Dynamic Evolution of Avian RNA Virus Sensors: Repeated Loss of RIG-I and RIPLET
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Avian MDA5, RIG-I and RIPLET Coding Sequences
2.2. Positive Selection Analysis
2.3. Structural and Surface Charge Analysis of MDA5
3. Results
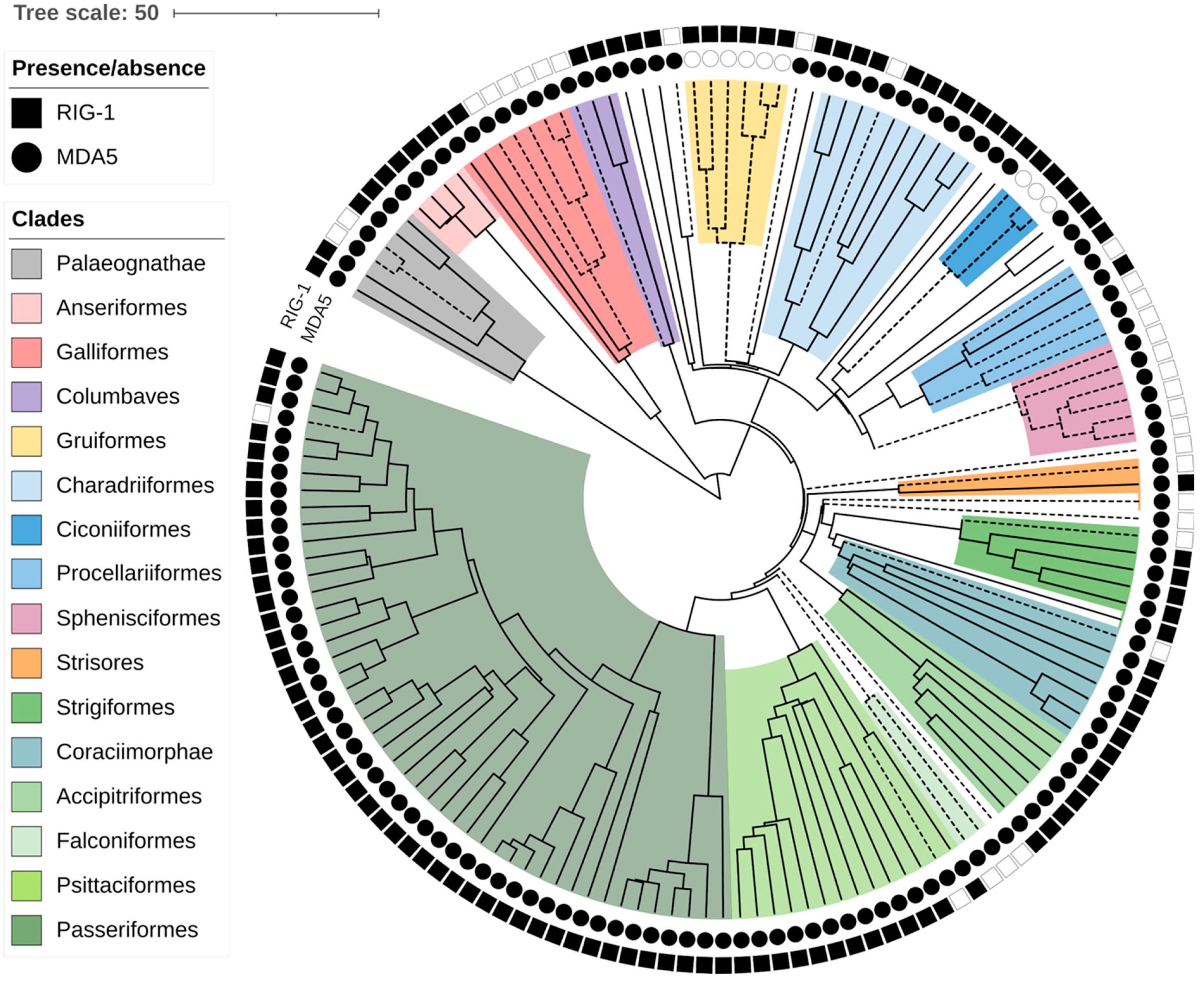
3.1. Multiple Losses of RIG-I and RIPLET in Birds
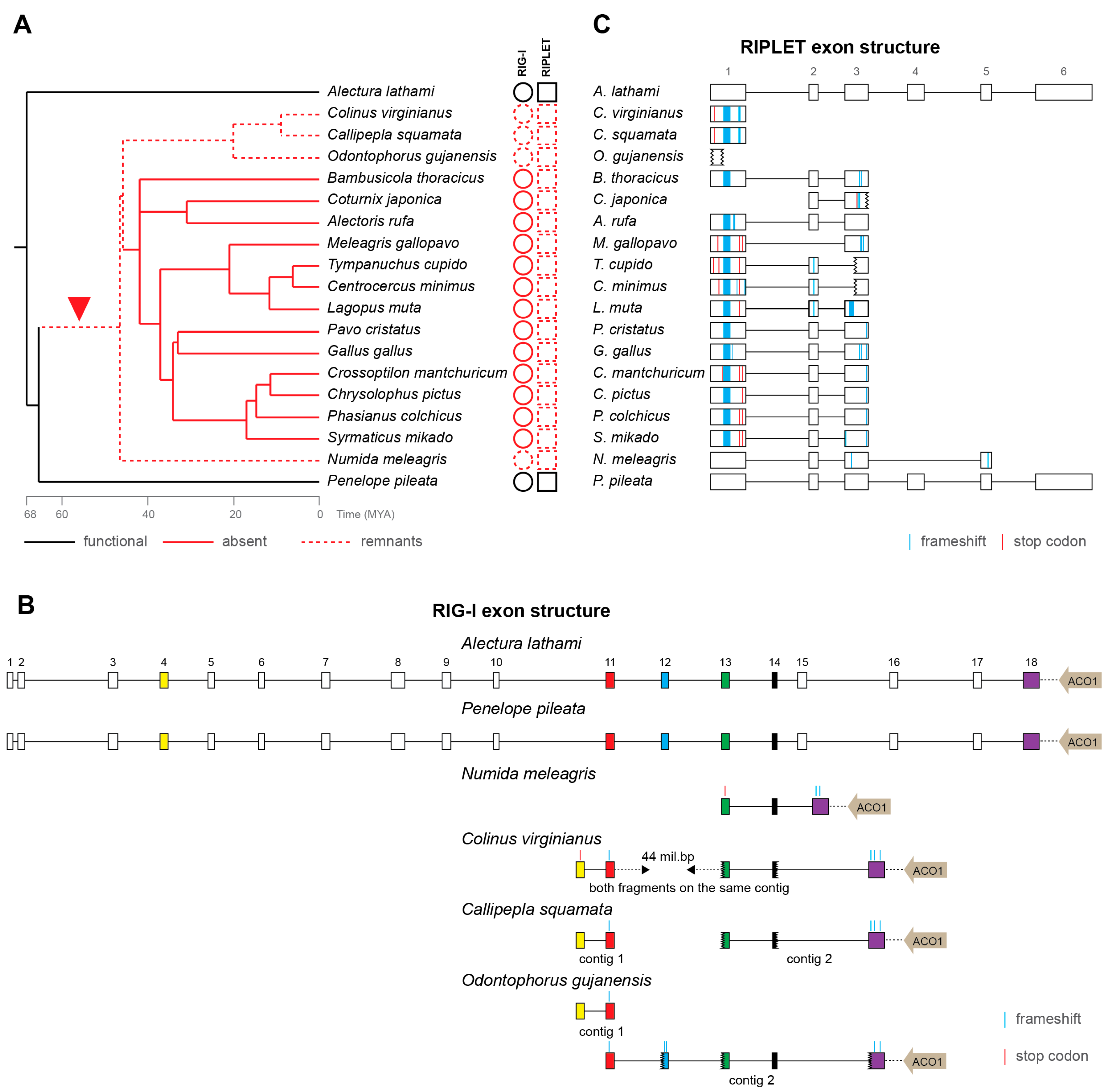
3.2. Loss of RIG-I in Galliformes
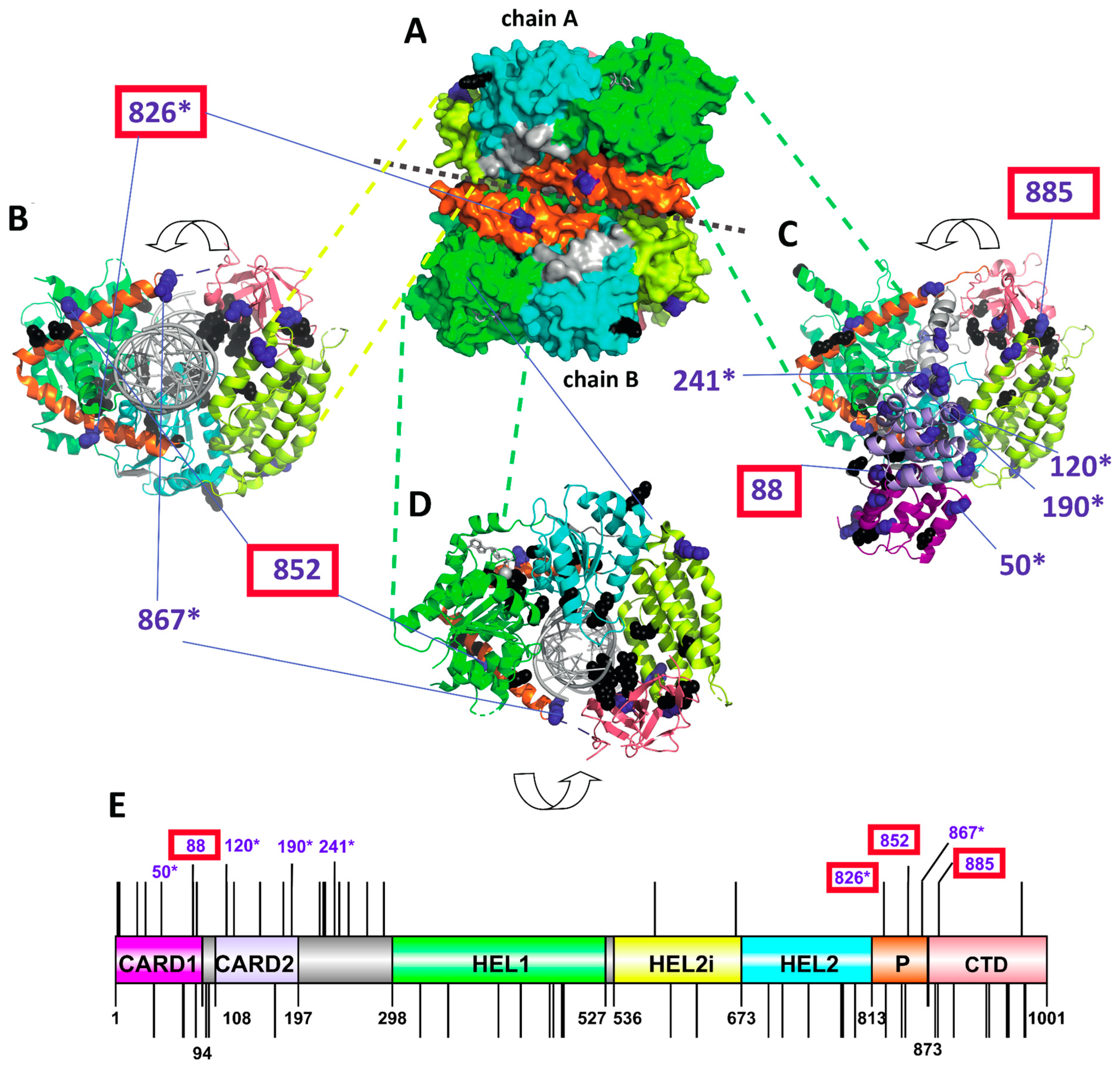
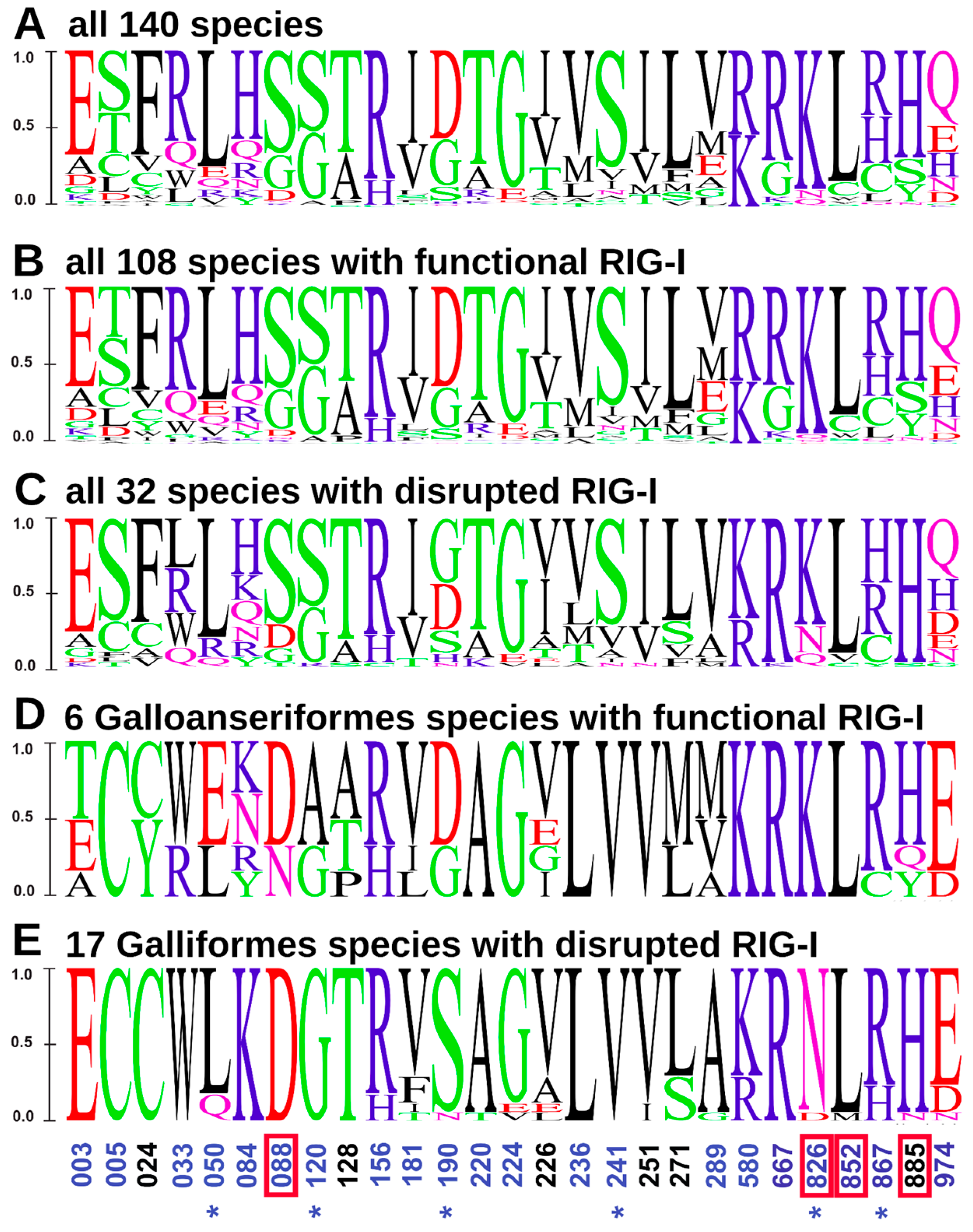
3.3. Positive Selection Acting on MDA5 and Its Potential for Compensation of RIG-I Dysfunction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehwinkel, J.; Gack, M.U. RIG-I-like Receptors: Their Regulation and Roles in RNA Sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; Medzhitov, R. Pattern Recognition Receptors and Control of Adaptive Immunity. Immunol. Rev. 2009, 227, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Magor, K.E. Evolution of RNA Sensing Receptors in Birds. Immunogenetics 2022, 74, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The Roles of TLRs, RLRs and NLRs in Pathogen Recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Building an Immune System from Nine Domains. Biochem. Soc. Trans. 2007, 35, 1437–1444. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA Helicase RIG-I Has an Essential Function in Double-Stranded RNA-Induced Innate Antiviral Responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef]
- Andrejeva, J.; Childs, K.S.; Young, D.F.; Carlos, T.S.; Stock, N.; Goodbourn, S.; Randall, R.E. The V Proteins of Paramyxoviruses Bind the IFN-Inducible RNA Helicase, Mda-5, and Inhibit Its Activation of the IFN-Beta Promoter. Proc. Natl. Acad. Sci. USA 2004, 101, 17264–17269. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kikuchi, M.; Matsumoto, K.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Foy, E.; Loo, Y.-M.; Gale, M., Jr.; Akira, S.; et al. Shared and Unique Functions of the DExD/H-Box Helicases RIG-I, MDA5, and LGP2 in Antiviral Innate Immunity. J. Immunol. 2005, 175, 2851–2858. [Google Scholar] [CrossRef]
- Rodriguez, K.R.; Bruns, A.M.; Horvath, C.M. MDA5 and LGP2: Accomplices and Antagonists of Antiviral Signal Transduction. J. Virol. 2014, 88, 8194–8200. [Google Scholar] [CrossRef]
- Yu, M.; Levine, S.J. Toll-like Receptor, RIG-I-like Receptors and the NLRP3 Inflammasome: Key Modulators of Innate Immune Responses to Double-Stranded RNA Viruses. Cytokine Growth Factor Rev. 2011, 22, 63–72. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential Roles of MDA5 and RIG-I Helicases in the Recognition of RNA Viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Ly, H. Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front. Immunol. 2019, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzózka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.-K.; Schlee, M.; et al. 5′-Triphosphate RNA Is the Ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-Mediated Antiviral Responses to Single-Stranded RNA Bearing 5′-Phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Goubau, D.; Schlee, M.; Deddouche, S.; Pruijssers, A.J.; Zillinger, T.; Goldeck, M.; Schuberth, C.; Van der Veen, A.G.; Fujimura, T.; Rehwinkel, J.; et al. Antiviral Immunity via RIG-I-Mediated Recognition of RNA Bearing 5′-Diphosphates. Nature 2014, 514, 372–375. [Google Scholar] [CrossRef]
- Schuberth-Wagner, C.; Ludwig, J.; Bruder, A.K.; Herzner, A.-M.; Zillinger, T.; Goldeck, M.; Schmidt, T.; Schmid-Burgk, J.L.; Kerber, R.; Wolter, S.; et al. A Conserved Histidine in the RNA Sensor RIG-I Controls Immune Tolerance to N1-2′O-Methylated Self RNA. Immunity 2015, 43, 41–51. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Mikamo-Satoh, E.; Hirai, R.; Kawai, T.; Matsushita, K.; Hiiragi, A.; Dermody, T.S.; Fujita, T.; Akira, S. Length-Dependent Recognition of Double-Stranded Ribonucleic Acids by Retinoic Acid-Inducible Gene-I and Melanoma Differentiation-Associated Gene 5. J. Exp. Med. 2008, 205, 1601–1610. [Google Scholar] [CrossRef]
- Wu, B.; Peisley, A.; Richards, C.; Yao, H.; Zeng, X.; Lin, C.; Chu, F.; Walz, T.; Hur, S. Structural Basis for dsRNA Recognition, Filament Formation, and Antiviral Signal Activation by MDA5. Cell 2013, 152, 276–289. [Google Scholar] [CrossRef]
- Chiang, C.; Gack, M.U. Post-Translational Control of Intracellular Pathogen Sensing Pathways. Trends Immunol. 2017, 38, 39–52. [Google Scholar] [CrossRef]
- Lemos de Matos, A.; McFadden, G.; Esteves, P.J. Positive Evolutionary Selection on the RIG-I-like Receptor Genes in Mammals. PLoS ONE 2013, 8, e81864. [Google Scholar] [CrossRef]
- Cagliani, R.; Forni, D.; Tresoldi, C.; Pozzoli, U.; Filippi, G.; Rainone, V.; De Gioia, L.; Clerici, M.; Sironi, M. RIG-I-like Receptors Evolved Adaptively in Mammals, with Parallel Evolution at LGP2 and RIG-I. J. Mol. Biol. 2014, 426, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Albalat, R.; Cañestro, C. Evolution by Gene Loss. Nat. Rev. Genet. 2016, 17, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Clarke, C.; Holland, P.W.H.; Paps, J. Widespread Patterns of Gene Loss in the Evolution of the Animal Kingdom. Nat. Ecol. Evol. 2020, 4, 519–523. [Google Scholar] [CrossRef]
- Xu, L.; Yu, D.; Fan, Y.; Peng, L.; Wu, Y.; Yao, Y.-G. Loss of RIG-I Leads to a Functional Replacement with MDA5 in the Chinese Tree Shrew. Proc. Natl. Acad. Sci. USA 2016, 113, 10950–10955. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Tschachler, E.; Eckhart, L. Pangolins Lack IFIH1/MDA5, a Cytoplasmic RNA Sensor That Initiates Innate Immune Defense Upon Coronavirus Infection. Front. Immunol. 2020, 11, 939. [Google Scholar] [CrossRef]
- Sharma, V.; Hecker, N.; Walther, F.; Stuckas, H.; Hiller, M. Convergent Losses of TLR5 Suggest Altered Extracellular Flagellin Detection in Four Mammalian Lineages. Mol. Biol. Evol. 2020, 37, 1847–1854. [Google Scholar] [CrossRef]
- Velová, H.; Gutowska-Ding, M.W.; Burt, D.W.; Vinkler, M. Toll-Like Receptor Evolution in Birds: Gene Duplication, Pseudogenization, and Diversifying Selection. Mol. Biol. Evol. 2018, 35, 2170–2184. [Google Scholar] [CrossRef]
- Bainová, H.; Králová, T.; Bryjová, A.; Albrecht, T.; Bryja, J.; Vinkler, M. First Evidence of Independent Pseudogenization of Toll-like Receptor 5 in Passerine Birds. Dev. Comp. Immunol. 2014, 45, 151–155. [Google Scholar] [CrossRef]
- Zou, J.; Chang, M.; Nie, P.; Secombes, C.J. Origin and Evolution of the RIG-I like RNA Helicase Gene Family. BMC Evol. Biol. 2009, 9, 85. [Google Scholar] [CrossRef]
- Barber, M.R.W.; Aldridge, J.R., Jr.; Webster, R.G.; Magor, K.E. Association of RIG-I with Innate Immunity of Ducks to Influenza. Proc. Natl. Acad. Sci. USA. 2010, 107, 5913–5918. [Google Scholar] [CrossRef]
- Zheng, W.; Satta, Y. Functional Evolution of Avian RIG-I-like Receptors. Genes 2018, 9, 456. [Google Scholar] [CrossRef]
- Krchlíková, V.; Hron, T.; Těšický, M.; Li, T.; Hejnar, J.; Vinkler, M.; Elleder, D. Repeated MDA5 Gene Loss in Birds: An Evolutionary Perspective. Viruses 2021, 13, 2131. [Google Scholar] [CrossRef] [PubMed]
- Fiddaman, S.R.; Vinkler, M.; Spiro, S.G.; Levy, H.; Emerling, C.A.; Boyd, A.C.; Dimopoulos, E.A.; Vianna, J.A.; Cole, T.L.; Pan, H.; et al. Adaptation and Cryptic Pseudogenization in Penguin Toll-like Receptors. Mol. Biol. Evol. 2022, 39, msab354. [Google Scholar] [CrossRef] [PubMed]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The Global Diversity of Birds in Space and Time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Redding, D.W.; Hartmann, K.; Mooers, A.O. Global Distribution and Conservation of Evolutionary Distinctness in Birds. Curr. Biol. 2014, 24, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Kuma, K.-I.; Toh, H.; Miyata, T. MAFFT Version 5: Improvement in Accuracy of Multiple Sequence Alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nielsen, R.; Yang, Z. Evaluation of an Improved Branch-Site Likelihood Method for Detecting Positive Selection at the Molecular Level. Mol. Biol. Evol. 2005, 22, 2472–2479. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Frost, S.D.W. Not so Different after All: A Comparison of Methods for Detecting Amino Acid Sites under Selection. Mol. Biol. Evol. 2005, 22, 1208–1222. [Google Scholar] [CrossRef]
- Murrell, B.; Wertheim, J.O.; Moola, S.; Weighill, T.; Scheffler, K.; Kosakovsky Pond, S.L. Detecting Individual Sites Subject to Episodic Diversifying Selection. PLoS Genet. 2012, 8, e1002764. [Google Scholar] [CrossRef]
- Zamyatnin, A.A. Amino Acid, Peptide, and Protein Volume in Solution. Annu. Rev. Biophys. Bioeng. 1984, 13, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, E.; Lethier, M.; Malet, H.; Brunel, J.; Gerlier, D.; Cusack, S. Structural Analysis of dsRNA Binding to Anti-Viral Pattern Recognition Receptors LGP2 and MDA5. Mol. Cell 2016, 62, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. DOG 1.0: Illustrator of Protein Domain Structures. Cell Res. 2009, 19, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein Complex Prediction with AlphaFold-Multimer. BioRxiv 2022. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Aldowsari, F.M.F.; Alharbi, S.M.A.; Adiyaman, R. ModFOLD8: Accurate Global and Local Quality Estimates for 3D Protein Models. Nucleic Acids Res. 2021, 49, W425–W430. [Google Scholar] [CrossRef]
- Těšický, M.; Velová, H.; Novotný, M.; Kreisinger, J.; Beneš, V.; Vinkler, M. Positive Selection and Convergent Evolution Shape Molecular Phenotypic Traits of Innate Immunity Receptors in Tits (Paridae). Mol. Ecol. 2020, 29, 3056–3070. [Google Scholar] [CrossRef]
- Donald, J.E.; Kulp, D.W.; DeGrado, W.F. Salt Bridges: Geometrically Specific, Designable Interactions. Proteins Struct. Funct. Bioinform. 2011, 79, 898–915. [Google Scholar] [CrossRef]
- Onofrio, A.; Parisi, G.; Punzi, G.; Todisco, S.; Di Noia, M.A.; Bossis, F.; Turi, A.; De Grassi, A.; Pierri, C.L. Distance-Dependent Hydrophobic-Hydrophobic Contacts in Protein Folding Simulations. Phys. Chem. Chem. Phys. 2014, 16, 18907–18917. [Google Scholar] [CrossRef]
- Tien, M.Z.; Meyer, A.G.; Sydykova, D.K.; Spielman, S.J.; Wilke, C.O. Maximum Allowed Solvent Accessibilites of Residues in Proteins. PLoS ONE 2013, 8, e80635. [Google Scholar] [CrossRef]
- Richter, S.; Wenzel, A.; Stein, M.; Gabdoulline, R.R.; Wade, R.C. webPIPSA: A Web Server for the Comparison of Protein Interaction Properties. Nucleic Acids Res. 2008, 36, W276–W280. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Shimodaira, H. Pvclust: An R Package for Assessing the Uncertainty in Hierarchical Clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Magor, K.E.; Miranzo Navarro, D.; Barber, M.R.W.; Petkau, K.; Fleming-Canepa, X.; Blyth, G.A.D.; Blaine, A.H. Defense Genes Missing from the Flight Division. Dev. Comp. Immunol. 2013, 41, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Watanabe, C.; Suzuki, Y.; Tanikawa, T.; Uchida, Y.; Saito, T. Chicken MDA5 Senses Short Double-Stranded RNA with Implications for Antiviral Response against Avian Influenza Viruses in Chicken. J. Innate Immun. 2014, 6, 58–71. [Google Scholar] [CrossRef]
- Xu, L.; Yu, D.; Fan, Y.; Liu, Y.-P.; Yao, Y.-G. Evolutionary Selection on MDA5 and LGP2 in the Chicken Preserves Antiviral Competence in the Absence of RIG-I. J. Genet. Genom. 2019, 46, 499–503. [Google Scholar] [CrossRef]
- Karpala, A.J.; Stewart, C.; McKay, J.; Lowenthal, J.W.; Bean, A.G.D. Characterization of Chicken Mda5 Activity: Regulation of IFN-β in the Absence of RIG-I Functionality. J. Immunol. 2011, 186, 5397–5405. [Google Scholar] [CrossRef]
- Sirén, J.; Imaizumi, T.; Sarkar, D.; Pietilä, T.; Noah, D.L.; Lin, R.; Hiscott, J.; Krug, R.M.; Fisher, P.B.; Julkunen, I.; et al. Retinoic Acid Inducible Gene-I and Mda-5 Are Involved in Influenza A Virus-Induced Expression of Antiviral Cytokines. Microbes Infect. 2006, 8, 2013–2020. [Google Scholar] [CrossRef]
- Lee, S.B.; Park, Y.H.; Chungu, K.; Woo, S.J.; Han, S.T.; Choi, H.J.; Rengaraj, D.; Han, J.Y. Targeted Knockout of MDA5 and TLR3 in the DF-1 Chicken Fibroblast Cell Line Impairs Innate Immune Response Against RNA Ligands. Front. Immunol. 2020, 11, 678. [Google Scholar] [CrossRef]
- Barber, M.R.W.; Aldridge, J.R., Jr.; Fleming-Canepa, X.; Wang, Y.-D.; Webster, R.G.; Magor, K.E. Identification of Avian RIG-I Responsive Genes during Influenza Infection. Mol. Immunol. 2013, 54, 89–97. [Google Scholar] [CrossRef]
- Hron, T.; Pajer, P.; Pačes, J.; Bartůněk, P.; Elleder, D. Hidden Genes in Birds. Genome Biol. 2015, 16, 164. [Google Scholar] [CrossRef]
- Kumar, S.; Suleski, M.; Craig, J.M.; Kasprowicz, A.E.; Sanderford, M.; Li, M.; Stecher, G.; Hedges, S.B. TimeTree 5: An Expanded Resource for Species Divergence Times. Mol. Biol. Evol. 2022, 39, msac174. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Matsumoto, M.; Hatakeyama, S.; Seya, T. Riplet/RNF135, a RING Finger Protein, Ubiquitinates RIG-I to Promote Interferon-Beta Induction during the Early Phase of Viral Infection. J. Biol. Chem. 2009, 284, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Hayman, T.J.; Hsu, A.C.; Kolesnik, T.B.; Dagley, L.F.; Willemsen, J.; Tate, M.D.; Baker, P.J.; Kershaw, N.J.; Kedzierski, L.; Webb, A.I.; et al. RIPLET, and Not TRIM25, Is Required for Endogenous RIG-I-Dependent Antiviral Responses. Immunol. Cell Biol. 2019, 97, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Buckmaster, M.V.; Goff, S.P. Riplet Binds the Zinc Finger Antiviral Protein (ZAP) and Augments ZAP-Mediated Restriction of HIV-1. J. Virol. 2022, 96, e0052622. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ahmad, S.; Zhu, Z.; Young, J.M.; Mu, X.; Park, S.; Malik, H.S.; Hur, S. Structural Analysis of RIG-I-like Receptors Reveals Ancient Rules of Engagement between Diverse RNA Helicases and TRIM Ubiquitin Ligases. Mol. Cell 2021, 81, 599–613.e8. [Google Scholar] [CrossRef]
- Dascher, C.C.; Brenner, M.B. Evolutionary Constraints on CD1 Structure: Insights from Comparative Genomic Analysis. Trends Immunol. 2003, 24, 412–418. [Google Scholar] [CrossRef]
- Dias Junior, A.G.; Sampaio, N.G.; Rehwinkel, J. A Balancing Act: MDA5 in Antiviral Immunity and Autoinflammation. Trends Microbiol. 2019, 27, 75–85. [Google Scholar] [CrossRef]
- Lei, Y.; Fei, P.; Song, B.; Shi, W.; Luo, C.; Luo, D.; Li, D.; Chen, W.; Zheng, J. A Loosened Gating Mechanism of RIG-I Leads to Autoimmune Disorders. Nucleic Acids Res. 2022, 50, 5850–5863. [Google Scholar] [CrossRef]
| Hypothesis | Test of Positive Selection (PAML) a | |
|---|---|---|
| dN/dS (%) b | p Value c | |
| Positive selection acting on avian MDA5 | 2.7 (2.8%) | <0.0001 |
| Positive selection acting on MDA5 of RIG-I-missing avian species | 3.5 (1.6%) | <0.0001 |
| Positive selection acting on MDA5 of RIG-I-missing avian species exclusively | - | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krchlíková, V.; Hron, T.; Těšický, M.; Li, T.; Ungrová, L.; Hejnar, J.; Vinkler, M.; Elleder, D. Dynamic Evolution of Avian RNA Virus Sensors: Repeated Loss of RIG-I and RIPLET. Viruses 2023, 15, 3. https://doi.org/10.3390/v15010003
Krchlíková V, Hron T, Těšický M, Li T, Ungrová L, Hejnar J, Vinkler M, Elleder D. Dynamic Evolution of Avian RNA Virus Sensors: Repeated Loss of RIG-I and RIPLET. Viruses. 2023; 15(1):3. https://doi.org/10.3390/v15010003
Chicago/Turabian StyleKrchlíková, Veronika, Tomáš Hron, Martin Těšický, Tao Li, Lenka Ungrová, Jiří Hejnar, Michal Vinkler, and Daniel Elleder. 2023. "Dynamic Evolution of Avian RNA Virus Sensors: Repeated Loss of RIG-I and RIPLET" Viruses 15, no. 1: 3. https://doi.org/10.3390/v15010003
APA StyleKrchlíková, V., Hron, T., Těšický, M., Li, T., Ungrová, L., Hejnar, J., Vinkler, M., & Elleder, D. (2023). Dynamic Evolution of Avian RNA Virus Sensors: Repeated Loss of RIG-I and RIPLET. Viruses, 15(1), 3. https://doi.org/10.3390/v15010003








