Abstract
The genus Pestivirus of the family Flaviviridae mainly comprises classical swine fever virus (CSFV), bovine viral diarrhea virus 1 (BVDV-1), BVDV-2, border disease virus (BDV), and multiple new pestivirus species such as atypical porcine pestivirus (APPV), giraffe pestivirus, and antelope pestivirus. Pestiviruses cause infectious diseases, resulting in tremendous economic losses to animal husbandry. Different types of pestivirus vaccines have been developed to control and prevent these important animal diseases. In recent years, pestiviruses have shown great potential as viral vectors for developing multivalent vaccines. This review analyzes the advantages and disadvantages of various pestivirus vaccines, including live attenuated pestivirus strains, genetically engineered marker pestiviruses, and pestivirus-based multivalent vaccines. This review provides new insights into the development of novel vaccines against emerging pestiviruses, such as APPV and ovine pestivirus.
1. Introduction
The genus Pestivirus (from the Latin pestis—plague), belonging to the family Flaviviridae, is responsible for infectious diseases in swine, cattle, sheep, goats, and other domestic and wild animals. According to the recent reclassification of the genus Pestivirus by the International Committee on Taxonomy of Viruses (ICTV), the pestivirus taxonomy resulted in the demarcation of eleven species designated pestiviruses A through K. Due to the increasing number of diverse pestiviruses, the taxonomy of the genus Pestivirus was revised in 2017 based on nucleotide or amino acid sequence distances of complete coding sequences, combined with antigenic differences, natural host ranges, and pathology. The number of pestivirus species was expanded to nineteen by identifying eight new species designated pestiviruses L through S, including atypical porcine pestivirus (APPV), giraffe pestivirus, antelope pestivirus, HoBi-like pestivirus, Bungowannah virus, and Linda virus [1,2,3,4,5,6,7,8,9]. Pestiviruses contain a single-stranded, positive-sense RNA [10,11,12]. The sizes of pestiviral genomes are approximately 12.3 kb, harboring a 5′ untranslated region (UTR), a single long open reading frame (ORF), and a 3′ UTR. The ORF encodes four structural and eight nonstructural proteins that are processed by viral and cellular proteases [13,14,15,16,17].
The pestivirus-associated epidemics have caused significant economic losses in many countries with intensive animal husbandry. The prevention and treatment of pestiviral infections have become quite challenging due to the lack of strict host specificity and the considerable variability among pestiviruses [15,18,19,20]. Live attenuated vaccines (LAVs) have been developed to prevent and control pestiviral infections. Some of the vaccines are commercially available. However, most LAVs do not allow differentiation by serology of infected from vaccinated animals (DIVA), complicating the eradication of the related disease [21]. Marker vaccines are highly preferred since they can avoid the economic loss caused by the ethically and economically disadvantageous slaughter and culling during the implementation of the “stamping-out policy” for CSF [22]. For the eradication of CSF, different types of marker vaccines that comply with the DIVA principle have been developed based on the E2 protein or chimeric pestiviruses. Additionally, co-infections of pestiviruses with other viruses, such as pseudorabies virus (PRV), African swine fever virus (ASFV), porcine parvovirus (PPV), and porcine circovirus type 2 (PCV2), pose a significant threat to the swine industry [23,24,25,26,27,28]. Therefore, the development of a multivalent vaccine is necessary for the prevention of co-infections.
In this review, we summarize the development of various pestivirus vaccines and discuss in detail the advantages and disadvantages of these vaccines in terms of the safety, efficacy, and feasibility to express foreign genes in the backbone of pestiviruses.
3. Development of Multivalent Vaccines Based on Pestiviruses
At present, various foreign genes of varied sizes have been engineered into different positions of the pestiviral genome (Figure 1), suggesting that pestiviruses can be used as vectors to deliver foreign genes. The pestiviral genome replicates in the cytoplasm, and the viral RNA cannot be integrated into the host cell genome, ensuring the biosafety of pestiviruses. However, whether a pestivirus can be used as a classic viral vector to express foreign genes needs further exploration. The marker pestivirus vaccines such as engineered marker C-strain or CP7_E2alf can be used as viral vectors for developing bivalent/multivalent vaccines. Further studies are needed to determine whether the LAV-based recombinant vaccines expressing foreign genes are safe.
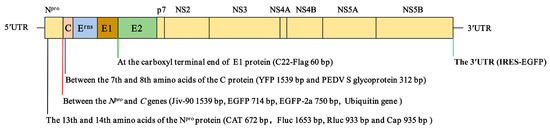
Figure 1.
Schematic diagram of the foreign gene insertion in the pestiviral genome. The insertion sites and sizes of foreign genes in the pestiviral genome are shown. The foreign genes can be inserted between the 13th and 14th amino acids of the Npro protein, downstream of the Npro protein and the 7th and 8th amino acids of the C protein, between E1 and E2, and upstream of the 3′ UTR of the pestiviral genome.
3.1. The Sites Suitable for the Insertion of Foreign Genes
Various strategies associated with foreign gene expression have been attempted. A widely used expression strategy is to fuse the foreign gene with one of the viral proteins. It was reported that the gene coding for bacterial chloramphenicol acetyltransferase (CAT) could be inserted into the viral Npro gene of the CSFV strain Alfort/187 [114]. Then, EGFP, Fluc, and Rluc were fused to the viral Npro protein of the CSFV Shimen strain, respectively [75,76,77]. It has been shown that the Npro and C genes of CSFV can allow the insertion of foreign genes [114]. Our previous study also demonstrated that the EGFP gene could be inserted between the 13th and 14th amino acids of the Npro protein of the highly virulent CSFV Shimen strain [115]. Based on the safety and efficacy of attenuated vaccines against pestiviral infections, these vaccine strains have great potential as viral vectors for developing multivalent vaccines to control co-infections in pigs.
The vaccines based on viral vectors can activate the humoral and cellular immunity of the hosts [62,116,117,118]. For example, the double-strand RNA produced during genome replication can enhance the efficacy [119,120]. The foreign genes in the viral vector are expressed continually and induce persistent immunity [121]. The functions of foreign proteins are minimally affected due to the smaller size of the structural proteins.
However, this is no evidence that this strategy can be suitable for C-strain. The foreign gene can be expressed separately by introducing an internal ribosome entry site (IRES) or the foot-and-mouth disease virus (FMDV) 2A self-cleaving peptide, which can undergo self-cleavage and not affect the expression of other proteins on the vector. For example, a recombinant C-strain expressing the PCV2 Cap by 2A-mediated cleavage was generated and evaluated in vitro and in vivo [122]. Notably, the same strategy might lead to different effects on the growth of the same virus due to the different insertion sites. For example, the use of the 2A peptide to achieve expression of a separate reporter might constitute a promising approach as the 2A peptide is small and can readily be self-cleaving while minimizing the possibility of the loss of functions of the viral proteins.
It is well-documented that the Npro gene is not required for CSFV replication, and the CSFV mutant lacking the full-length Npro gene is attenuated in pigs [123,124,125]. Remarkably, the CSFV mutant with the C gene deleted and the NS3 gene mutated was rescued and attenuated, suggesting that the C gene was dispensable for viral replication and able to be replaced by foreign genes [126]. Furthermore, the Erns-deleted CSFV mutant was developed as a potential non-transmissible, live-attenuated marker vaccine [72]. Therefore, foreign genes might be introduced by substituting the CSFV Npro, C, or Erns genes.
The EGFP and FMDV 2A fusion gene was inserted between the Npro and C genes of the noncytopathic BVDV strain SD1. In addition, the reporter virus was similar to wt SD1 in viral RNA replication and protein expression and comparable to wt SD1 in growth kinetics; however, this virus had a peak virus titer approximately 0.5 log10 lower and a maximum yield about 4 h later than wt SD1. The study has indicated that BVDV is a suitable viral vector for the stable expression of heterologous genes when inserted between the Npro and C genes [127]. The foreign gene encoding the PEDV S glycoprotein was inserted between the seventh and eighth amino acids of the C protein of the BVDV genome by homologous recombination vASH-dS312, which could successfully express the PEDV S glycoprotein. The immunized mice were healthy and showed no clinical symptoms. The antigen S499-602 was inserted into the infectious cDNA clone pASH28 of pig-originated BVDV-2 in tandem by overlapping PCR, located between the seventh and eighth amino acids of the C protein. IgG antibodies against BVDV and PEDV could be detected in the mice administered with vASH-dS312 by intramuscular injection, which had neutralizing activity against BVDV and PEDV [128]. However, none of these experimental vaccines have been subjected to an immune evaluation in pigs, and thus it remains unknown whether they can provide protection.
3.2. The Size and Genetic Stability of the Inserted Foreign Genes
Though much smaller than the genomes of popular vectors like PRV and vaccinia virus, the pestiviral genome also allows the insertion of foreign genes. EGFP (714 bp), Fluc (1653 bp), and Rluc (933 bp) have each been inserted into the genome of the CSFV Shimen strain [115,129,130]. The insertion of 1,539 bp was achieved in the CSFV strain Alfort-p447, and the insertion derived from the cytopathic BVDV strain CP8 encoded a 513-aa fusion peptide, encompassing fragments from viral sequences (C, Erns, and Npro) and cellular sequences [JivI, JivII, and bovine homolog to human nuclear protein Hcc-1 (Hcc-1 *)] [131]. Whether the attenuated vaccine strains (such as C-strain) can tolerate similar insertions warrants experimentation. Since the pestiviral genome is relatively small, the delivery of antigens with smaller molecular weights may be more suitable for pestiviruses.
3.3. The Expression Levels of Foreign Genes in the Pestiviruses
The expression levels of the foreign genes in the pestiviral vectors can be affected by various factors, such as the insertion sites and expression strategy. For example, the titers of the engineered C-strain and its mutants are relatively low, which might affect the expression levels of foreign genes. The robust adaptation of the attenuated viruses to the cells and the manufacturing techniques are required to improve the viral titers [132].
Our group has generated three C-strain-based recombinant viruses expressing the capsid (Cap) gene of PCV2 by reverse genetic manipulation (Figure 1). The Cap protein is a major immunogenic protein of PCV2, which can induce protective immunity [133]. The data showed that rHCLV-uspCap and rHCLV-pspCap rather than rHCLV-2ACap elicited detectable anti-Cap antibodies in rabbits, which demonstrated that C-strain could be used as a viral vector to develop bivalent vaccines [122]. Furthermore, it has been shown that the recombinant BVDV expressing the PEDV spike protein, as a recombinant virus vector, can induce higher titers of NAbs and provide protection against virulent challenge [128,134].
4. The Limitations and Prospects of Pestivirus Vaccines
In summary, a number of vaccine strategies have been explored to combat pestivirus infections of livestock and wildlife. However, there are several limitations in the pestivirus vaccines. Firstly, the safety of engineered vaccines based on pestiviral vectors should be evaluated in the field. This is relevant for vaccination against infectious diseases and potentially exploiting virus-based vectors in vaccination strategies where individuals are sometimes immunocompromised. Secondly, maternally derived antibodies may inhibit the immune responses of LAVs. Thirdly, subunit vaccines usually cannot provide rapid-onset and complete protection. Additionally, new-type pestivirus vaccines need a long time before they can be commercially available.
Replicons are replication-competent RNA molecules that are incapable of generating infectious progeny viruses due to the loss of one or more structural proteins. The genome sequences encoding NS3-NS5B together with the 5′ and 3′ UTRs are the minimal elements required for autonomous pestiviral RNA replication [135]. Significantly, the viral E2-coding region can enhance the replication efficiency of the CSFV RNA replicon [136]. The RNA replicon activates the cellular immunity of the hosts and has potential as a vector to express foreign genes. For instance, chimeric CSF-Japanese encephalitis (JE) viral replicon as a non-transmissible vaccine candidate has been proven effective against CSFV and JEV infections [137]. Other studies have confirmed that activated specific cellular immunity (CD8+/CD4+ T cells) contributes to the protection against ASFV infection [138,139]. Considering the safety and efficacy of the pestivirus RNA replicons, multivalent vaccines can be developed based on the RNA replicons.
Viral vectors hold great promise for development of multivalent vaccines to counter co-infectious diseases. Compared with other viral vectors, pestivirus-based vectors have the following advantages: (1) The infectious clones of the RNA viruses are easier to be engineered than those of the DNA viruses due to their smaller genome size of 7 to 19 kb; (2) The double-stranded RNA produced by the genome replication can enhance the immunization efficacy; (3) Viral proteins are expressed with high efficacy; (4) The replication of the RNA occurs in the cytoplasm rather than in the nucleus, thus avoiding the RNA degradation; (5) No RNA can be integrated into the DNA of the host; (6) Since they have fewer viral structural proteins and cause less immune responses against the vector, the pestiviral vectors have great potential for developing safe and effective vaccines against the animal diseases.
Several CSF vaccines have been developed for oral immunization of wild boars [140,141,142,143,144,145,146,147,148]. For example, immunization with an oral bait vaccine based on C-strain proved to be safe and efficacious. It has been demonstrated that CP7_E2alf is a safe and efficient marker vaccine strain for oral immunization of wild boars against CSF [106,149,150]. New immunization regimes of pestivirus vaccines may be developed in the future.
Collectively, various pestivirus vaccines have been developed by different attractive strategies and have shown the advantages and disadvantages in terms of the safety, efficacy involved in the rapid-onset protection, cross-protection, and DIVA potential, which will provide new insights into the development of novel vaccines against emerging pestiviruses.
Author Contributions
Conceptualization, H.-J.Q. and Y.L.; writing—original draft preparation, M.Y. and X.Y.; writing—review and revision, M.Y., X.Y., X.Z. (Xiaotian Zhao), X.Z. (Xin Zhang), and M.A.; figure preparation, M.Y.; manuscript revision and supervision, H.-J.Q. and Y.L.; and funding acquisition, H.-J.Q. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (grant no. 2021YFD1801401), the National Natural Science Foundation of China (no. 31972673), and the Natural Science Foundation of Heilongjiang Province of China (no. YQ2021C037).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hause, B.; Collin, E.A.; Peddireddi, L.; Yuan, F.; Chen, Z.; Hesse, R.A.; Gauger, P.C.; Clement, T.; Fang, Y.; Anderson, G. Discovery of a novel putative atypical porcine pestivirus in pigs in the United States. J. Gen. Virol. 2015, 96, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Smith, D.B.; Becher, P. Proposed update to the taxonomy of pestiviruses: Eight additional species within the genus Pestivirus, Family Flaviviridae. Viruses 2021, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Bolin, S.R. The genomic sequence of a virulent bovine viral diarrhea virus (BVDV) from the type 2 genotype: Detection of a large genomic insertion in a noncytopathic BVDV. Virology 1995, 212, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ruggli, N.; Tratschin, J.D.; Mittelholzer, C.; Hofmann, M.A. Nucleotide sequence of classical swine fever virus strain Alfort/187 and transcription of infectious RNA from stably cloned full-length cDNA. J. Virol. 1996, 70, 3478–3487. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.; Shannon, A.D.; Tautz, N.; Thiel, H.J. Molecular characterization of border disease virus, a pestivirus from sheep. Virology 1994, 198, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.; Orlich, M.; Kosmidou, A.; König, M.; Baroth, M.; Thiel, H.J. Genetic diversity of pestiviruses: Identification of novel groups and implications for classification. Virology 1999, 262, 64–71. [Google Scholar] [CrossRef]
- Schirrmeier, H.; Strebelow, G.; Depner, K.; Hoffmann, B.; Beer, M. Genetic and antigenic characterization of an atypical pestivirus isolate, a putative member of a novel pestivirus species. J. Gen. Virol. 2004, 85, 3647–3652. [Google Scholar] [CrossRef]
- Kirkland, P.D.; Frost, M.J.; Finlaison, D.S.; King, K.R.; Ridpath, J.F.; Gu, X. Identification of a novel virus in pigs--Bungowannah virus: A possible new species of pestivirus. Virus Res. 2007, 129, 26–34. [Google Scholar] [CrossRef]
- Lamp, B.; Schwarz, L.; Högler, S.; Riedel, C.; Sinn, L.; Rebel-Bauder, B.; Weissenböck, H.; Ladinig, A.; Rümenapf, T. Novel pestivirus species in pigs, Austria, 2015. Emerg. Infect. Dis. 2017, 23, 1176–1179. [Google Scholar] [CrossRef]
- Moennig, V. The hog cholera virus. Comp. Immunol. Microbiol. Infect. Dis. 1992, 15, 189–201. [Google Scholar] [CrossRef]
- Collett, M.S.; Larson, R.; Gold, C.; Strick, D.; Anderson, D.K.; Purchio, A.F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology 1988, 165, 191–199. [Google Scholar] [CrossRef]
- Renard, A.; Guiot, C.; Schmetz, D.; Dagenais, L.; Pastoret, P.P.; Dina, D.; Martial, J.A. Molecular cloning of bovine viral diarrhea viral sequences. DNA 1985, 4, 429–438. [Google Scholar] [CrossRef]
- Collett, M.S.; Moennig, V.; Horzinek, M.C. Recent advances in pestivirus research. J. Gen. Virol. 1989, 70, 253–266. [Google Scholar] [CrossRef]
- Thiel, H.J.; Stark, R.; Weiland, E.; Rümenapf, T.; Meyers, G. Hog cholera virus: Molecular composition of virions from a pestivirus. J. Virol. 1991, 65, 4705–4712. [Google Scholar] [CrossRef]
- Becher, P.; Thiel, H.J.; Collins, M.; Brownlie, J.; Orlich, M. Cellular sequences in pestivirus genomes encoding gamma-aminobutyric acid (A) receptor-associated protein and Golgi-associated ATPase enhancer of 16 kilodaltons. J. Virol. 2002, 76, 13069–13076. [Google Scholar] [CrossRef]
- Stark, R.; Meyers, G.; Rümenapf, T.; Thiel, H.J. Processing of pestivirus polyprotein: Cleavage site between autoprotease and nucleocapsid protein of classical swine fever virus. J. Virol. 1993, 67, 7088–7095. [Google Scholar] [CrossRef]
- Rümenapf, T.; Unger, G.; Strauss, J.H.; Thiel, H.J. Processing of the envelope glycoproteins of pestiviruses. J. Virol. 1993, 67, 3288–3294. [Google Scholar] [CrossRef]
- Pinior, B.; Firth, C.L.; Richter, V.; Lebl, K.; Trauffler, M.; Dzieciol, M.; Hutter, S.E.; Burgstaller, J.; Obritzhauser, W.; Winter, P.; et al. A systematic review of financial and economic assessments of bovine viral diarrhea virus (BVDV) prevention and mitigation activities worldwide. Prev. Vet. Med. 2017, 137, 77–92. [Google Scholar] [CrossRef]
- Evans, C.A.; Pinior, B.; Larska, M.; Graham, D.; Schweizer, M.; Guidarini, C.; Decaro, N.; Ridpath, J.; Gates, M.C. Global knowledge gaps in the prevention and control of bovine viral diarrhoea (BVD) virus. Transbound. Emerg. Dis. 2019, 66, 640–652. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Neill, J.D. Pestiviruses: Old enemies and new challenges. Anim. Health Res. Rev. 2015, 16, 1–3. [Google Scholar] [CrossRef][Green Version]
- van Oirschot, J.T. DIVA vaccines that reduce virus transmission. J. Biotechnol. 1999, 73, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.N.; Chen, Y.H. Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine 2007, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Cabezón, O.; Muñoz-González, S.; Colom-Cadena, A.; Pérez-Simó, M.; Rosell, R.; Lavín, S.; Marco, I.; Fraile, L.; de la Riva, P.M.; Rodríguez, F.; et al. African swine fever virus infection in classical swine fever subclinically infected wild boars. BMC Vet. Res. 2017, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Huang, Y.; Ye, M.; Li, S.; Xiao, Y.; Cui, B.; Zhu, J. Co-infection status of classical swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circoviruses (PCV2 and PCV3) in eight regions of China from 2016 to 2018. Infect. Genet. Evol. 2019, 68, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Pan, Y.; Liu, M.; Han, Z. Prevalence of porcine pseudorabies virus and its co-infection rate in Heilongjiang province in China from 2013 to 2018. Viral Immunol. 2020, 33, 550–554. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, M.; Liu, Z.; Meng, F.; Wang, H.; Cao, L.; Li, Y.; Jiao, Q.; Han, Z.; Liu, S. Epidemiological investigation of porcine circovirus type 2 and its co-infection rate in Shandong province in China from 2015 to 2018. BMC Vet. Res. 2021, 17, 17. [Google Scholar] [CrossRef]
- Casciari, C.; Sozzi, E.; Bazzucchi, M.; Moreno Martin, A.M.; Gaffuri, A.; Giammarioli, M.; Lavazza, A.; De Mia, G.M. Serological relationship between a novel ovine pestivirus and classical swine fever virus. Transbound. Emerg. Dis. 2020, 67, 1406–1410. [Google Scholar] [CrossRef]
- Toplu, N.; Oguzoglu, T.C.; Albayrak, H. Dual infection of fetal and neonatal small ruminants with border disease virus and peste des petits ruminants virus (PPRV): Neuronal tropism of PPRV as a novel finding. J. Comp. Pathol. 2012, 146, 289–297. [Google Scholar] [CrossRef]
- Blome, S.; Moß, C.; Reimann, I.; König, P.; Beer, M. Classical swine fever vaccines-state-of-the-art. Vet. Microbiol. 2017, 206, 10–20. [Google Scholar] [CrossRef]
- Griebel, P.J. BVDV vaccination in North America: Risks versus benefits. Anim. Health Res. Rev. 2015, 16, 27–32. [Google Scholar] [CrossRef]
- Al-Kubati, A.A.G.; Hussen, J.; Kandeel, M.; Al-Mubarak, A.I.A.; Hemida, M.G. Recent advances on the bovine viral diarrhea virus molecular pathogenesis, immune response, and vaccines development. Front. Vet. Sci. 2021, 8, 665128. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, Y.; Zhang, G. Research progress and challenges in vaccine development against classical swine fever virus. Viruses 2021, 13, 445. [Google Scholar] [CrossRef]
- Tong, C.; Liu, H.; Wang, J.; Sun, Y.; Chen, N. Safety, efficacy, and DIVA feasibility on a novel live attenuated classical swine fever marker vaccine candidate. Vaccine 2022, 40, 7219–7229. [Google Scholar] [CrossRef]
- Fulton, R.W. Impact of species and subgenotypes of bovine viral diarrhea virus on control by vaccination. Anim. Health Res. Rev. 2015, 16, 40–54. [Google Scholar] [CrossRef]
- Qiu, H.J.; Tong, G.Z.; Shen, R.X. The lapinized Chinese strain of classical swine fever virus: A retrospective review spanning half a century. J. Integr. Agr. 2005, 38, 1675–1685. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Wang, Z.; Sun, J.; Mi, S.; Xu, J.; Cao, J.; Hou, Y.; Wang, D.; Huo, X.; et al. Commercial E2 subunit vaccine provides full protection to pigs against lethal challenge with 4 strains of classical swine fever virus genotype 2. Vet. Microbiol. 2019, 237, 108403. [Google Scholar] [CrossRef]
- Eblé, P.L.; Quak, S.; Geurts, Y.; Moonen-Leusen, H.W.; Loeffen, W.L. Efficacy of CSF vaccine CP7_E2alf in piglets with maternally derived antibodies. Vet. Microbiol. 2014, 174, 27–38. [Google Scholar] [CrossRef]
- Renson, P.; Le Dimna, M.; Keranflech, A.; Cariolet, R.; Koenen, F.; Le Potier, M.F. CP7_E2alf oral vaccination confers partial protection against early classical swine fever virus challenge and interferes with pathogeny-related cytokine responses. Vet. Res. 2013, 44, 9. [Google Scholar] [CrossRef]
- Beer, M.; Hehnen, H.R.; Wolfmeyer, A.; Poll, G.; Kaaden, O.R.; Wolf, G. A new inactivated BVDV genotype I and II vaccine. An immunisation and challenge study with BVDV genotype I. Vet. Microbiol. 2000, 77, 195–208. [Google Scholar] [CrossRef]
- Abd, E.l.; Fadeel, M.R.; El-Dakhly, A.T.; Allam, A.M.; Farag, T.K.; El-Kholy, A.A. Preparation and efficacy of freeze-dried inactivated vaccine against bovine viral diarrhea virus genotypes 1 and 2, bovine herpes virus type 1.1, bovine parainfluenza-3 virus, and bovine respiratory syncytial virus. Clin. Exp. Vaccine Res. 2020, 9, 119–125. [Google Scholar]
- Makoschey, B.; Janssen, M.; Vrijenhoek, M.P.; Korsten, J.H.; Marel, P. An inactivated bovine virus diarrhoea virus (BVDV) type 1 vaccine affords clinical protection against BVDV type 2. Vaccine 2001, 19, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, K.; Schnakel, J.; Chase, C.C. Evaluation of a modified live virus type-l a bovine viral diarrhea virus vaccine (Singer strain) against a type-2 (strain 890) challenge. Vet. Ther. 2003, 4, 24–34. [Google Scholar] [PubMed]
- Zhou, W.; Gao, S.; Podgórska, K.; Stadejek, T.; Qiu, H.J.; Yin, H.; Drew, T.; Liu, L. Rovac is the possible ancestor of the Russian lapinized vaccines LK-VNIVViM and CS strains but not the Chinese strain (C-strain) vaccine against classical swine fever. Vaccine 2014, 32, 6639–6642. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, J.T. Vaccinology of classical swine fever: From lab to field. Vet. Microbiol. 2003, 96, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Björklund, H.V.; Stadejek, T.; Vilcek, S.; Belák, S. Molecular characterization of the 3′ noncoding region of classical swine fever virus vaccine strains. Virus Genes 1998, 16, 307–312. [Google Scholar] [CrossRef]
- Oláh, P.; Palatka, Z. Immunobiological study of lapinized Hog cholera virus strains. Acta Vet. Acad. Sci. Hung. 1967, 17, 239–247. [Google Scholar]
- Corthier, G.; Aynaud, J.M. Comparison of the immune response in serum and bucco-pharyngeal secretions following immunization by different routes with a live hog cholera virus vaccine (Thiverval strain). Ann. Rech. Vet. 1977, 8, 159–165. [Google Scholar]
- Lamothe-Reyes, Y.; Bohórquez, J.A.; Wang, M.; Alberch, M.; Pérez-Simó, M.; Rosell, R.; Ganges, L. Early and solid protection afforded by the Thiverval vaccine provides novel vaccination alternatives against classical swine fever virus. Vaccines 2021, 9, 464. [Google Scholar] [CrossRef]
- Terpstra, C.; Wensvoort, G. The protective value of vaccine-induced neutralising antibody titers in swine fever. Vet. Microbiol. 1988, 16, 123–128. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Everett, H.E.; Graham, S.P.; Steinbach, F.; Crooke, H.R. Head start immunity: Characterizing the early protection of C-strain vaccine against subsequent classical swine fever virus infection. Front. Immunol. 2019, 10, 1584. [Google Scholar] [CrossRef]
- Tamura, T.; Sakoda, Y.; Yoshino, F.; Nomura, T.; Yamamoto, N.; Sato, Y.; Okamatsu, M.; Ruggli, N.; Kida, H. Selection of classical swine fever virus with enhanced pathogenicity reveals synergistic virulence determinants in E2 and NS4B. J. Virol. 2012, 86, 8602–8613. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ji, S.; Lei, J.L.; Xiang, G.T.; Liu, Y.; Gao, Y.; Meng, X.Y.; Zheng, G.; Zhang, E.Y.; Wang, Y.; et al. Efficacy evaluation of the C-strain-based vaccines against the subgenotype 2.1d classical swine fever virus emerging in China. Vet. Microbiol. 2017, 201, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kosmidou, A.; Ahl, R.; Thiel, H.J.; Weiland, E. Differentiation of classical swine fever virus (CSFV) strains using monoclonal antibodies against structural glycoproteins. Vet. Microbiol. 1995, 47, 111–118. [Google Scholar] [CrossRef] [PubMed]
- van Gennip, H.G.; van Rijn, P.A.; Widjojoatmodjo, M.N.; de Smit, A.J.; Moormann, R.J. Chimeric classical swine fever viruses containing envelope protein Erns or E2 of bovine viral diarrhoea virus protect pigs against challenge with CSFV and induce a distinguishable antibody response. Vaccine 2000, 19, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Madera, R.; Gong, W.; Wang, L.; Burakova, Y.; Lleellish, K.; Galliher-Beckley, A.; Nietfeld, J.; Henningson, J.; Jia, K.; Li, P.; et al. Pigs immunized with a novel E2 subunit vaccine are protected from subgenotype heterologous classical swine fever virus challenge. BMC Vet. Res. 2016, 12, 197. [Google Scholar] [CrossRef]
- Lin, M.; Trottier, E.; Pasick, J. Antibody responses of pigs to defined Erns fragments after infection with classical swine fever virus. Clin. Diagn. Lab. Immunol. 2005, 12, 180–186. [Google Scholar]
- Zhang, H.; Li, X.; Peng, G.; Tang, C.; Zhu, S.; Qian, S.; Xu, J.; Qian, P. Glycoprotein E2 of classical swine fever virus expressed by baculovirus induces the protective immune responses in rabbits. Vaccine 2014, 32, 6607–6613. [Google Scholar] [CrossRef]
- Suárez-Pedroso, M.; Sordo-Puga, Y.; Sosa-Teste, I.; Rodriguez-Molto, M.P.; Naranjo-Valdés, P.; Sardina-González, T.; Santana-Rodríguez, E.; Montero-Espinosa, C.; Frías-Laporeaux, M.T.; Fuentes-Rodríguez, Y.; et al. Novel chimeric E2-CD154 subunit vaccine is safe and confers long lasting protection against classical swine fever virus. Vet. Immunol. Immunopathol. 2021, 234, 110222. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Han, G.; Fang, W.; He, F. Identification of E2 with improved secretion and immunogenicity against CSFV in piglets. BMC Microbiol. 2020, 20, 26. [Google Scholar] [CrossRef]
- Suárez, M.; Sordo, Y.; Prieto, Y.; Rodríguez, M.P.; Méndez, L.; Rodríguez, E.M.; Rodríguez-Mallon, A.; Lorenzo, E.; Santana, E.; González, N.; et al. A single dose of the novel chimeric subunit vaccine E2-CD154 confers early full protection against classical swine fever virus. Vaccine 2017, 35, 4437–4443. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Sordo, Y.; Pérez-Simó, M.; Suárez, M.; Canturri, A.; Rodriguez, M.P.; Frías-Lepoureau, M.T.; Domingo, M.; Estrada, M.P.; Ganges, L. Efficacy of E2 glycoprotein fused to porcine CD154 as a novel chimeric subunit vaccine to prevent classical swine fever virus vertical transmission in pregnant sows. Vet. Microbiol. 2017, 205, 110–116. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.Y.; Zhang, X.J.; Chang, T.M.; He, F.; Wang, X.P.; Liu, D.F.; Qiu, H.J. Comparison of the protective efficacy of recombinant adenoviruses against classical swine fever. Immunol. Lett. 2011, 135, 43–49. [Google Scholar] [CrossRef]
- Xia, S.L.; Lei, J.L.; Du, M.; Wang, Y.; Cong, X.; Xiang, G.T.; Li, L.F.; Yu, S.; Du, E.; Liu, S.; et al. Enhanced protective immunity of the chimeric vector-based vaccine rAdV-SFV-E2 against classical swine fever in pigs by a salmonella bacterial ghost adjuvant. Vet. Res. 2016, 47, 64. [Google Scholar] [CrossRef]
- Laughlin, R.C.; Madera, R.; Peres, Y.; Berquist, B.R.; Wang, L.; Buist, S.; Burakova, Y.; Palle, S.; Chung, C.J.; Rasmussen, M.V.; et al. Plant-made E2 glycoprotein single-dose vaccine protects pigs against classical swine fever. Plant Biotechnol. J. 2019, 17, 410–420. [Google Scholar] [CrossRef]
- Ganges, L.; Barrera, M.; Núñez, J.I.; Blanco, I.; Frias, M.T.; Rodríguez, F.; Sobrino, F. A DNA vaccine expressing the E2 protein of classical swine fever virus elicits T cell responses that can prime for rapid antibody production and confer total protection upon viral challenge. Vaccine 2005, 23, 3741–3752. [Google Scholar] [CrossRef]
- Postel, A.; Becher, P. Genetically distinct pestiviruses pave the way to improved classical swine fever marker vaccine candidates based on the chimeric pestivirus concept. Emerg. Microbes Infect. 2020, 9, 2180–2189. [Google Scholar] [CrossRef]
- de Smit, A.J.; Bouma, A.; van Gennip, H.G.; de Kluijver, E.P.; Moormann, R.J. Chimeric (marker) C-strain viruses induce clinical protection against virulent classical swine fever virus (CSFV) and reduce transmission of CSFV between vaccinated pigs. Vaccine 2001, 19, 1467–1476. [Google Scholar] [CrossRef]
- Reimann, I.; Depner, K.; Trapp, S.; Beer, M. An avirulent chimeric pestivirus with altered cell tropism protects pigs against lethal infection with classical swine fever virus. Virology 2004, 322, 143–157. [Google Scholar] [CrossRef]
- Reimann, I.; Depner, K.; Utke, K.; Leifer, I.; Lange, E.; Beer, M. Characterization of a new chimeric marker vaccine candidate with a mutated antigenic E2 epitope. Vet. Microbiol. 2010, 142, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yuan, Y.; Ankenbauer, R.G.; Nelson, L.D.; Witte, S.B.; Jackson, J.A.; Welch, S.K. construction of chimeric bovine viral diarrhea viruses containing glycoprotein Erns of heterologous pestiviruses and evaluation of the chimeras as potential marker vaccines against BVDV. Vaccine 2012, 30, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Henke, J.; Carlson, J.; Zani, L.; Leidenberger, S.; Schwaiger, T.; Schlottau, K.; Teifke, J.P.; Schröder, C.; Beer, M.; Blome, S. Protection against transplacental transmission of moderately virulent classical swine fever virus using live marker vaccine “CP7-E2alf”. Vaccine 2018, 36, 4181–4187. [Google Scholar] [CrossRef] [PubMed]
- Widjojoatmodjo, M.N.; van Gennip, H.G.; Bouma, A.; van Rijn, P.A.; Moormann, R.J. Classical swine fever virus Erns deletion mutants: Trans-complementation and potential use as non-transmissible, modified, live-attenuated marker vaccines. J. Virol. 2000, 74, 2973–2980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Gennip, H.G.; Bouma, A.; van Rijn, P.A.; Widjojoatmodjo, M.N.; Moormann, R.J. Experimental non-transmissible marker vaccines for classical swine fever (CSF) by trans-complementation of Erns or E2 of CSFV. Vaccine 2002, 20, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.I.; Choe, S.; Kim, K.S.; Jeoung, H.Y.; Cha, R.M.; Park, G.S.; Shin, J.; Park, G.N.; Cho, I.S.; Song, J.Y.; et al. Assessment of the efficacy of an attenuated live marker classical swine fever vaccine (Flc-LOM-BErns) in pregnant sows. Vaccine 2019, 37, 3598–3604. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, L.; Yuan, M.; Ma, Y.; Sun, H.; Sun, Y.; Li, Y.; Qiu, H.J. Development of a marker vaccine candidate against classical swine fever based on the live attenuated vaccine C-strain. Vet. Microbiol. 2020, 247, 108741. [Google Scholar] [CrossRef]
- Kim, T.; Huynh, L.T.; Hirose, S.; Igarashi, M.; Hiono, T.; Isoda, N.; Sakoda, Y. Characteristics of classical swine fever virus variants derived from live attenuated GPE− vaccine seed. Viruses 2021, 13, 1672. [Google Scholar] [CrossRef]
- Beer, M.; Reimann, I.; Hoffmann, B.; Depner, K. Novel marker vaccines against classical swine fever. Vaccine 2007, 25, 5665–5670. [Google Scholar] [CrossRef]
- Holinka, L.G.; Fernandez-Sainz, I.; O'Donnell, V.; Prarat, M.V.; Gladue, D.P.; Lu, Z.; Risatti, G.R.; Borca, M.V. Development of a live attenuated antigenic marker classical swine fever vaccine. Virology 2009, 384, 106–113. [Google Scholar] [CrossRef]
- Kortekaas, J.; Vloet, R.P.; Weerdmeester, K.; Ketelaar, J.; van Eijk, M.; Loeffen, W.L. Rational design of a classical swine fever C-strain vaccine virus that enables the differentiation between infected and vaccinated animals. J. Virol. Methods 2010, 163, 175–185. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Chen, S.; Li, W.; Yin, X.; Li, S.; Xiao, P.; Han, J.; Li, X.; Sun, L.; et al. Generation of an attenuated Tiantan vaccinia virus strain by deletion of multiple genes. Front. Cell. Infect. Microbiol. 2017, 7, 462. [Google Scholar] [CrossRef]
- Makoschey, B.; Sonnemans, D.; Bielsa, J.M.; Franken, P.; Mars, M.; Santos, L.; Alvarez, M. Evaluation of the induction of NS3 specific BVDV antibodies using a commercial inactivated BVDV vaccine in immunization and challenge trials. Vaccine 2007, 25, 6140–6145. [Google Scholar] [CrossRef]
- Meyer, D.; Fritsche, S.; Luo, Y.; Engemann, C.; Blome, S.; Beyerbach, M.; Chang, C.Y.; Qiu, H.J.; Becher, P.; Postel, A. The double-antigen ELISA concept for early detection of Erns-specific classical swine fever virus antibodies and application as an accompanying test for differentiation of infected from marker vaccinated animals. Transbound. Emerg. Dis. 2017, 64, 2013–2022. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, D.Y.; Li, S.; Meng, Q.L. Comprehensive evaluation of the adenovirus/alphavirus-replicon chimeric vector-based vaccine rAdV-SFV-E2 against classical swine fever. Vaccine 2013, 31, 538–544. [Google Scholar] [CrossRef]
- Bohórquez, J.A.; Defaus, S.; Muñoz-González, S.; Perez-Simó, M.; Rosell, R.; Fraile, L.; Sobrino, F.; Andreu, D.; Ganges, L. A bivalent dendrimeric peptide bearing a T-cell epitope from foot-and-mouth disease virus protein 3A improves humoral response against classical swine fever virus. Virus Res. 2017, 238, 8–12. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, L.; Luo, Y.; Zhao, D.; Mi, S.; Yu, X.; Zheng, J.; Tu, C.; Yu, X. A novel combined vaccine against classical swine fever and porcine epidemic diarrhea viruses elicits a significant Th2-favored humoral response in mice. Vaccine 2021, 39, 4573–4576. [Google Scholar] [CrossRef]
- Carlson, J.; Kammerer, R.; Teifke, J.P.; Sehl-Ewert, J.; Pfarrer, C.; Meyers, G. A double deletion prevents replication of the pestivirus bovine viral diarrhea virus in the placenta of pregnant heifers. PLoS Pathog. 2021, 17, e1010107. [Google Scholar] [CrossRef]
- Hansen, T.R.; Smirnova, N.P.; Van Campen, H.; Shoemaker, M.L.; Ptitsyn, A.A.; Bielefeldt-Ohmann, H. Maternal and fetal response to fetal persistent infection with bovine viral diarrhea virus. Am. J. Reprod. Immunol. 2010, 64, 295–306. [Google Scholar] [CrossRef]
- Fairbanks, K.K.; Rinehart, C.L.; Ohnesorge, W.C.; Loughin, M.M.; Chase, C.C. Evaluation of fetal protection against experimental infection with type 1 and type 2 bovine viral diarrhea virus after vaccination of the dam with a bivalent modified-live virus vaccine. J. Am. Vet. Med. Assoc. 2004, 225, 1898–1904. [Google Scholar] [CrossRef]
- Kovács, F.; Magyar, T.; Rinehart, C.; Elbers, K.; Schlesinger, K.; Ohnesorge, W.C. The live attenuated bovine viral diarrhea virus components of a multi-valent vaccine confer protection against fetal infection. Vet. Microbiol. 2003, 96, 117–131. [Google Scholar] [CrossRef]
- Ridpath, J.F. Practical significance of heterogeneity among BVDV strains: Impact of biotype and genotype on U.S. control programs. Prev. Vet. Med. 2005, 72, 215–219. [Google Scholar] [CrossRef]
- Bolin, S.R. Control of bovine viral diarrhea infection by use of vaccination. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Endsley, J.J.; Roth, J.A.; Ridpath, J.; Neill, J. Maternal antibody blocks humoral but not T cell responses to BVDV. Biologicals 2003, 31, 123–125. [Google Scholar] [CrossRef] [PubMed]
- González, A.M.; Arnaiz, I.; Yus, E.; Eiras, C.; Sanjuán, M.; Diéguez, F.J. Evaluation of long-term antibody responses to two inactivated bovine viral diarrhoea virus (BVDV) vaccines. Vet. J. 2014, 199, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Clarke, M.C.; Sopp, P.; Brownlie, J. Systemic vaccination with inactivated bovine virus diarrhoea virus protects against respiratory challenge. Vet. Microbiol. 1994, 42, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Dean, H.J.; Leyh, R. Cross-protective efficacy of a bovine viral diarrhea virus (BVDV) type 1 vaccine against BVDV type 2 challenge. Vaccine 1999, 17, 1117–1124. [Google Scholar] [CrossRef]
- Potgieter, L.N. Immunology of bovine viral diarrhea virus. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 501–520. [Google Scholar] [CrossRef]
- Fulton, R.W.; Ridpath, J.F.; Saliki, J.T.; Briggs, R.E.; Confer, A.W.; Burge, L.J.; Purdy, C.W.; Loan, R.W.; Duff, G.C.; Payton, M.E. bovine viral diarrhea virus (BVDV) 1b: Predominant BVDV subtype in calves with respiratory disease. Can. J. Vet. Res. 2002, 66, 181–190. [Google Scholar]
- Meyers, G.; Tautz, N.; Becher, P. Recovery of cytopathogenic and noncytopathogenic bovine viral diarrhea viruses from cDNA constructs. J. Virol. 1996, 70, 8606–8613. [Google Scholar] [CrossRef]
- Makoschey, B.; Becher, P.; Janssen, M.G. Bovine viral diarrhea virus with deletions in the 5′-nontranslated region: Reduction of replication in calves and induction of protective immunity. Vaccine 2004, 22, 3285–3294. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Schmeiser, S.; Meyer, D.; Meindl-Bohmer, A.; Koenen, F.; Beer, M. Efficacy of marker vaccine candidate CP7_E2alf against challenge with classical swine fever virus isolates of different genotypes. Vet. Microbiol. 2014, 169, 8–17. [Google Scholar] [CrossRef]
- Koethe, S.; König, P.; Wernike, K.; Schulz, J.; Reimann, I.; Beer, M. Bungowannah pestivirus chimeras as novel double marker vaccine strategy against bovine viral diarrhea virus. Vaccines 2022, 10, 88. [Google Scholar] [CrossRef]
- Bellido, D.; Baztarrica, J.; Rocha, L.; Pecora, A.; Acosta, M.; Escribano, J.M.; Parreño, V.; Wigdorovitz, A. A novel MHC-II targeted BVDV subunit vaccine induces a neutralizing immunological response in guinea pigs and cattle. Transbound. Emerg. Dis. 2021, 68, 3474–3481. [Google Scholar] [CrossRef]
- Paton, D.J.; McGoldrick, A.; Greiser-Wilke, I.; Parchariyanon, S.; Song, J.Y.; Liou, P.P.; Stadejek, T.; Lowings, J.P.; Björklund, H.; Belák, S. Genetic typing of classical swine fever virus. Vet. Microbiol. 2000, 73, 137–157. [Google Scholar] [CrossRef]
- Vandeputte, J.; Too, H.L.; Ng, F.K.; Chen, C.; Chai, K.K.; Liao, G.A. Adsorption of colostral antibodies against classical swine fever, persistence of maternal antibodies, and effect on response to vaccination in baby pigs. Am. J. Vet. Res. 2001, 62, 1805–1811. [Google Scholar] [CrossRef]
- Tu, C.; Lu, Z.; Li, H.; Yu, X.; Liu, X.; Li, Y.; Zhang, H.; Yin, Z. Phylogenetic comparison of classical swine fever virus in China. Virus Res. 2001, 81, 29–37. [Google Scholar] [CrossRef]
- König, P.; Lange, E.; Reimann, I.; Beer, M. CP7_E2alf: A safe and efficient marker vaccine strain for oral immunisation of wild boar against classical swine fever virus (CSFV). Vaccine 2007, 25, 3391–3399. [Google Scholar] [CrossRef]
- Deng, M.; Ji, S.; Fei, W.; Raza, S.; He, C.; Chen, Y.; Chen, H.; Guo, A. Prevalence study and genetic typing of bovine viral diarrhea virus (BVDV) in four bovine species in China. PLoS ONE 2015, 10, e0121718. [Google Scholar] [CrossRef]
- Kelling, C.L. Evolution of bovine viral diarrhea virus vaccines. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 115–129. [Google Scholar] [CrossRef]
- Xue, W.; Mattick, D.; Smith, L. Protection from persistent infection with a bovine viral diarrhea virus (BVDV) type 1b strain by a modified-live vaccine containing BVDV types 1a and 2, infectious bovine rhinotracheitis virus, parainfluenza 3 virus and bovine respiratory syncytial virus. Vaccine 2011, 29, 4657–4662. [Google Scholar] [CrossRef]
- Nardelli, S.; Decaro, N.; Belfanti, I.; Lucente, M.S.; Giammarioli, M.; Mion, M.; Lucchese, L.; Martini, M.; Cecchinato, M.; Schiavo, M.; et al. Do modified live virus vaccines against bovine viral diarrhea induce fetal cross-protection against HoBi-like pestivirus? Vet. Microbiol. 2021, 260, 109178. [Google Scholar] [CrossRef]
- Wang, W.; Shi, X.; Wu, Y.; Li, X.; Ji, Y.; Meng, Q.; Zhang, S.; Wu, H. Immunogenicity of an inactivated Chinese bovine viral diarrhea virus 1a (BVDV 1a) vaccine cross protects from BVDV 1b infection in young calves. Vet. Immunol. Immunopathol. 2014, 160, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Hamers, C.; Couvreur, B.; Dehan, P.; Letellier, C.; Fischer, L.; Brun, A.J.; Lewalle, P.; Michaux, C.; Pastoret, P.P.; Kerkhofs, P. Assessment of the clinical and virological protection provided by a commercial inactivated bovine viral diarrhoea virus genotype 1 vaccine against a BVDV genotype 2 challenge. Vet. Rec. 2003, 153, 236–240. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, P.A. A common neutralizing epitope on envelope glycoprotein E2 of different pestiviruses: Implications for improvement of vaccines and diagnostics for classical swine fever (CSF)? Vet. Microbiol. 2007, 125, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Tratschin, J.D.; Hofmann, M.A. A recombinant classical swine fever virus stably expresses a marker gene. J. Virol. 1998, 72, 5318–5322. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, L.; Sun, Y.; Yuan, J.; Huang, J.; Li, C.; Li, S.; Luo, Y.; Qiu, H.J. Simplified serum neutralization test based on enhanced green fluorescent protein-tagged classical swine fever virus. J. Clin. Microbiol. 2013, 51, 2710–2712. [Google Scholar] [CrossRef]
- Zhu, F.C.; Wurie, A.H.; Hou, L.H.; Liang, Q.; Li, Y.H.; Russell, J.B.; Wu, S.P.; Li, J.X.; Hu, Y.M.; Guo, Q.; et al. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: A single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 389, 621–628. [Google Scholar] [CrossRef]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2018, 153, 1–9. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune responses to viral gene therapy vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Okahira, S.; Nishikawa, F.; Nishikawa, S.; Akazawa, T.; Seya, T.; Matsumoto, M. Interferon-beta induction through toll-like receptor 3 depends on double-stranded RNA structure. DNA Cell Biol. 2005, 24, 614–623. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Si, L.; Bai, H.; Oh, C.Y.; Jiang, A.; Hong, F.; Zhang, T.; Ye, Y.; Jordan, T.X.; Logue, J.; McGrath, M.; et al. Self-assembling short immunostimulatory duplex RNAs with broad-spectrum antiviral activity. Mol. Ther. Nucleic Acids 2022, 29, 923–940. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Xie, L.; Wang, X.; Gao, X.; Sun, Y.; Qiu, H.J. Secreted expression of the Cap gene of porcine circovirus type 2 in classical swine fever virus C-strain: Potential of C-strain used as a vaccine vector. Viruses 2017, 9, 298. [Google Scholar] [CrossRef]
- Tratschin, J.D.; Moser, C.; Ruggli, N.; Hofmann, M.A. classical swine fever virus leader proteinase Npro is not required for viral replication in cell culture. J. Virol. 1998, 72, 7681–7684. [Google Scholar] [CrossRef]
- Ruggli, N.; Tratschin, J.D.; Schweizer, M.; McCullough, K.C.; Hofmann, M.A.; Summerfield, A. Classical swine fever virus interferes with cellular antiviral defense: Evidence for a novel function of Npro. J. Virol. 2003, 77, 7645–7654. [Google Scholar] [CrossRef]
- Mayer, D.; Hofmann, M.A.; Tratschin, J.D. Attenuation of classical swine fever virus by deletion of the viral Npro gene. Vaccine 2004, 22, 317–328. [Google Scholar] [CrossRef]
- Riedel, C.; Lamp, B.; Heimann, M.; König, M.; Blome, S.; Moennig, V.; Schüttler, C.; Thiel, H.J.; Rümenapf, T. The core protein of classical swine fever virus is dispensable for virus propagation in vitro. PLoS Pathog. 2012, 8, e1002598. [Google Scholar] [CrossRef]
- Fan, Z.C.; Dennis, J.C.; Bird, R.C. Bovine viral diarrhea virus is a suitable viral vector for stable expression of heterologous gene when inserted in between N pro and C genes. Virus Res. 2008, 138, 97–104. [Google Scholar] [CrossRef]
- Tao, J.; Li, B.; Shi, Y.; Chen, J.; Zhu, G.; Shen, X.; Liu, H. Attenuated porcine-derived type 2 bovine viral diarrhea virus as vector stably expressing viral gene. J. Virol. Methods 2020, 279, 113842. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Chen, J.; Li, C.; Huang, J.; Luo, Y.; Sun, Y.; Li, S.; Qiu, H.J. Generation of a recombinant classical swine fever virus stably expressing the firefly luciferase gene for quantitative antiviral assay. Antiviral Res. 2014, 109, 15–21. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, L.; Shen, L.; Zhang, L.; Yu, J.; Luo, Y.; Sun, Y.; Li, S.; Qiu, H.J. RNA interference screening of interferon-stimulated genes with antiviral activities against classical swine fever virus using a reporter virus. Antiviral Res. 2016, 128, 49–56. [Google Scholar] [CrossRef]
- Gallei, A.; Blome, S.; Gilgenbach, S.; Tautz, N.; Moennig, V.; Becher, P. Cytopathogenicity of classical swine fever virus correlates with attenuation in the natural host. J. Virol. 2008, 82, 9717–9729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hulst, M.M.; van Gennip, H.G.; Moormann, R.J. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparin sulfate due to a single amino acid change in envelope protein Erns. J. Virol. 2000, 74, 9553–9561. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.G.; Coimbra, E.C.; Jesus, A.L. Secretory expression of porcine circovirus type 2 capsid protein in Pichia pastoris. J. Virol. Methods 2014, 207, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Arenhart, S.; Silva, J.V.; Flores, E.F.; Weiblen, R.; Gil, L.H. Use of homologous recombination in yeast to create chimeric bovine viral diarrhea virus cDNA clones. Braz. J. Microbiol. 2016, 47, 993–999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moser, C.; Stettler, P.; Tratschin, J.D.; Hofmann, M.A. Cytopathogenic and noncytopathogenic RNA replicons of classical swine fever virus. J. Virol. 1999, 73, 7787–7794. [Google Scholar] [CrossRef]
- Risager, P.C.; Fahnøe, U.; Gullberg, M.; Rasmussen, T.B.; Belsham, G.J. Analysis of classical swine fever virus RNA replication determinants using replicons. J. Gen. Virol. 2013, 94, 1739–1748. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, R.; Li, R.W.; Li, L.; Xiong, Z.; Zhao, H.; Guo, D.; Pan, Z. Chimeric classical swine fever (CSF) Japanese encephalitis (JE) viral replicon as anon-transmissible vaccine candidate against CSF and JE infections. Virus Res. 2012, 165, 61–70. [Google Scholar] [CrossRef]
- Oura, C.A.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 2005, 86, 2445–2450. [Google Scholar] [CrossRef]
- Denyer, M.S.; Wileman, T.E.; Stirling, C.M.A.; Zuber, B.; Takamatsu, H.H. Perforin expression can define CD8+ lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet. Immunol. Immunopathol. 2006, 110, 279–292. [Google Scholar] [CrossRef]
- Maurer, R.; Stettler, P.; Ruggli, N.; Hofmann, M.A.; Tratschin, J.D. Oronasal vaccination with classical swine fever virus (CSFV) replicon particles with either partial or complete deletion of the E2 gene induces partial protection against lethal challenge with highly virulent CSFV. Vaccine 2005, 23, 3318–3328. [Google Scholar] [CrossRef]
- Kaden, V.; Lange, B. Oral immunisation against classical swine fever (CSF): Onset and duration of immunity. Vet. Microbiol. 2001, 82, 301–310. [Google Scholar] [CrossRef]
- Kaden, V.; Schurig, U.; Steyer, H. Oral immunization of pigs against classical swine fever. Course of the disease and virus transmission after simultaneous vaccination and infection. Acta Virol. 2001, 45, 23–29. [Google Scholar]
- Kaden, V.; Heyne, H.; Kiupel, H.; Letz, W.; Kern, B.; Lemmer, U.; Gossger, K.; Rothe, A.; Böhme, H.; Tyrpe, P. Oral immunisation of wild boar against classical swine fever: Concluding analysis of the recent field trials in Germany. Berl. Munch Tierarztl. Wochenschr. 2002, 115, 179–185. [Google Scholar]
- Kaden, V.; Lange, E.; Müller, T.; Teuffert, J.; Teifke, J.P.; Riebe, R. Protection of gruntlings against classical swine fever virus-infection after oral vaccination of sows with C-strain vaccine. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 455–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Cheng, J.; Liu, X.; Miao, S.; Tan, W.S.; Zhao, L. Designing a novel E2-IFN-γ fusion protein against CSFV by immunoinformatics and structural vaccinology approaches. Appl. Microbiol. Biotechnol. 2022, 106, 3611–3623. [Google Scholar] [CrossRef]
- Liu, Z.H.; Xu, H.L.; Han, G.W.; Tao, L.N.; Lu, Y.; Zheng, S.Y.; Fang, W.H.; He, F. Self-assembling nanovaccine enhances protective efficacy against CSFV in pigs. Front. Immunol. 2021, 12, 689187. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, Y.; Yang, J.; Ma, F.; Wang, Y.; Zhang, S.; Li, X.; Qu, X.; Bai, Y.; Jia, R.; et al. An improved immunochromatographic strip based on plant-derived E2 for detection of antibodies against classical swine fever virus. Microbiol. Spectr. 2022, 10, e0105022. [Google Scholar] [CrossRef]
- Huang, Y.L.; Deng, M.C.; Wang, F.I.; Huang, C.C.; Chang, C.Y. The challenges of classical swine fever control: Modified live and E2 subunit vaccines. Virus Res. 2014, 179, 1–11. [Google Scholar] [CrossRef]
- Lin, G.J.; Deng, M.C.; Chen, Z.W.; Liu, T.Y.; Wu, C.W.; Cheng, C.Y.; Chien, M.S.; Huang, C. Yeast expressed classical swine fever E2 subunit vaccine candidate provides complete protection against lethal challenge infection and prevents horizontal virus transmission. Vaccine 2012, 30, 2336–2341. [Google Scholar] [CrossRef]
- Blome, S.; Wernike, K.; Reimann, I.; König, P.; Moß, C.; Beer, M. A decade of research into classical swine fever marker vaccine CP7_E2alf (Suvaxyn® CSF Marker): A review of vaccine properties. Vet. Res. 2017, 48, 51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
