Trend of HPV Molecular Epidemiology in the Post-Vaccine Era: A 10-Year Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Data Source
2.2. HPV DNA Extraction, PCR Amplification, and Genotyping
2.3. Statistical Analysis
3. Results
3.1. General Descriptive Statistics of HPV in 10 Years
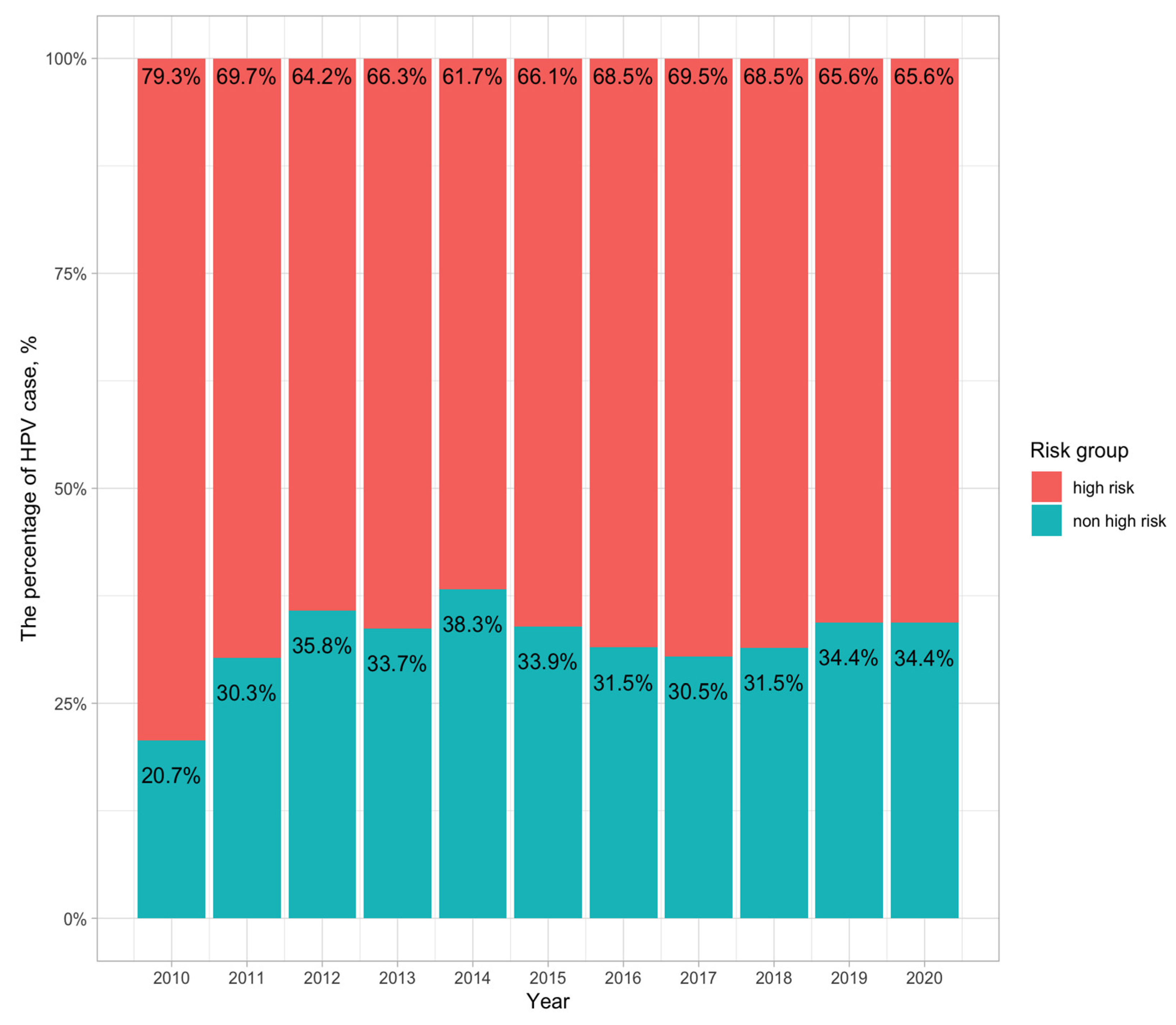
3.2. Distribution of High-Risk and Non-High-Risk HPV Groups
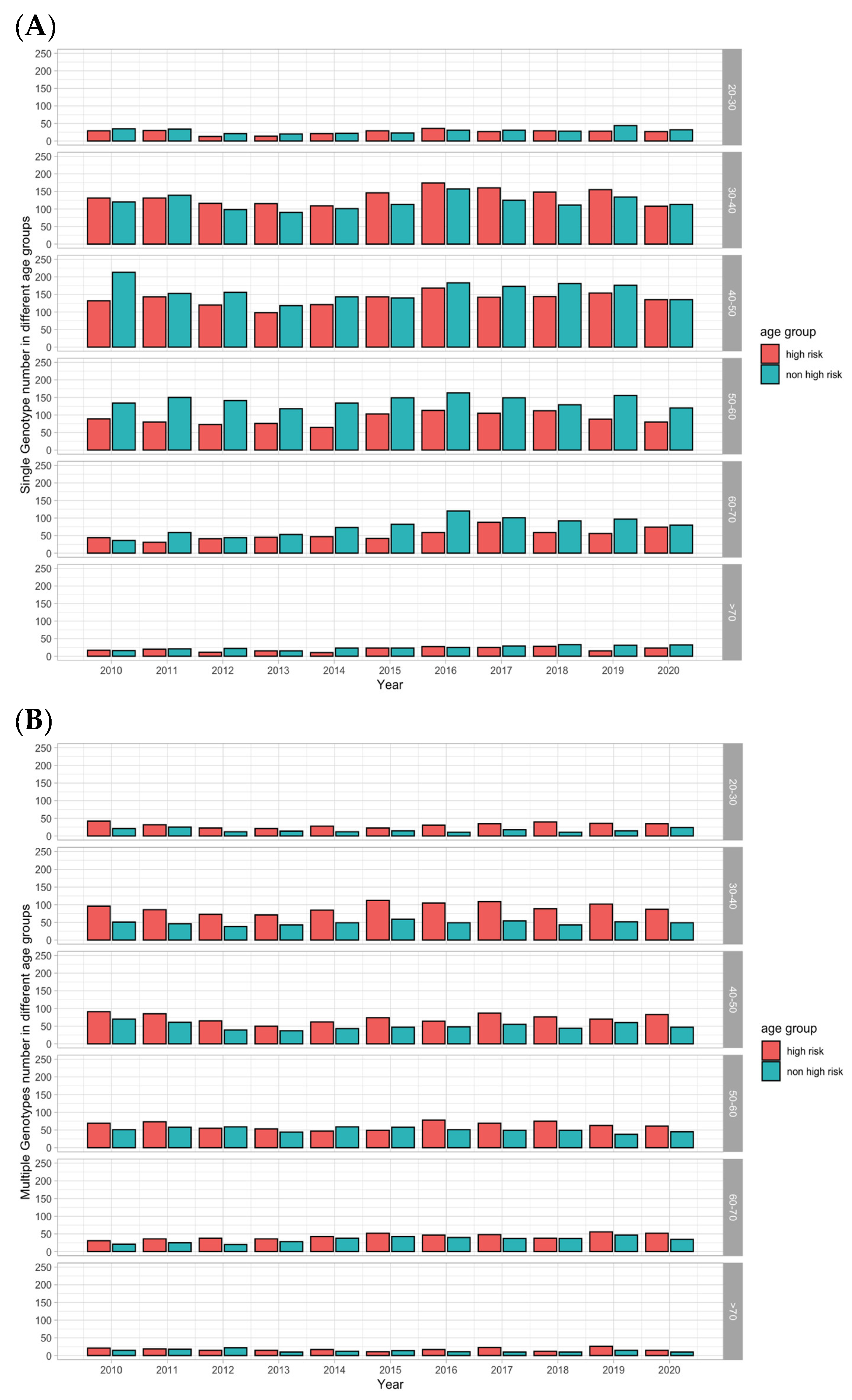
3.3. HPV Infection in Different Age Groups
3.4. Single-Type and Mixed-Type HPV Infection in Different Age Groups
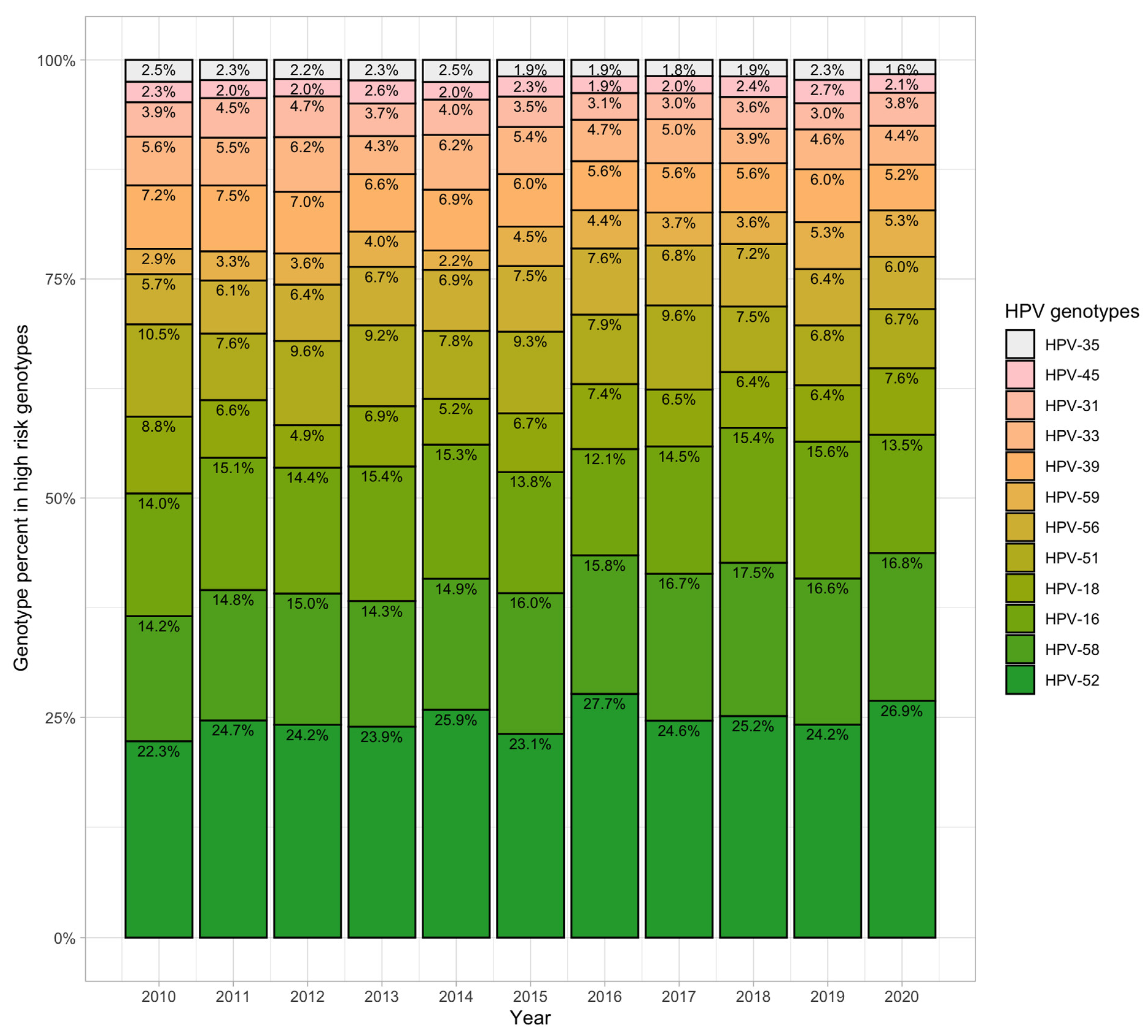
3.5. Distribution of the 12 High-Risk Genotypes
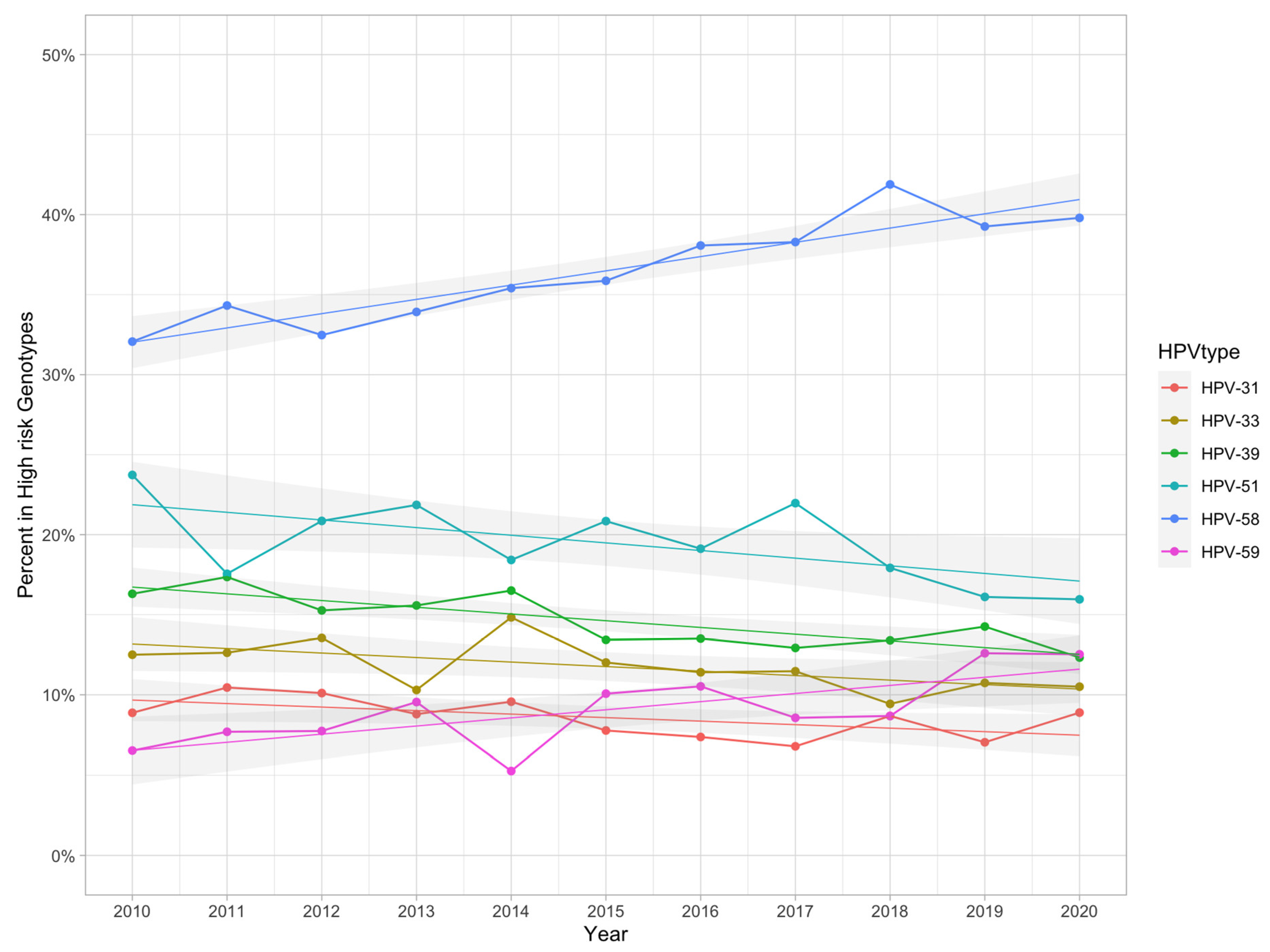
3.6. Change in Trends for the 12 High-Risk Genotypes
4. Discussion
4.1. HPV Prevalence and Genotype Distribution
4.2. High Risk Ratio in Different Age Group
4.3. Single- and Mixed-Type Infections in Different Age Groups
4.4. The Changing Trends of the 12 High-Risk Types
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Munoz, N.; Bosch, F.X.; de Sanjose, S.; Herrero, R.; Castellsague, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Cancer 2007, 121, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef]
- Lai, C.H.; Huang, H.J.; Hsueh, S.; Chao, A.; Lin, C.T.; Huang, S.L.; Chao, F.Y.; Qiu, J.T.; Hong, J.H.; Chou, H.H.; et al. Human papillomavirus genotype in cervical cancer: A population-based study. Int. J. Cancer 2007, 120, 1999–2006. [Google Scholar] [CrossRef]
- WHO. Human papillomavirus vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2017, 92, 241–268. [Google Scholar]
- Stanley, M.; Pinto, L.A.; Trimble, C. Human papillomavirus vaccinesi—Immune responses. Vaccine 2012, 30, F83–F87. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Herweijer, E.; Sundström, K.; Ploner, A.; Uhnoo, I.; Sparén, P.; Arnheim-Dahlström, L. Erratum: Quadrivalent HPV vaccine effectiveness against high-grade cervical lesions by age at vaccination: A population-based study. Int. J. Cancer 2017, 141, E1–E4. [Google Scholar]
- Silverberg, M.J.; Leyden, W.A.; Lam, J.O.; Gregorich, S.E.; Huchko, M.J.; Kulasingam, S.; Kuppermann, M.; Smith-McCune, K.K.; Sawaya, G.F. Effectiveness of catch-up human papillomavirus vaccination on incident cervical neoplasia in a US health-care setting: A population-based case-control study. Lancet Child Adolesc. Health 2018, 2, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vaccine in National Immunization Programme Update October 2020. Available online: https://cdn.who.int/media/docs/default-source/immunization/hpv/vaccineintrostatus.pdf (accessed on 4 October 2020).
- PATH. Global HPV Vaccine Introduction Overview: Projected and Current National Introductions, Demonstration/Pilot Projects, Gender-Neutral Vaccination Programs, and Global HPV Vaccine Introduction Maps (2006–2023). 2020. Available online: https://path.azureedge.net/media/documents/Global_HPV_Vaccine_Intro_Overview_Slides_webversion_2020May.pdf (accessed on 4 May 2020.).
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef]
- Bao, Y.P.; Li, N.; Smith, J.S.; Qiao, Y.L.; ACCPAB members. Human papillomavirus type distribution in women from Asia: A meta-analysis. Int. J. Gynecol. Cancer 2008, 18, 71–79. [Google Scholar] [CrossRef]
- Han, X.; Song, G.; Li, Y.; Dong, Z.; Yan, X.; Wang, S.; Tian, H.; Wu, X.; Li, C.; Huo, Y. Prevalence and genotype distribution of human papillomavirus infection among women aged 30–65 years in Xi’an, China: A population-based study of 14,655 women. Hum. Vaccines Immunother. 2021, 17, 5439–5446. [Google Scholar] [CrossRef]
- Teka, B.; Gizaw, M.; Ruddies, F.; Addissie, A.; Chanyalew, Z.; Skof, A.S.; Thies, S.; Mihret, A.; Kantelhardt, E.J.; Kaufmann, A.M.; et al. Population-based human papillomavirus infection and genotype distribution among women in rural areas of South Central Ethiopia. Int. J. Cancer 2021, 148, 723–730. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Wei, X.; Wang, G.; Sun, R.; Wang, M.; Zhao, L. Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol. J. 2022, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Herrero, R.; Clifford, G.M.; Snijders, P.J.; Arslan, A.; Anh, P.T.; Bosch, F.X.; Ferreccio, C.; Hieu, N.T.; Lazcano-Ponce, E.; et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int. J. Cancer 2006, 119, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Melendy, A.; Rana, R.K.; Pimenta, J.M. Age-specific prevalence of infection with human papillomavirus in females: A global review. J. Adolesc. Health 2008, 43, S5–S25.e41. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, R.S.; Olovsson, M.; Hoxell, E.; Lindström, A.K. HPV prevalence and HPV-related dysplasia in elderly women. PLoS ONE 2018, 13, e0189300. [Google Scholar] [CrossRef] [PubMed]
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef]
- Lukic, A.; De Vincenzo, R.; Ciavattini, A.; Ricci, C.; Senatori, R.; Ruscito, I.; Frega, A. Are We Facing a New Colposcopic Practice in the HPV Vaccination Era? Opportunities, Challenges, and New Perspectives. Vaccines 2021, 9, 1081. [Google Scholar] [CrossRef]
- Dickson, E.L.; Vogel, R.I.; Geller, M.A.; Downs, L.S., Jr. Cervical cytology and multiple type HPV infection: A study of 8182 women ages 31–65. Gynecol. Oncol. 2014, 133, 405–408. [Google Scholar] [CrossRef]
- Song, F.; Yan, P.; Huang, X.; Wang, C.; Du, H.; Qu, X.; Wu, R. Roles of extended human papillomavirus genotyping and multiple infections in early detection of cervical precancer and cancer and HPV vaccination. BMC Cancer 2022, 22, 42. [Google Scholar] [CrossRef]
- Li, P.; Ma, J.; Zhang, X.; Guo, Y.; Liu, Y.; Li, X.; Zhao, D.; Wang, Z. Cervical small cell carcinoma frequently presented in multiple high risk HPV infection and often associated with other type of epithelial tumors. Diagn. Pathol. 2018, 13, 31. [Google Scholar] [CrossRef]
- Crow, J.M. HPV: The global burden. Nature 2012, 488, S2–S3. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Song, L.; Wang, D.; Yin, R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer 2019, 125, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Fappani, C.; Bianchi, S.; Panatto, D.; Petrelli, F.; Colzani, D.; Scuri, S.; Gori, M.; Amendola, A.; Grappasonni, I.; Tanzi, E.; et al. HPV Type-Specific Prevalence a Decade after the Implementation of the Vaccination Program: Results from a Pilot Study. Vaccines 2021, 9, 336. [Google Scholar] [CrossRef]
- Rollison, D.E.; Viarisio, D.; Amorrortu, R.P.; Gheit, T.; Tommasino, M. An Emerging Issue in Oncogenic Virology: The Role of Beta Human Papillomavirus Types in the Development of Cutaneous Squamous Cell Carcinoma. J. Virol. 2019, 93, e01003-18. [Google Scholar] [CrossRef]
- Beesley, L.J.; Moran, K.R.; Wagh, K.; Castro, L.A.; Theiler, J.; Yoon, H.; Fischer, W.; Hengartner, N.W.; Korber, B.; Del Valle, S.Y. SARS-CoV-2 variant transition dynamics are associated with vaccination rates, number of co-circulating variants, and convalescent immunity. EBioMedicine 2023, 91, 104534. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Yousefi, Z.; Khosravi, G.; Malayeri, F.E.; Golabi, M.; Askarzadeh, M.; Shams, M.H.; Ghezelbash, B.; Eskandari, N. Human papillomavirus vaccination in low- and middle-income countries: Progression, barriers, and future prospective. Front. Immunol. 2023, 14, 1150238. [Google Scholar] [CrossRef]
- Ver, A.T.; Notarte, K.I.; Velasco, J.V.; Buac, K.M.; Nazareno, J., 3rd; Lozañes, J.A.; Antonio, D.; Bacorro, W. A systematic review of the barriers to implementing human papillomavirus vaccination programs in low- and middle-income countries in the Asia-Pacific. Asia Pac. J. Clin. Oncol. 2021, 17, 530–545. [Google Scholar] [CrossRef]
- Romero-Hernández, B.; Martínez-García, L.; Rodríguez-Dominguez, M.; Martínez-Sanz, J.; Vélez-Díaz-Pallarés, M.; Mies, B.P.; Muriel, A.; Gea, F.; Pérez-Elías, M.J.; Galán, J.C. The Negative Impact of COVID-19 in HCV, HIV, and HPV Surveillance Programs During the Different Pandemic Waves. Front. Public Health 2022, 10, 880435. [Google Scholar] [CrossRef]

| HPV Genotype Distribution (N = 40,561) | |||
|---|---|---|---|
| No. of Patients (%) | Mixed-Type Infection | Single-Type Infection | |
| Total | 40,561 (100.0) | ||
| HPV(−) | 23,513 (58.0) | NA | NA |
| HPV(+) | 17,048 (42.0) | 34.8% (5932/17,048) | 65.2% (11,116/17,048) |
| HPV-52* | 3260 (8.0) | 44.1% (1439/3260) | 55.9% (1821/3260) |
| HPV-58* | 2074 (5.1) | 46.9% (972/2074) | 53.1% (1102/2074) |
| HPV-16* | 1894 (4.7) | 44.3% (839/1894) | 55.7% (1055/1894) |
| HPV-53 | 1622 (4.0) | 58.8% (954/1622) | 41.2% (668/1622) |
| HPV-51* | 1106 (2.7) | 57.6% (637/1106) | 42.4% (469/1106) |
| HPV-62 | 1022 (2.5) | 63.8% (652/1022) | 36.0% (368/1022) |
| HPV-18* | 884 (2.2) | 46.4% (410/884) | 53.6% (474/884) |
| HPV-56* | 878 (2.2) | 60.9% (535/878) | 39.1% (343/878) |
| HPV-70 | 859 (2.1) | 56.0% (481/859) | 44.0% (378/859) |
| HPV-54 | 850 (2.1) | 70.8% (602/850) | 29.2% (248/850) |
| HPV-39* | 823 (2.0) | 61.1% (503/823) | 38.9% (320/823) |
| HPV-42 | 814 (2.0) | 61.5% (501/814) | 38.5% (313/814) |
| HPV-84(MM8) | 737 (1.8) | 70.6% (520/737) | 29.4% (217/737) |
| HPV-81(CP8304) | 716 (1.8) | 67.0% (480/716) | 33.0% (236/716) |
| HPV-66 | 705 (1.7) | 63.0% (444/705) | 37.0% (261/705) |
| HPV-33* | 664 (1.6) | 52.9% (351/664) | 47.1% (313/664) |
| HPV-61 | 627 (1.5) | 68.1% (427/627) | 31.9% (200/627) |
| HPV-44 | 580 (1.4) | 66.9% (388/580) | 33.1% (192/580) |
| HPV-72 | 554 (1.4) | 57.9% (321/554) | 42.1% (233/554) |
| HPV-68 | 548 (1.4) | 60.9% (334/548) | 38.5% (211/548) |
| HPV-59* | 517 (1.3) | 66.9% (346/517) | 33.1% (171/517) |
| HPV-71(CP8061) | 502 (1.2) | 62.5% (314/502) | 37.5% (188/502) |
| HPV-31* | 480 (1.2) | 58.3% (280/480) | 41.7% (200/480) |
| HPV-43 | 476 (1.2) | 67.9% (323/476) | 32.1% (153/476) |
| HPV-82(MM4) | 415 (1.0) | 77.3% (321/415) | 22.7% (94/415) |
| HPV-6 | 410 (1.0) | 66.8% (274/410) | 33.2% (136/410) |
| HPV-45* | 290 (0.7) | 66.6% (193/290) | 33.4% (97/290) |
| HPV-35* | 275 (0.7) | 58.2% (160/275) | 41.8% (115/275) |
| HPV-67 | 265 (0.7) | 75.1% (199/265) | 24.9% (66/265) |
| HPV-55 | 257 (0.6) | 75.1% (193/257) | 24.9% (64/257) |
| HPV-74 | 248 (0.6) | 54.0% (134/248) | 46.0% (114/248) |
| HPV-69 | 199 (0.5) | 65.8% (131/199) | 34.2% (68/199) |
| HPV-11 | 193 (0.5) | 49.2% (95/193) | 50.8% (98/193) |
| HPV-32 | 164 (0.4) | 73.8% (121/164) | 26.2% (43/164) |
| HPV-L1AE5 | 95 (0.2) | 73.7% (70/95) | 26.3% (25/95) |
| HPV-83(MM7) | 82 (0.2) | 42.7% (35/82) | 57.3% (47/82) |
| HPV-26 | 64 (0.2) | 76.6% (49/64) | 23.4% (15/64) |
| HPV-37 | 2 (0.0) | 100.0% (2/2) | 0.0% (0/2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Lin, W.-Y.; Lin, T.-W.; Tseng, Y.-J.; Wang, Y.-C.; Yu, J.-R.; Chung, C.-R.; Wang, H.-Y. Trend of HPV Molecular Epidemiology in the Post-Vaccine Era: A 10-Year Study. Viruses 2023, 15, 2015. https://doi.org/10.3390/v15102015
Lin Y, Lin W-Y, Lin T-W, Tseng Y-J, Wang Y-C, Yu J-R, Chung C-R, Wang H-Y. Trend of HPV Molecular Epidemiology in the Post-Vaccine Era: A 10-Year Study. Viruses. 2023; 15(10):2015. https://doi.org/10.3390/v15102015
Chicago/Turabian StyleLin, Yueh, Wan-Ying Lin, Ting-Wei Lin, Yi-Ju Tseng, Yu-Chiang Wang, Jia-Ruei Yu, Chia-Ru Chung, and Hsin-Yao Wang. 2023. "Trend of HPV Molecular Epidemiology in the Post-Vaccine Era: A 10-Year Study" Viruses 15, no. 10: 2015. https://doi.org/10.3390/v15102015
APA StyleLin, Y., Lin, W.-Y., Lin, T.-W., Tseng, Y.-J., Wang, Y.-C., Yu, J.-R., Chung, C.-R., & Wang, H.-Y. (2023). Trend of HPV Molecular Epidemiology in the Post-Vaccine Era: A 10-Year Study. Viruses, 15(10), 2015. https://doi.org/10.3390/v15102015






