The Development of the SARS-CoV-2 Epidemic in Different Regions of Siberia in the 2020–2022 Period
Abstract
:1. Introduction
2. Materials and Methods
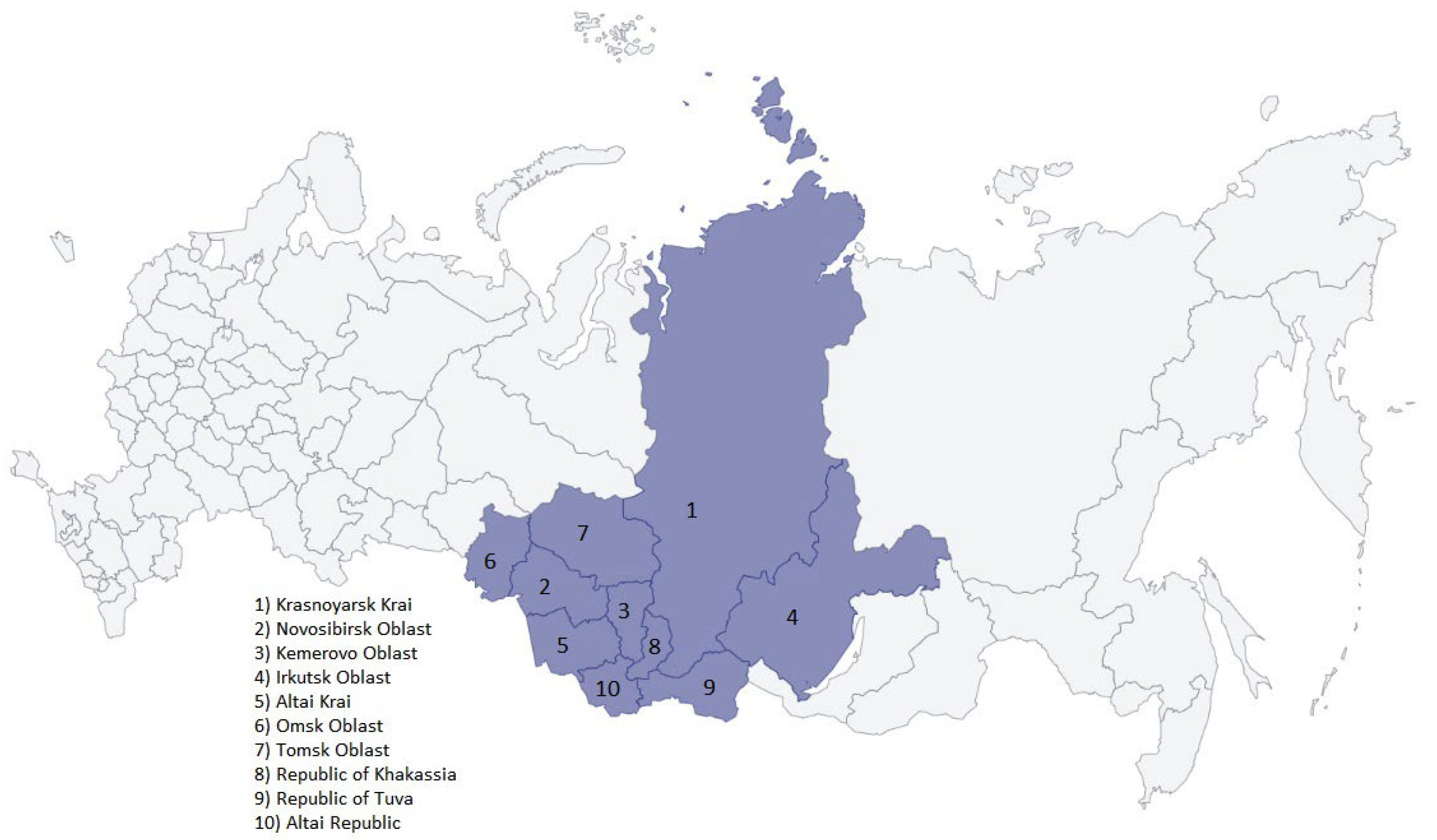
2.1. Collecting Epidemiological Data in the Siberian Federal District
2.2. The Next-Generation Sequensing
2.3. The Analysis of Genetic Data
3. Results
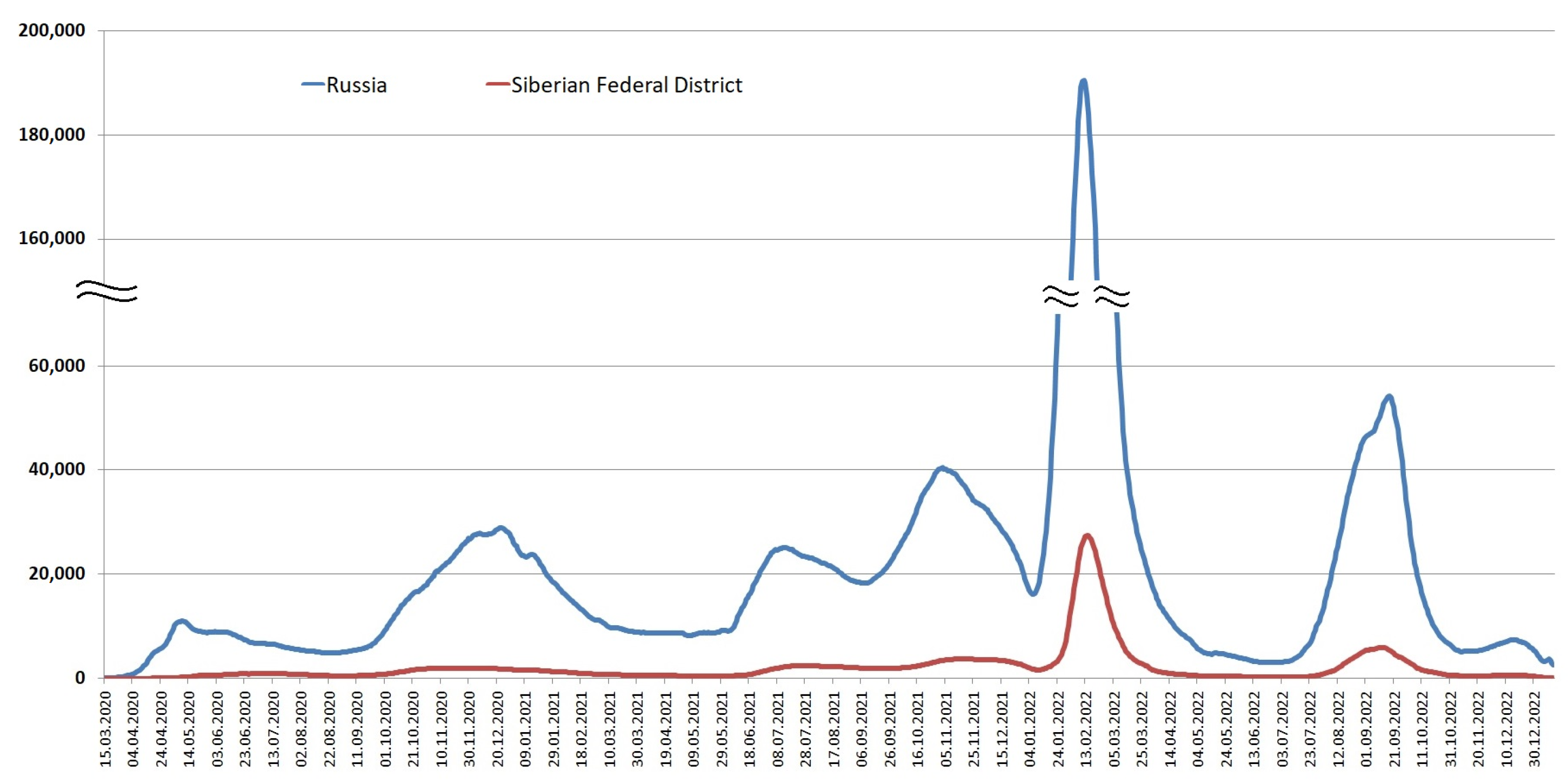
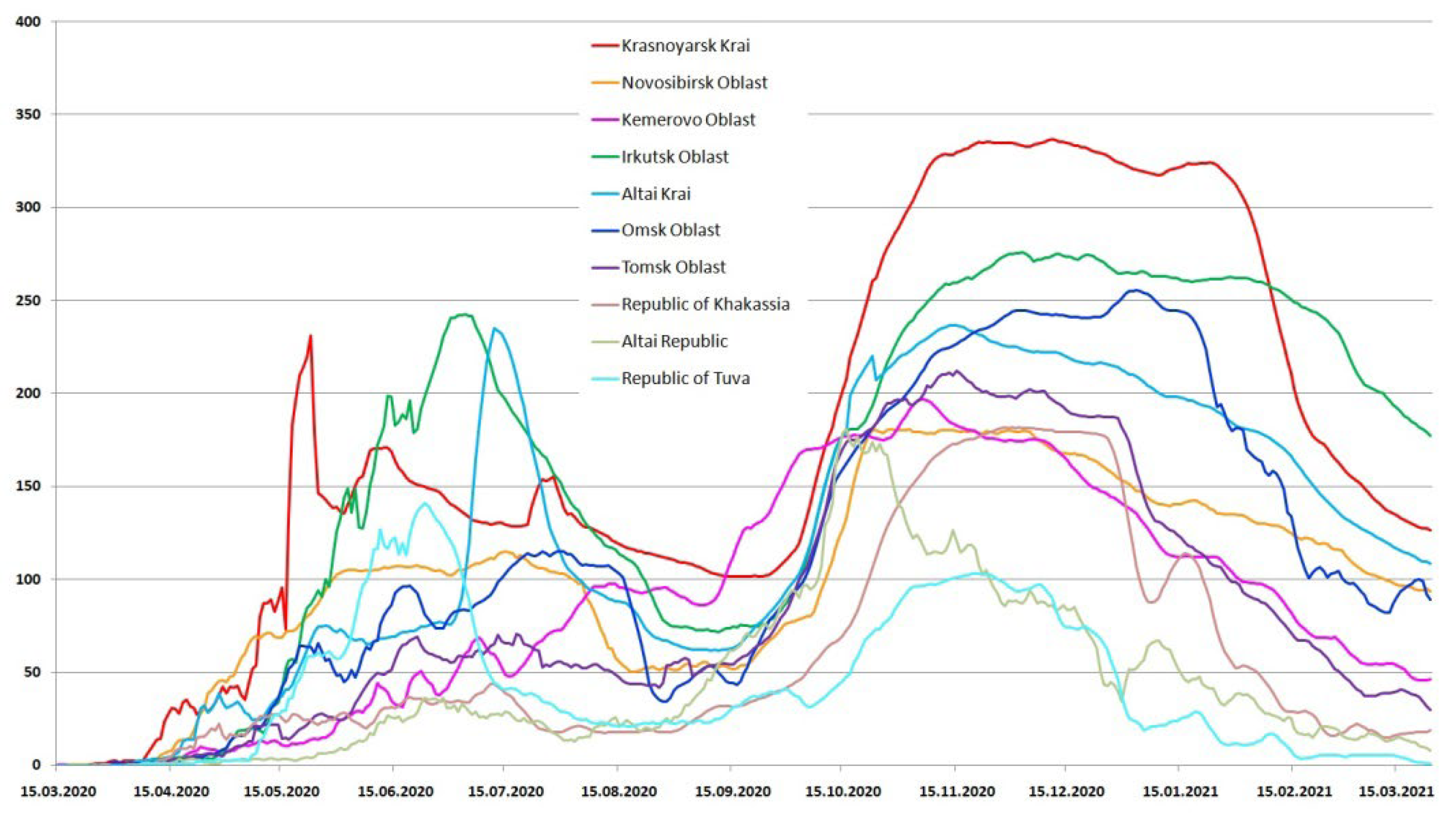
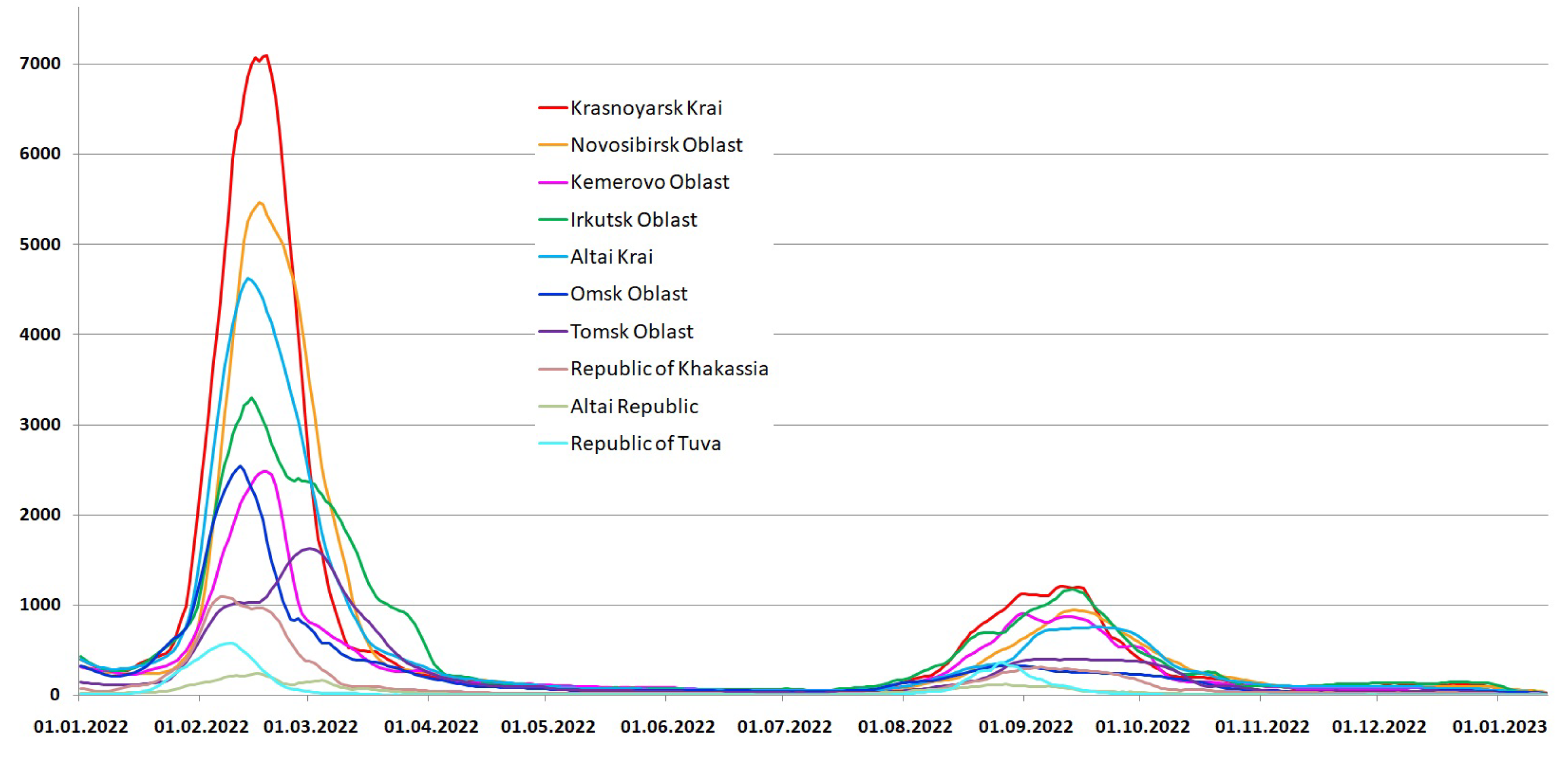
3.1. Detection of SARS-CoV-2 in the Siberian Federal District
3.2. COVID-19 Mortality in the Siberian Federal District
3.3. The Diversity of Genetic Variants of SARS-CoV-2 in the Siberian Federal District
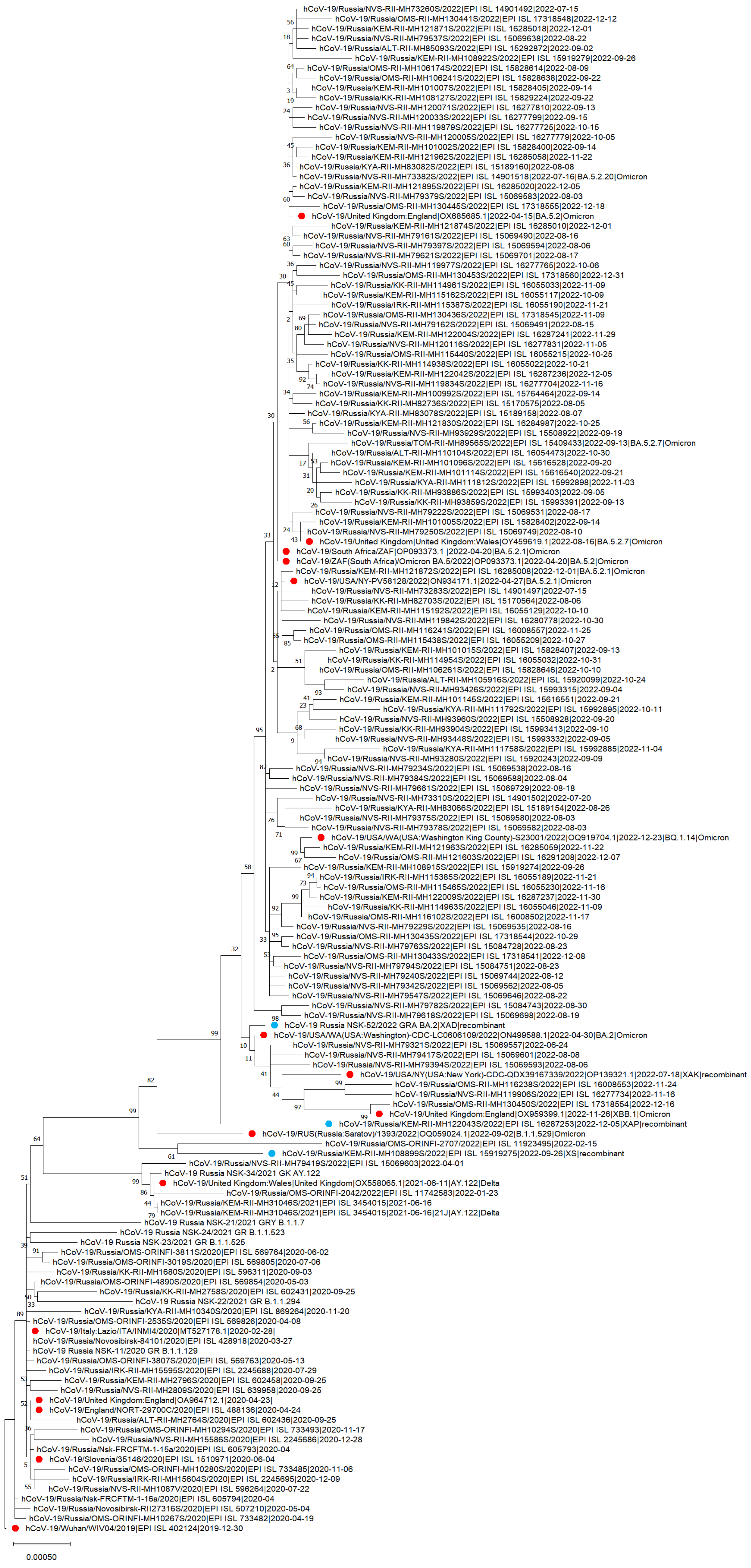
3.4. The Phylogenetic Tree of Genetic Variants of SARS-CoV-2 in the Siberian Federal District
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Komissarov, A.B.; Safina, K.R.; Garushyants, S.K.; Fadeev, A.V.; Sergeeva, M.V.; Ivanova, A.A.; Danilenko, D.M.; Lioznov, D.; Shneider, O.V.; Shvyrev, N.; et al. Genomic epidemiology of the early stages of the SARS-CoV-2 outbreak in Russia. Nat. Commun. 2021, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- The Siberian Federal District Population and the Map. Available online: http://sfo.gov.ru/okrug/ (accessed on 9 August 2023).
- The Number of SARS-CoV-2 Cases. Available online: https://datalens.yandex/7o7is1q6ikh23?tab=X1&state=f7075b93275 (accessed on 9 August 2023).
- Scientific Brief: SARS-CoV-2 Transmission. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/sciencebriefs/sars-cov-2-transmission.html (accessed on 1 September 2022).
- Popova, A.Y.; Smirnov, V.S.; Andreeva, E.E.; Babura, E.A.; Balakhonov, S.V.; Bashketova, N.S.; Bugorkova, S.A.; Bulanov, M.V.; Valeullina, N.N.; Vetrov, V.V.; et al. SARS-CoV-2 Seroprevalence Structure of the Russian Population during the COVID-19 Pandemic. Viruses 2021, 13, 1648. [Google Scholar] [CrossRef] [PubMed]
- Borisova, A.A.; Popova, N.D.; Kychkina, A.I.; Rozhina, A.A.; Fedulova, A.G. Epidemiological characteristics of the dynamics of a new coronavirus infection (COVID-19) in the republic of Sakha (Yakutia). Int. Res. J. 2022, 1, 84–87. (In Russian) [Google Scholar]
- Ochkasova, Y.V.; Korotkov, V.V.; Saveliev, S.I.; Zubchonok, N.V.; Shchukina, I.A.; Yarkovskaya, I.V.; Khodyakova, I.A.; Bondarev, V.A. Epidemiological features of the novel coronavirus disease in the Lipetsk region in 2020. Public Health Life Environ. 2021, 29, 63–68. (In Russian) [Google Scholar] [CrossRef]
- Gladkikh, A.; Dedkov, V.; Sharova, A.; Klyuchnikova, E.; Sbarzaglia, V.; Kanaeva, O.; Arbuzova, T.; Tsyganova, N.; Popova, A.; Ramsay, E.; et al. Epidemiological Features of COVID-19 in Northwest Russia in 2021. Viruses 2022, 14, 931. [Google Scholar] [CrossRef]
- Raghwani, J.; du Plessis, L.; McCrone, J.T.; Hill, S.C.; Parag, K.V.; Thézé, J.; Kumar, D.; Puvar, A.; Pandit, R.; Pybus, O.G.; et al. Genomic Epidemiology of Early SARS-CoV-2 Transmission Dynamics, Gujarat, India. Emerg. Infect. Dis. 2022, 28, 751–758. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Rahn, S.; Gödel, M.; Köster, G.; Hofinger, G. Modelling airborne transmission of SARS-CoV-2 at a local scale. PLoS ONE 2022, 17, e0273820. [Google Scholar] [CrossRef]
- Popova, A.Y.; Ezhlova, E.B.; Melnikova, A.A.; Smirnov, V.S.; Lyalina, L.V.; Ermakov, A.V.; Solomashchenko, N.I.; Kovalchuk, I.V.; Vasilenko, E.A.; Romanenko, E.N.; et al. Characteristic of herd immunity among the population of Stavropol region amid the COVID-19 epidemic. J. Infectol. 2021, 13, 79–89. (In Russian) [Google Scholar] [CrossRef]
- Popova, A.Y.; Smirnov, V.S.; Ezhlova, E.B.; Mel’nikova, A.A.; Samoilova, L.V.; Lyalina, L.V.; Semenova, E.V.; Gurskiy, M.A.; Aksenova, E.A.; Arbuzova, T.B.; et al. Herd immunity to SARS-CoV-2 in the Novosibirsk Region population amid the COVID-19 pandemic. Probl. Virol. 2021, 66, 299–309. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.; Piniaeva, A.; Ignatyev, G.; Selivanov, A.; Shishova, A.; Kovpak, A.; Gordeychuk, I.; Ivin, Y.; Berestovskaya, A.; Prokhortchouk, E.; et al. Isolation and phylogenetic analysis of SARS-CoV-2 variants collected in Russia during the COVID-19 outbreak. Int. J. Infect. Dis. 2020, 99, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Palyanova, N.; Sobolev, I.; Alekseev, A.; Glushenko, A.; Kazachkova, E.; Markhaev, A.; Kononova, Y.; Gulyaeva, M.; Adamenko, L.; Kurskaya, O.; et al. Genomic and Epidemiological Features of COVID-19 in the Novosibirsk Region during the Beginning of the Pandemic. Viruses 2022, 14, 2036. [Google Scholar] [CrossRef] [PubMed]
- Population of the Russian Federation by Gender and Age. Available online: https://rosstat.gov.ru/storage/mediabank/Bul_chislen_nasel-pv_01-01-2022.pdf (accessed on 9 August 2023).
- Arctic Primer Set. Available online: https://github.com/joshquick/artic-ncov2019/tree/master/primer_schemes/nCoV-2019 (accessed on 1 September 2022).
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Pango Lineages: Latest Epidemiological Lineages of SARS-CoV-2. Available online: https://cov-lineages.org (accessed on 1 September 2023).
- Aksamentov, I.; Roemer, C.; Hodcroft, E.B.; Neher, R.A. Nextclade: Clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 2021, 6, 3773. [Google Scholar] [CrossRef]
- Nextclade. Available online: https://clades.nextstrain.org/ (accessed on 9 August 2023).
- Mohapatra, R.K.; Kandi, V.; Tuli, H.S.; Chakraborty, C.; Dhama, K. The recombinant variants of SARS-CoV-2: Concerns continues amid COVID-19 pandemic. J. Med. Virol. 2022, 94, 3506–3508. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2008, 10, 421. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Akimkin, V.G.; Popova, A.Y.; Khafizov, K.F.; Dubodelov, D.V.; Ugleva, S.V.; Semenenko, T.A.; Ploskireva, A.A.; Gorelov, A.V.; Pshenichnaya, N.Y.; Yezhlova, E.B.; et al. COVID-19: Evolution of the pandemic in Russia. Report II: Dynamics of the circulation of SARS-CoV-2 genetic variants. J. Microbiol. Epidemiol. Immunobiol. 2022, 99, 381–396. [Google Scholar] [CrossRef]
- Karpova, L.S.; Komissarov, A.B.; Stolyarov, K.A.; Popovtseva, N.M.; Stolyarova, T.P.; Pelikh, M.Y.; Lioznov, D.A. Features of the COVID-19 Epidemic Process in Each of the of the Five Waves of Morbidity in Russia. Epidemiol. Vaccinal Prev. 2023, 22, 23–36. [Google Scholar] [CrossRef]
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). ArcGIS. Johns Hopkins University. Available online: https://coronavirus.jhu.edu/map.html (accessed on 9 August 2023).
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Salzberger, B.; Buder, F.; Lampl, B.; Ehrenstein, B.; Hitzenbichler, F.; Holzmann, T.; Schmidt, B.; Hanses, F. Epidemiology of SARS-CoV-2. Infection 2021, 49, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]

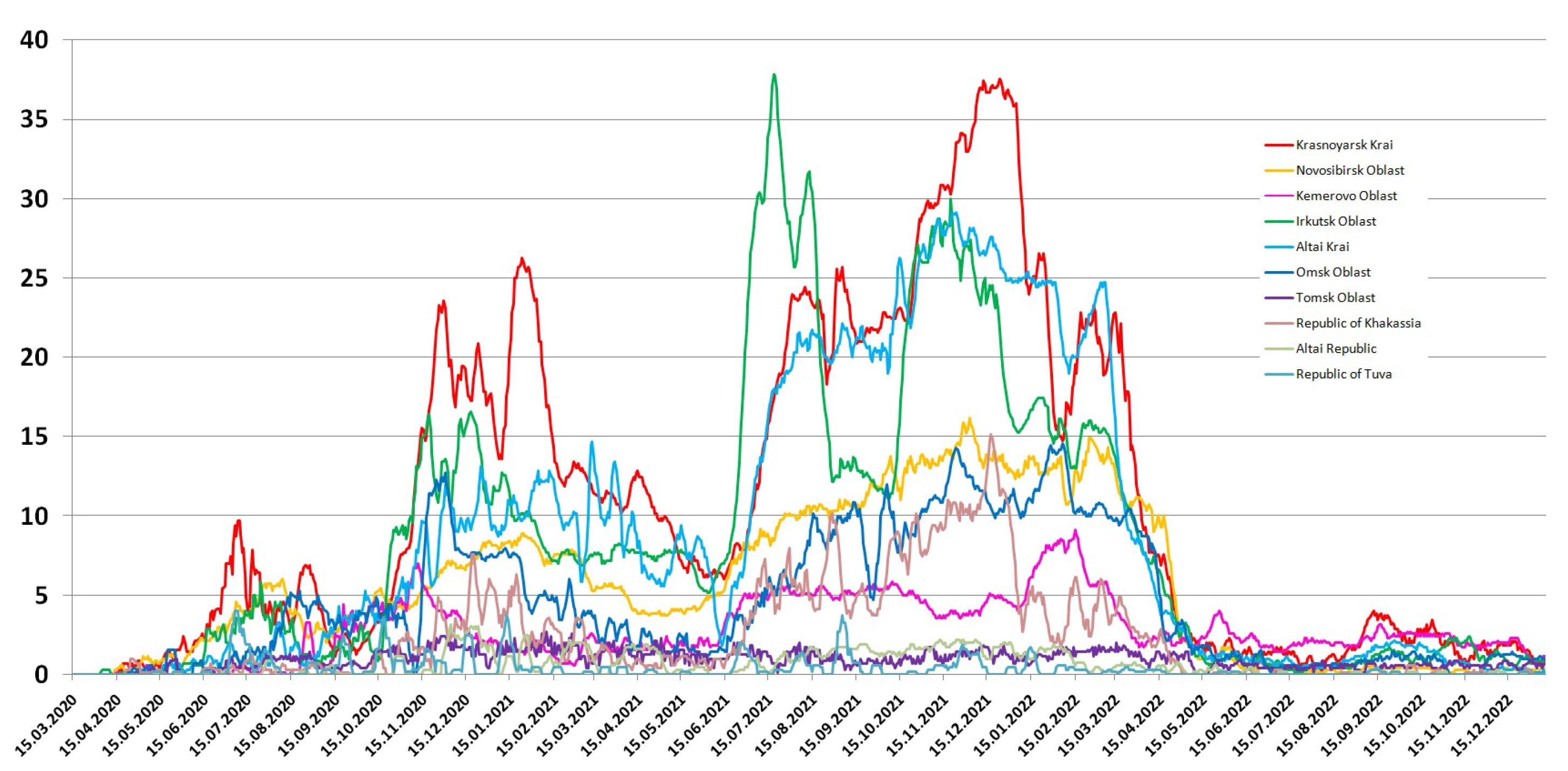
| Siberian Federal District | Population Size | Population Density | Cases | Cases per 100,000 | Deaths | Mortality, % | Cases Max per Day | Deaths Max per Day |
|---|---|---|---|---|---|---|---|---|
| Krasnoyarsk Krai | 2,849,169 | 1.2 | 427,197 | 14,993.74 | 11,227 | 2.63 | 8192 | 39 |
| Novosibirsk Oblast | 2,780,292 | 15.74 | 310,720 | 11,175.8 | 5749 | 1.85 | 5824 | 18 |
| Kemerovo Oblast | 2,604,272 | 28.3 | 213,336 | 8191.771 | 2862 | 1.34 | 2585 | 12 |
| Irkutsk Oblast | 2,357,134 | 3.05 | 358,369 | 15,203.59 | 8685 | 2.42 | 3715 | 42 |
| Altai Krai | 2,268,179 | 14.1 | 316,416 | 13,950.22 | 8853 | 2.8 | 4817 | 32 |
| Omsk Oblast | 1,879,548 | 13.12 | 231,764 | 12,330.84 | 4567 | 1.97 | 2668 | 17 |
| Tomsk Oblast | 1,068,304 | 3.4 | 158,808 | 14,865.43 | 963 | 0.61 | 1673 | 8 |
| Republic of Khakassia | 528,338 | 8.66 | 103,230 | 19,538.63 | 2427 | 2.35 | 1202 | 17 |
| Republic of Tuva | 332,609 | 1.9 | 52,384 | 15,749.42 | 358 | 0.68 | 586 | 10 |
| Altai Republic | 221,559 | 2.27 | 40,066 | 18,083.67 | 630 | 1.57 | 306 | 8 |
| Wave | First | Second | Third | Fourth | Fifth | Sixth | |
|---|---|---|---|---|---|---|---|
| Date | 15 March 2020–31 August 2020 | 1 September 2020–31 May 2021 | 1 June 2021–13 September 2021 | 14 September 2021–12 January 2022 | 13 January 2022–12 July 2022 | 24 June 2022–1 January 2023 | |
| Number of Sequences | 174 | 73 | 16 | 8 | 13 | 1605 | |
| Dominating clade | B.1.1 (20B) | B.1.1 (20B) | AY.122 (21J) Delta | AY.122 (21J) Delta | BA.1.1 (21K) Omicron | BA.5.2 (22B) Omicron | |
| Number of Sequences | 124 (71.3%) | 46 (63%) | 12 (75%) | 8 (100%) | 5 (38.5%) | 988 (61.5%) | |
| Also found | B.1 (20A), B.1.1.317 (20B), B.1.1.294 (20B), B.1.1.348 (20B), B.1.1.163 (20B), B.1.1.83 (20B), B.1.1.141 (20B) | B.1.1.141 (20B) B.1.1.294 (20B) B.1.1.317 (20B) B.1.1.348 (20B) B.1.1.397 (20B) B.1.1.398 (20B) B.1.1.525 (20B) B.1.1.523 (20B) AT.1 (20B) B.1.1.7 (20I)—Alpha | B.1.1 (20B) | no | AY.126 (21J) AY.122 (21J) BA.1.15 (21K) BA.2 (21L) | BA.1 (21K) BA.1.1 (21K) BA.2 (21L) BA.2.37 (21L) BA.2.37 (21L) BA.2.38.1 (21L) BA.2.40.1 (21L) BA.2.75.2 (22D) BA.2.75.2 (22D) BA.4.4 (22A) BA.4.5 (22A) BA.4.6 (22A) BA.5 (22B) BA.5.1 (22B) BA.5.1.10 (22B) BA.5.1.12 (22B) BA.5.1.19 (22B) BA.5.1.29 (22B) BA.5.1.3 (22B) BA.5.1.30 (22B) BA.5.1.5 (22B) BA.5.2 (22B) BA.5.2.1 (22B) BA.5.2.12 (22B) BA.5.2.16 (22B) BA.5.2.20 (22B) BA.5.2.21 (22B) BA.5.2.21 (22B) BA.5.2.24 (22B) BA.5.2.27 (22B) BA.5.2.28 (22B) BA.5.2.31 (22B) BA.5.2.32 (22B) BA.5.2.32 (22B) BA.5.2.33 (22B) | BA.5.2.41 (22B) BA.5.2.44 (22B) BA.5.2.48 (22B) BA.5.2.52 (22B) BA.5.2.54 (22B) BA.5.2.56 (22B) BA.5.2.6 (22B) BA.5.2.60 (22B) BA.5.2.62 (22B) BA.5.2.7 (22B) BA.5.3.1 (22B) BA.5.5 (22B) BA.5.6 (22B) BE.1 (22B) BE.1.1 (22B) BE.1.1.2 (22B) BF.11.5 (22B) BF.28 (22B) BF.36 (22B) BF.5 (22B) BF.7 (22B) BN.1.3 (22D) BN.1.3.1 (22D) BQ.1.1 (22E) BQ.1.2.1 (22E) BQ.1.23 (22E) CK.1 (22B) CL.1.2 (22B) CT.1 (22B) XAD (recombinant) XAP (recombinant) XBB (22F) XBB.1 (22F) XBB.1.25 (22F) XS (recombinant) |
| SeqName | Nextclade–Pango | Clade | Region | |
|---|---|---|---|---|
| 1 | hCoV-19_Russia_NSK-52/2022_GRA_BA.2 (not deposited) | XAD | recombinant | Novosibirsk Oblast |
| 2 | hCoV-19/Russia/KEM-RII-MH108899S/2022|EPI_ISL_15919275|2022-09-26 | XS | recombinant | Kemerovo Oblast |
| 3 | hCoV-19/Russia/KEM-RII-MH122043S/2022|EPI_ISL_16287253|2022-12-05 | XAP | recombinant | Kemerovo Oblast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palyanova, N.V.; Sobolev, I.A.; Palyanov, A.Y.; Kurskaya, O.G.; Komissarov, A.B.; Danilenko, D.M.; Fadeev, A.V.; Shestopalov, A.M. The Development of the SARS-CoV-2 Epidemic in Different Regions of Siberia in the 2020–2022 Period. Viruses 2023, 15, 2014. https://doi.org/10.3390/v15102014
Palyanova NV, Sobolev IA, Palyanov AY, Kurskaya OG, Komissarov AB, Danilenko DM, Fadeev AV, Shestopalov AM. The Development of the SARS-CoV-2 Epidemic in Different Regions of Siberia in the 2020–2022 Period. Viruses. 2023; 15(10):2014. https://doi.org/10.3390/v15102014
Chicago/Turabian StylePalyanova, Natalia V., Ivan A. Sobolev, Andrey Yu. Palyanov, Olga G. Kurskaya, Andrey B. Komissarov, Daria M. Danilenko, Artem V. Fadeev, and Alexander M. Shestopalov. 2023. "The Development of the SARS-CoV-2 Epidemic in Different Regions of Siberia in the 2020–2022 Period" Viruses 15, no. 10: 2014. https://doi.org/10.3390/v15102014
APA StylePalyanova, N. V., Sobolev, I. A., Palyanov, A. Y., Kurskaya, O. G., Komissarov, A. B., Danilenko, D. M., Fadeev, A. V., & Shestopalov, A. M. (2023). The Development of the SARS-CoV-2 Epidemic in Different Regions of Siberia in the 2020–2022 Period. Viruses, 15(10), 2014. https://doi.org/10.3390/v15102014






