analysis of the virus isolation in molecular epidemiology studies. Onderstepoort J. Vet. Res. 2019, 86, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Maherchandani, S.; Kashyap, S.K.; Singh, S.V.; Sharma, S.; Chaubey, K.K.; Ly, H. Peste Des Petits Ruminants Virus Infection of Small Ruminants: A Comprehensive Review. Viruses 2014, 6, 2287–2327. [Google Scholar] [CrossRef]
- Balamurugan, V.; Kumar, K.V.; Dheeraj, R.; Kurli, R.; Suresh, K.P.; Govindaraj, G.; Shome, B.R.; Roy, P. Temporal and Spatial Epidemiological Analysis of Peste Des Petits Ruminants Outbreaks from the Past 25 Years in Sheep and Goats and Its Control in India. Viruses 2021, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Lundervold, M.; Milner-Gulland, E.; O’Callaghan, C.; Hamblin, C.; Corteyn, A.; Macmillan, A. A Serological Survey of Ruminant Livestock in Kazakhstan During Post-Soviet Transitions in Farming and Disease Control. Acta Vet. Scand. 2004, 45, 211–224. [Google Scholar] [CrossRef]
- Abduraimov, Y.O.; Yershebulov, Z.D.; Zhugunisov, K.D.; Ye, A.B.; Nurgaziyev, R.Z.; Ye, D.K. The study of immunobiological properties of the vaccine against ovine rinderpest. Bull ASAU 2016, 2, 121–125. (In Russian) [Google Scholar]
- Legnardi, M.; Raizman, E.; Beltran-Alcrudo, D.; Cinardi, G.; Robinson, T.; Falzon, L.C.; Djomgang, H.K.; Okori, E.; Parida, S.; Njeumi, F.; et al. Peste des Petits Ruminants in Central and Eastern Asia/West Eurasia: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Programme (2017–2021). Animals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Kock, R.A.; Orynbayev, M.B.; Sultankulova, K.T.; Strochkov, V.M.; Omarova, Z.D.; Shalgynbayev, E.K.; Rametov, N.M.; Sansyzbay, A.R.; Parida, S. Detection and Genetic Characterization of Lineage IV Peste Des Petits Ruminant Virus in Kazakhstan. Transbound. Emerg. Dis. 2015, 62, 470–479. [Google Scholar] [CrossRef]
- Benfield, C.T.O.; Legnardi, M.; Mayen, F.; Almajali, A.; Cinardi, G.; Wisser, D.; Chaka, H.; Njeumi, F. Peste Des Petits Ruminants in the Middle East: Epidemiological Situation and Status of Control and Eradication Activities after the First Phase of the PPR Global Eradication Program (2017–2021). Animals 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Shatar, M.; Khanui, B.; Purevtseren, D.; Khishgee, B.; Loitsch, A.; Unger, H.; Settypalli, T.B.K.; Cattoli, G.; Damdinjav, B.; Dundon, W.G. First genetic characterization of peste des petits ruminants virus from Mongolia. Arch. Virol. 2017, 162, 3157–3160. [Google Scholar] [CrossRef] [PubMed]
- Amanova, Z.; Zhugunissov, K.; Barakbayev, K.; Kondybaeva, Z.; Sametova, Z.; Shayakhmetov, Y.; Kaissenov, D.; Dzhekebekov, K.; Zhunushov, A.; Abduraimov, Y.; et al. Duration of Protective Immunity in Sheep Vaccinated with a Combined Vaccine against Peste des Petits Ruminants and Sheep Pox. Vaccines 2021, 9, 912. [Google Scholar] [CrossRef]
- Balamurugan, V.; Sen, A.; Venkatesan, G.; Rajak, K.K.; Bhanuprakash, V.; Singh, R.K. Study on passive immunity: Time of vaccination in kids born to goats vaccinated against Peste des petits ruminants. Virol. Sin. 2012, 27, 228–233. [Google Scholar] [CrossRef]
- OIE. Peste des petits ruminants (infection with peste des petits ruminant’s virus). In Testerial Manual 2018; OIE: Paris, France, 2018; Chapter 3.7.9; pp. 1–16. [Google Scholar]
- Libeau, G.; Préhaud, C.; Lancelot, R.; Colas, F.; Guerre, L.; Bishop, D.H.; Diallo, A. Development of a competitive ELISA for detecting antibodies to the Peste des Petits Ruminants virus using a recombinant nucleoprotein. Res. Vet. Sci. 1995, 58, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Mamadaliev, S.M.; Koshemetov, Z.K.; Nurabaev, S.S.; Orynbayev, M.B.; Kasenov, M.M.; Bulatov, Y.A.; Mambetaliev, M. Kentau-7 PK-4-05D Strain of Peste des Petits Ruminants Virus, Suitable for Manufacturing Diagnostic Drugs and Monitoring the Immunogenicity of Vaccine Strains. Patent #20025, 29 January 2007. (In Kazakhstan). [Google Scholar]
- Bamouh, Z.; Fakri, F.; Jazouli, M.; Safini, N.; Tadlaoui, K.O.; Elharrak, M. Peste des petits ruminants pathogenesis on experimental infected goats by the Moroccan 2015 isolate. BMC Vet. Res. 2019, 15, 452. [Google Scholar] [CrossRef]
- Hamdi, J.; Bamouh, Z.; Jazouli, M.; Alhyane, M.; Safini, N.; Omari Tadlaoui, K.; Fassi Fihri, O.; El Harrak, M. Experimental infection of indigenous North African goats with goatpox virus. Acta Vet. Scand. 2021, 63, 9. [Google Scholar] [CrossRef]
- Markus, T.P.; Adamu, J.; Kazeem, H.M.; Olaolu, O.S.; Woma, T.Y. Assessment of Peste des petits ruminants antibodies in vaccinated pregnant Kano brown does from Nigeria and subsequent maternal immunity in their kids. Small Rumin. Res. 2019, 174, 53–56. [Google Scholar] [CrossRef]
- Bodjo, S.C.; Couacy-Hymann, E.; Koffi, M.Y.; Danho, T. Assessment of the duration of maternal antibodies specific to the homologous peste des petits ruminant vaccine “Nigeria 75/1” in Djallonké lambs. Biokemistri 2006, 18, 99–103. [Google Scholar] [CrossRef]
- Abdollahi, M.; Lotfi, M.; Lotfollahzadeh, S.; Adibi, M.; Kamalzadeh, M.; Firuzyar, S. Determining the decreasing trend of maternal immunity against small ruminant morbillivirus in goat kids. Vet. Med. Sci. 2023, 9, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Olaolu, O.; Kazeem, H.; Adamu, J.; Markus, T.; Woma, T. Assessment of Peste Des Petits Ruminants Antibodies in Vaccinated Yankasa Pregnant Ewes from Nigeria and the Duration of Maternal Immunity in Their Lambs. Vaccine Res. 2021, 8, 47–51. [Google Scholar] [CrossRef]
- Begum, S.S.; Mahato, G.; Sharma, P.; Hussain, M.; Saleque, A. Assessment of immune response to a lyophilized peste-des-petitsruminants virus vaccine in three different breeds of goats. Vet. World 2016, 9, 568–571. [Google Scholar] [CrossRef]
- Rossitter, P.B.; Jessett, D.M.; Taylor, W.P. Microneutralisation systems for use with different strains of peste des petits ruminants virus and rinderpest virus. Trop. Anim. Health Prod. 1985, 17, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Sen, A.; Balamurugan, V.; Rajak, K.; Bhanuprakash, V.; Palaniswami, K.; Nachimuthu, K.; Thangavelu, A.; Dhinakarraj, G.; Hegde, R.; et al. Comparative efficacy of peste des petits ruminants (PPR) vaccines. Biologicals 2010, 38, 479–485. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanova, Z.; Turyskeldy, S.; Kondybaeva, Z.; Sametova, Z.; Usembai, A.; Kerimbayev, A.; Bulatov, Y. Assessment of Peste des Petits Ruminants Antibodies in Vaccinated Pregnant Ewes of Kazakh Breed Fine-Fleeced and Determination of the Decreasing Trend of Maternal Immunity in Their Lambs. Viruses 2023, 15, 2054. https://doi.org/10.3390/v15102054
Amanova Z, Turyskeldy S, Kondybaeva Z, Sametova Z, Usembai A, Kerimbayev A, Bulatov Y. Assessment of Peste des Petits Ruminants Antibodies in Vaccinated Pregnant Ewes of Kazakh Breed Fine-Fleeced and Determination of the Decreasing Trend of Maternal Immunity in Their Lambs. Viruses. 2023; 15(10):2054. https://doi.org/10.3390/v15102054
Chicago/Turabian StyleAmanova, Zhanat, Sholpan Turyskeldy, Zhanat Kondybaeva, Zhanna Sametova, Abdurakhman Usembai, Aslan Kerimbayev, and Yerbol Bulatov. 2023. "Assessment of Peste des Petits Ruminants Antibodies in Vaccinated Pregnant Ewes of Kazakh Breed Fine-Fleeced and Determination of the Decreasing Trend of Maternal Immunity in Their Lambs" Viruses 15, no. 10: 2054. https://doi.org/10.3390/v15102054
APA StyleAmanova, Z., Turyskeldy, S., Kondybaeva, Z., Sametova, Z., Usembai, A., Kerimbayev, A., & Bulatov, Y. (2023). Assessment of Peste des Petits Ruminants Antibodies in Vaccinated Pregnant Ewes of Kazakh Breed Fine-Fleeced and Determination of the Decreasing Trend of Maternal Immunity in Their Lambs. Viruses, 15(10), 2054. https://doi.org/10.3390/v15102054








