1. Introduction
Influenza viruses, type A and B, circulate pervasively in the global human population and upon infection, induce a contagious upper respiratory illness. Influenza virus infection often results in fatigue, fever, sneezing, body aches, nausea, and, in severe cases, pneumonia and death. Type A influenza viruses has a broad host species range, whereas Type B influenza viruses are mainly human isolated. Avian, swine, and other host species act as reservoirs for zoonotic transmission and lead to constant reintroduction of influenza viruses with pandemic potential due to individuals lacking pre-existing immunity to novel strains. The two major surface proteins, hemagglutinin (HA) and neuraminidase (NA), classify the influenza A viruses into subtypes. The sequentially numbered protein subtypes denote antigenically distinct groups. In humans, the H1N1 and H3N2 viral subtypes co-circulate seasonally with occasional zoonotic spillover infections, most commonly with avian-origin H5N1 and H7N9 viruses [
1]. Broadly protective influenza virus vaccines are currently unavailable for seasonal human influenza or zoonotic pandemic viral variants [
2]. Governmental agencies prioritized the funding of the development of such a vaccine in 2019 [
3,
4].
Influenza viruses transmit, primarily, through airborne transmission or direct contact with infectious individuals and surfaces. Ideally, an effective influenza virus vaccine will prevent infection and prevent transmission to another person. Vaccination can also lower viral shedding from virally exposed vaccinated individuals by either reducing the peak viral load or decreasing the shedding timeframe [
5]. Currently, split-inactivated vaccines are non-sterilizing and infection-permissive, but vaccination reduces disease symptoms and adverse outcomes following infection [
6,
7].
Influenza virus vaccine development has often overlooked the influenza virus neuraminidase as a potential vaccine candidate antigen. Split-inactivated vaccines are standardized based upon HA content and are not quantified or standardized for NA content. The immunodominance of the HA further dampens the immune response to NA [
8,
9]. The HA protein of the virus then uses sialic acid receptors to mediate entry into cells, whereas the NA protein cleaves the sialic acid receptors. This function improves viral motility, allows the release nascent virions from host cells and prevents self-aggregation [
10]. Anti-NA polyclonal and monoclonal antibodies protect mice and ferrets from influenza virus infection [
11,
12,
13]. NA-inhibiting antibodies decrease influenza virus disease severity; vaccine effectiveness can be enhanced through the synergy of NA and HA inhibiting antibodies [
4,
14,
15].
The goal of the current study was to evaluate a next-generation neuraminidase vaccine based upon computationally optimized broadly reactive antigen (COBRA) methodology in ferrets [
16,
17]. The NA antigen was designed for the N1 influenza subtype, designated N1-I [
16,
17]. This COBRA NA antigen elicited inhibitory antibody responses to a panel of HxN1 viruses encompassing all three genetic lineages of N1: N1.1 (avian; human pandemic), N1.2 (human seasonal), and N1.3 (classical swine). In contrast, wildtype N1.1 and N1.3 antigens elicited cross-reactive NAI antibodies among the lineages, but could not inhibit the NA of H1N1 N1.2 viruses. Likewise, the antisera to the N1.2 NA did not inhibit the N1.1 or N1.3 clade viruses. Previously, our group demonstrated that mice vaccinated with a recombinant N1-I COBRA NA protein were protected against viral influenza. The infection results of the N1-I COBRA NA-vaccinated groups and homologous vaccine groups were similar. Further, the N1-I COBRA NA groups maintained lower viral titers than the mock-vaccinated animals. Consequently, the protective efficacy of the NA COBRA vaccine was quantified in the ferret model.
The ferret model is the gold standard for vaccine efficacy testing due to its natural susceptibility to human influenza, the ferret’s sizeable respiratory system, and similar immunological and physiological responses to vaccination and infection [
18]. The ferret is an excellent model for pre-immunity studies that more closely model human infection due to humans experiencing immune imprinting from previous viral infections [
19]. In this study, we tested the ability of the N1-I COBRA NA to elicit protective immune responses in a naïve and H1N1 pre-immune ferret model. The N1-I COBRA NA results were compared to ferrets vaccinated with homologous and heterologous wildtype NA and HA antigens following challenges with A/California/07/2009 (H1N1), A/Vietnam/1203/2004 (H5N1), or A/Brisbane/59/2007 (H1N1). Vaccines were evaluated for elicitation of broadly reactive antibodies, protection against both morbidity and mortality, and the inhibition of viral transmission between ferrets.
2. Materials and Methods
2.1. Viruses
The historical influenza virus A/Singapore/06/1986 (Sing/86; H1N1; BSL-2) was used for establishing pre-immunity, while A/California/07/2009 (CA/09; H1N1; BSL-2), A/Vietnam/1203/2004 (Viet/04; H5N1; BSL-3 select agent), and A/Brisbane/59/2007 (Bris/07; H1N1; BSL-2) were used for infections. In addition to Sing/86 and CA/09, the following viruses were used in the hemagglutinin inhibition assay (HAI) and enzyme-linked lectin assay (ELLA): A/Vietnam/1203/2004 PR8 reassortant (Viet/04xPR8; H5N1; BSL-2; 6:2 reassortant virus with A/Puerto Rico/8/1934 internal genes and Viet/04 HA and NA gene segments), Bris/07, and A/swine/North Carolina/154704/2015 (Sw/NC/15; H1N1; BSL-2). Sw/NC/15 was propagated in Madin–Darby canine kidney (MDCK) cells; all other viruses were propagated in specific pathogen-free (SPF) 10-day-old embryonated chicken eggs. MDCK cells were maintained with Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS) with 1% penicillin-streptomycin (P/S) at 37 °C with 5% CO2.
2.2. Vaccines
Recombinant soluble proteins used for vaccination included: CA/09 NA, Viet/04 NA, Bris/07 NA, N1-I COBRA NA, and CA/09 HA. The optimized coding sequences for wildtype and COBRA proteins in pcDNA3.3 vectors were expressed in soluble proteins using a HEK-293T cell expression line, as described previously [
16,
20]. Proteins were extracted using HisTrapExcel columns with the AKTA Pure System (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Purified proteins were concentrated with phosphate-buffered saline + 0.1%
w/v sodium azide (PBSA). Protein concentration was determined using Micro BCA Protein Assay Reagent kits (Pierce Biotechnology, Rockford, IL, USA), and aliquots of each protein were stored at −80 °C until used for vaccination. To assess purity, 1 μg of each NA protein sample was mixed 3:1 with 4 × Laemmli Sample Buffer (Bio-Rad, Hercules, CA, USA) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The precast 10% SDS gel (Thermo Fisher Scientific, Waltham, MA, USA) with loaded NA protein samples were electrophoresed at 200 V for 30 min and then stained with PageBlue Protein Staining Solution (Thermo Scientific) for 1 h at room temperature and de-stained with distilled water to visualize the protein bands.
2.3. Animals
Female Fitch ferrets (Mustela putorius furo) between 6 and 15 months of age were sourced from Triple F Farms (Gillett, PA, USA) after de-scenting and spaying. Each animal was confirmed to be serologically naive to the A/California/07/2009 H1N1 influenza virus with sera collected prior to vaccination or pre-immune infection. When not infected, ferrets were pair housed with free access to food, water, and enrichment. Ferrets were anesthetized with vaporized isoflurane before bleeds, vaccination, infection, nasal washes, and euthanasia. All animal procedures were performed following the Guide for the Care and Use of Laboratory Animals, Animal Welfare Act, and Biosafety in Microbiological and Biomedical Laboratories (AUP: A2020 11-016-Y1-A6).
Ferrets were made pre-immune by infecting the naïve ferrets (serologically naive to CA/09 influenza virus) intranasally with the Sing/86 influenza virus 8 weeks prior to initial vaccination. Ferrets (pre-immune or naïve) were vaccinated intramuscularly in the thigh muscle with 15 μg of protein in a total volume of 500 μL (
Figure 1A). Addavax adjuvant (InvivoGen, San Diego, CA, USA) was mixed in a 1:1 ratio (250 μL sterile PBS with protein: 250 μL Addavax). Mock-vaccinated groups received 250 μL of sterile PBS with 250 μL of Addavax. Four weeks from the prime vaccination, the animals received a booster vaccine of the same mixture. At least two weeks after the boost, blood was collected in BD Vacutainer SST tubes. After 30 min at room temperature (RT), serum was separated by processing the tubes at 2500 rpm for 10 min. Purified serum was stored at −20 °C until analysis.
2.4. Direct and Contact Transmission Ferret Infections
Ferrets were directly infected either to establish pre-immunity before vaccination (Sing/86) or to challenge the vaccine groups for protection characteristics (CA/09, Viet/04, and Bris/07). Direct infection was performed intranasally with 1 mL total volume with 500 μL administered to each naris. The infection dose used for Sing/86, CA/09, and Bris/07 was 1 × 106 plaque-forming units (PFU)/mL. Whereas the infection dose used for Viet/04 was 1 × 105 PFU/mL. After infection, animals were observed twice daily for clinical signs and weighed once daily until two consecutive days without signs. On days 1, 3, 5, and 7 post-infection (p.i.) nasal washes were performed with 3 mL of sterile PBS.
Groups of influenza-naïve ferrets were primed and boosted with HA or NA vaccines. Approximately 4 weeks after the booster, two types of models were used to evaluate the virus transmission between vaccinated animals and unvaccinated animals: (i) the vaccinated ferrets (Transmitter) were infected with CA/09 influenza virus intranasally, and unvaccinated influenza-naïve ferrets (Receiver) were introduced 1-day p.i. (
Figure 1B). (ii) The vaccinated ferrets (Receiver) were exposed to the virus by co-housing with a donor, unvaccinated influenza-naïve ferrets (Transmitter), that had been infected with CA/09 one day previously (
Figure 1C). The receiving ferret was placed with the directly infected ferret on day 1 p.i. after nasal wash to evaluate contact transmission. The receiving ferret remained pair housed with the transmitting ferret until the end of the observation period. The receiving ferret was nasal washed on days 3, 5, 7, and 9 p.i. of the transmitting ferret, i.e., days 2, 4, 6, and 8 post-contact. All nasal wash samples were stored at −80 °C until viral titration.
When a cumulative clinical score of three was reached, the animal was humanely euthanized. Clinical signs with their scores were as follows: nasal discharge/sneezing/diarrhea (0.5; not used for humane endpoint calculation but used for graphical representation), lethargy (1), dyspnea (2), cyanosis (2), neurological signs (3), moribund (3), laterally recumbent (3), failure to respond to stimuli (3), weight loss of 20–25% (2), and weight loss of greater than 25% (3). The maximum of the two clinical scores recorded for each day was used for analysis.
2.5. Histopathological Analysis
Histopathological samples were collected from designated animals prior to infection and were euthanized with B-euthanasia on day 5 p.i. The left cranial and caudal lobes were sectioned into quarters, placed on dry ice, and stored at −80 °C until viral titration. The right cranial, middle, caudal lobe, and accessory lobe were infused intratracheally with neutral-buffered, 10% formalin fixative solution (BF). The trachea and right lung were extracted and placed in BF. The submandibular lymph node was extracted and set in BF. The head was removed at the junction of the cricoid cartilage and tracheal rings. The nasal cavity was fixed with BF administration through the nasopharynx until BF drained from both nares. All samples were stored in BF for one week, after which 70% ethanol solution replaced the BF. The skull was decalcified in Kristensen’s solution for two weeks. All tissues were embedded in paraffin, and sectioned as follows: coronal sections through the nasal cavity, transverse sections through the middle ear, and cross sections through the submandibular lymph node, trachea, and right lung lobes. The 5 μm thick sections were stained with hematoxylin and eosin (H&E). To identify T-cells in the submandibular lymph nodes, CD3 immunohistochemistry for T-cells (polyclonal rabbit anti-CD3 antibody (Dako A0452) was performed.
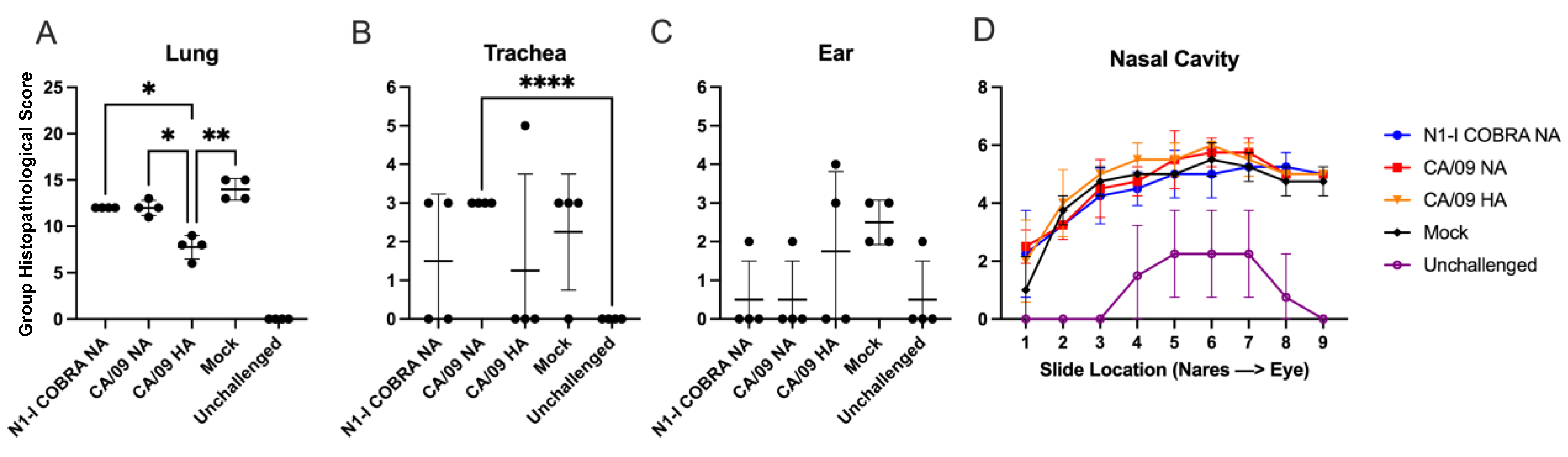
The microscopic exam consisted of the evaluation of the nasal cavity (at 9 levels), ear (middle), trachea, and the right lung lobes (cranial, middle, and caudal) for the presence or absence of inflammation. Microscopically, lesion (tissue change or alteration) incidence, severity, and distribution were recorded. If absent (i.e., histologically normal), a score of 0 was assigned. If present, the severity of the lesions was recorded as minimal, mild, moderate, or severe, with severity scores of 1 through 4, respectively, based on an increasing extent and/or complexity of change, unless otherwise specified. Lesion distribution was recorded as focal, multifocal, or diffuse, with distribution scores of 1, 2, or 3, respectively. A group histopathological score was calculated by adding individual animal severity and distribution scores. All histopathological work was conducted in the spirit of the US FDA Good Laboratory Practice regulations (21 CFR Part 58 and subsequent amendments) and all microscopic evaluations were performed on the H&E-stained sections by a board-certified pathologist (UBM).
2.6. Influenza Virus Plaque Assay
The nasal wash and lung samples were processed for viral titration. The nasal wash samples were diluted in 10-fold serial dilutions in DMEM + P/S before addition to the cells. The lung samples were weighed and then homogenized in a corresponding quantity of DMEM + P/S such that 0.1 g was resuspended in 1 mL DMEM + P/S. The homogenized lung was passed through a. 0.70 μm nylon filter (Corning Cell Strainer, Sigma Aldrich, St. Louis, MO, USA). The filtrate was then diluted in 10-fold serial dilutions in DMEM + P/S before addition to the cells. The upper right quadrant of the left cranial lobe and the lower left quadrant of the left caudal lobe were processed for viral lung titers.
MDCK cells were seeded at 2.5 × 105 cells per well of a 12-well tissue-culture treated plate. The next day the confluent cells were washed with DMEM + P/S and overlaid with 100 μL of the viral sample. Plates were incubated at RT with shaking every 15 min. The cells were then washed with DMEM + P/S and overlaid with 1 mL of plaque medium (minimum essential media with P/S, 2 mM L-glutamine,1.5 mg/mL NaHCO3, 10 mM HEPES, 5 μg/mL Gentamycin, and 1.2% Avicel RC-591 NF (MFC corporation, Philadelphia, PA, USA). For Viet/04 virus, trypsin was not added, but for CA/09 virus, 1.5 μg/mL TPCK-treated trypsin was added (Sigma-Aldrich). Plates were incubated at 37 °C with 5% CO2. After 48 h (Viet/04) or 72 h (CA/09), plates were removed, washed with PBS, and fixed with BF for 15 min. Afterwards, the plaques were visualized by staining with 1% crystal-violet for 10 min. The plaques were counted and back-calculated to determine the PFU/mL for nasal wash viral titers and the PFU/g for lung tissue viral titers. All plaques were conducted in duplicate for each sample, and the average value was taken for analysis. The limit of detection of nasal wash and viral lung titers were 1.0 log10(PFU/mL) and 2.0 log10(PFU/g). The limit of quantification was defined as greater than or equal to 10 countable plaques, which led to reliable lower limits of 2.0 log10(PFU/mL) and 3.0 log10(PFU/g) for nasal wash and viral lung titer values, respectively.
2.7. Hemagglutination Inhibition (HAI) Assay
Ferret sera were treated with three parts receptor destroying enzyme (RDE, DENKA SEIKEN, Tokyo, Japan). Sera and RDE were incubated at 37 °C for 18–20 h and then heat-inactivated at 56 °C for 1 h. After reaching RT, six parts PBS was added to the samples. The HAI assay was performed as previously described. The H1N1 viruses and Viet/04xPR8 virus were adjusted to 1:8 HA units/50 μL with 0.8% turkey erythrocytes (Lampire Biologicals, Pipersville, PA, USA) and 1% horse erythrocytes (Lampire Biologicals, Pipersville, PA, USA), respectively. The serum was diluted two-fold in V-bottom 96 well plates and incubated in equal volume with the virus for 20 min at RT. After which, an equal volume of the respective erythrocytes was added. After 30 min for H1N1 viruses and 60 min for Viet/04xPR8 H5N1 virus, the plates were tilted, and the reciprocal dilution of the last well to not be agglutinated was recorded as the HAI titer. The last column of the plate contained no sera—only PBS, virus, and erythrocytes—served as the negative control.
2.8. Neuraminidase Inhibition Assay (NAI); Enzyme-Linked Lectin Assay (ELLA)
Sera treatment for the ELLA assay was similar to the treatment for the HAI assay, except heat inactivation was performed for 8 h to completely deactivate the NA activity of the
Vibrio cholerae neuraminidase. The NA activity of the virus was determined as previously described and diluted to a concentration providing 90–95% NA activity [
21]. From an initial dilution of 1:100, sera were diluted two-fold in Dulbecco’s phosphate-buffered saline containing 0.133 g/L CaCl
2 and 0.1 g/L MgCl
2 (DPBS), 1% BSA, and 0.5% Tween-20 (DPBS-BT). The sera were added to a PBS + Tween-20 (PBS-T) washed fetuin plated coated previously overnight with 100 μL of 25 μg/mL fetuin. The serial dilutions were added in 25 μL in duplicate per ferret sera sample. In the control wells, 50 μL of DPBS-BT was added in substitution of sera. The control wells included at least six wells with no sera and no virus for the subtraction of the background absorbance and another minimum of six wells with no sera and only virus to serve as the 100% NA activity threshold. The diluted virus was added in 50 μL, and the plate was rocked to mix. Plates were incubated at 37 °C with 5% CO
2 for 16–18 h. After which, they were washed 6X with PBS-T, and 100 μL of peanut agglutinin-HRPO (Sigma-Aldrich, St. Louis, MO, USA) was added at a dilution of 1:1000 in DPBS-T. Plates were incubated in the dark for 2 h at RT. After washing 3X in PBS-T, 100 μL of o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich, St. Louis, MO, USA) in 0.05 M phosphate-citrate buffer with 0.03% sodium perborate pH 5.0 (Sigma-Aldrich, St. Louis, MO, USA) was added to the plates. They were incubated in the dark at RT for 10 min and stopped with 100 μL of 1 N sulfuric acid. The absorbance was read at 490 nm using a spectrophotometer (PowerWave XS; BioTek, Winooski, VT, USA). The background absorbance was subtracted, and the serum-containing wells were normalized with the average of the virus-only wells defining 100% NA activity. Non-linear regression was conducted in Prism 9.1 using the duplicates to provide the average estimated log
10 50% NI titer for individual ferrets.
2.9. Statistical Analysis
The data were analyzed by either a two-way ANOVA or a REML mixed effects model if data were missing. Repeated measures were used to account for the ferret variation. Initially, the interactions were fit between the two main effects (usually vaccine group and day p.i.). If the interaction was not significant with an F-test, the analysis was then conducted with only the main effects. Tukey’s multiple comparison test was conducted first. If there was no significant difference between the vaccinated groups to each other, a Dunnett’s multiple comparison test was conducted using the mock-vaccinated as the control group. Survival curves were analyzed using the log-rank Mantel–Cox test with asymmetrical 95% confidence intervals. The mean value with standard deviation error bars were depicted on all figures except for clinical scores. Clinical score figures depicted the standard error of the mean, with the individual values shown in the background. The offsetting values of the weight loss and viral nasal wash titers were determined by adjusting the days in increments of one, until the mock-vaccinated groups were visually aligned with each other. All the statistical data are available in the
Supplementary Materials.
4. Discussion
Antibodies elicited against the influenza NA protein is associated with decreased influenza H1N1 shedding and illness in humans [
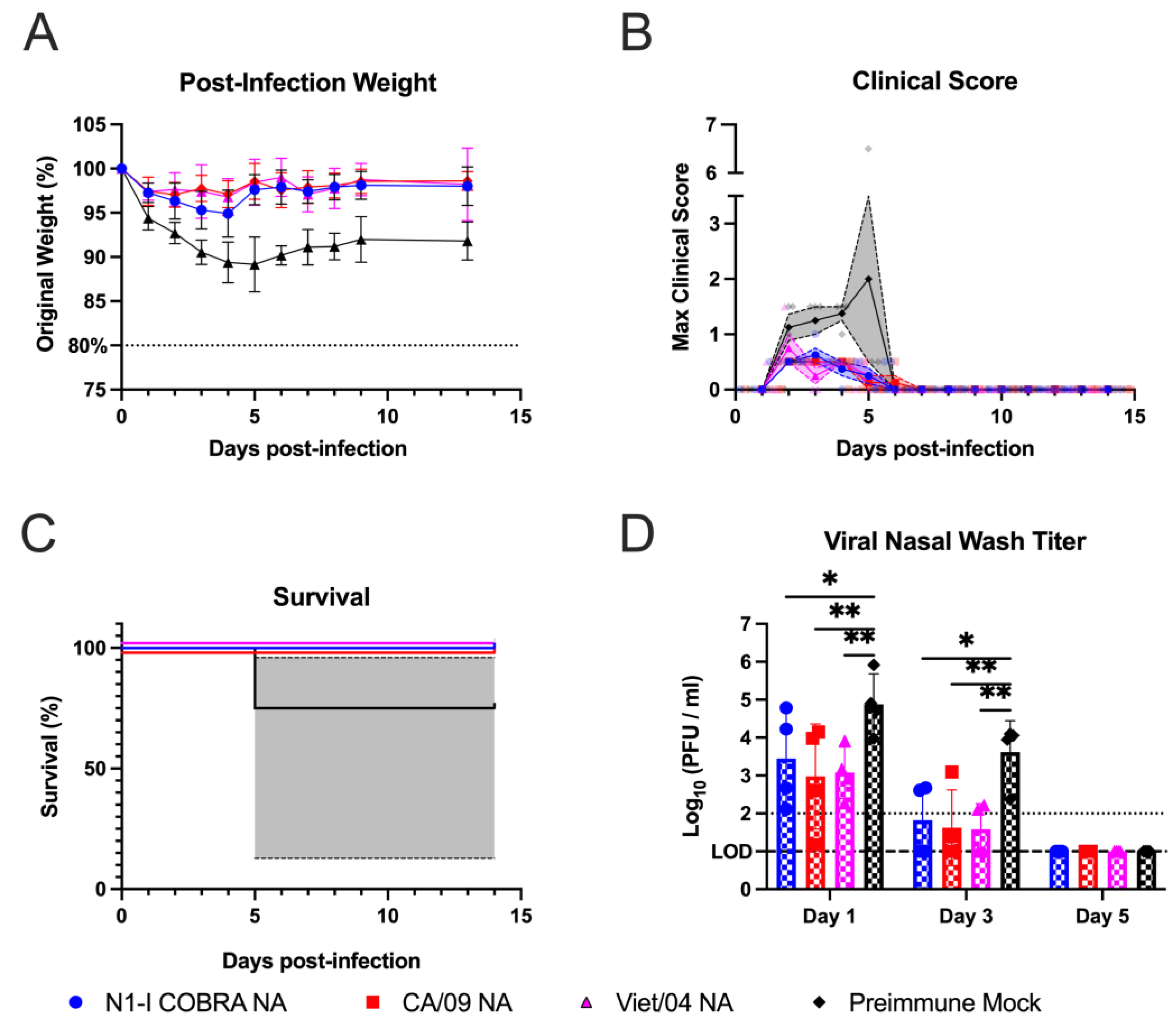
22]. Therefore, the N1-I COBRA NA was investigated for its potential as an influenza vaccine antigen. The naïve ferret model was used to thoroughly characterize the protective responses elicited by the N1-I COBRA NA after infection. The N1-I COBRA NA induced similar ferret responses and viral titers compared to the CA/09 HA and NA positive controls. The Bris/07 NA vaccine was included as a heterologous NA and does not elicit NAI antibodies to either the H1N1 or H5N1 viruses. The ferrets in N1-I COBRA NA and positive control vaccine groups consistently had lower mean viral titers in nasal washes and lung tissue compared to ferrets in both Bris/07 NA and mock vaccine groups. Although differences in the mean weight loss compared to ferrets in other vaccine groups were similar, only 50% of the Bris/07 NA-vaccinated ferrets survived infection. The severity and distribution of inflammation (histopathological scores) in the vaccinated animals were similar in the ear and nasal cavity. Even CA/09 HA-vaccinated ferrets, which had undetectable viral titers in both the nasal wash and lung tissue, the inflammation levels were similar to the ferrets in all other groups infected with CA/09 influenza virus. The N1-I COBRA NA vaccine reduced inflammation in ferrets as the CA/09 NA vaccine, but not as much as the CA/09 HA vaccine. These results correlate with the presence of virus still in the lungs in the NA-vaccinated animals on day 5 p.i., while the viral loads for the ferrets in CA/09 HA group was below the limit of detection.
The N1-I COBRA NA vaccine provided protection against seasonal and pandemic N1 viruses. The N1-I COBRA NA vaccine elicited antibodies with lower NAI titers than antibodies elicited by the Viet/04 NA, but both equally protected ferrets against Viet/04 virus infection. Therefore, the NAI titers and magnitude of protection against the H5N1 virus may not be directly correlated. There, potentially, is a minimum NAI antibody titer threshold for protection. Furthermore, ferrets vaccinated with the N1-I COBRA NA vaccine had little weight loss or mortality compared to the CA/09 NA-vaccinated ferrets over the course of infection.
The protective responses in ferrets corresponded with the elicited serological responses. The N1-I COBRA NA vaccine elicited strong responses against both CA/09 and Viet/04 viruses in 100% of the ferrets/group. The viral inhibition titer was lower against Bris/07, but was similar against the Sw/NC/15. The wildtype NA vaccines had antigenic profiles similar to their specific lineages, as previously observed [
16]. One contrasting observation was that the Bris/07 NA vaccine elicited antibodies that inhibited the NA of Sw/NC/15. Previously in the mouse model, there was no cross-reaction between clades (N1.2 and N1.3) [
16]. This difference in specificity may be due to the change in animal models, from mice to ferrets.
The majority of the human population has pre-existing immunity to influenza through both vaccination and infection. This pre-existing immunity biases the immune response recall response and is termed immune imprinting [
23,
24,
25,
26,
27,
28,
29]. The N1-I COBRA NA was tested in a pre-immune ferret model to mimic individuals exposed to H1N1 influenza viruses. The N1-I COBRA NA vaccine performed equally well, as the CA/09 NA-vaccinated control ferrets in both the naïve and pre-immune models. Therefore, even with the pre-existing anti-Sing/86 N1 NA antibodies, a protective response was elicited when vaccinated with the N1-I COBRA NA. This effect was prominent in the CA/09 virus infected ferrets. Following the Viet/04 virus infection in pre-immune ferrets, there was no weight loss for any group of ferrets. Contrary to expectations, the pre-immunity elicited by the Sing/86 H1N1 virus was more protective against the Viet/04 H5N1 infection than in the CA/09 H1N1 challenge. It was expected that the Sing/86 pre-immunity would be more protective in the CA/09 infection than in the Viet/04 infection because both Sing/86 and CA/09 are H1N1 subtype viruses. Since, the serum from Sing/86 pre-immunized ferrets did not have HAI activity for either of the viruses (all HAI titers were less than 1:10), the differences in protection may be a results of stem binding antibodies or T-cell responses induced from pre-immunization with live virus infection.
Vaccination should not only protect the individuals who are vaccinated, but also nearby associated people. In the contact transmission model, the receiving ferrets were co-housed with the transmitting ferrets for the entirety of the observation period. Our results indicated that vaccination with either NA- or HA-based vaccines did not inhibit the contact transmission by the CA/09 virus. This was previously observed in the pig model as well [
30]. This transmission model mimicked family transmission between individuals who are frequently in contact. One of the limitations of this model is that it does not capture shorter exposure periods. Since the viral dynamics differed in the vaccinated groups, it may suggest varying windows for transmission post-infection.
In addition, the viral dynamics after aerosol transmission in vaccinated ferrets may also differ due to the differences in inoculum particulate size. Additionally, inflammatory responses were only measured on day 5 p.i., that may be informative for collective lung tissue, but may have been past the time point to observe significant quantitative nasal cavity inflammation. Earlier time points of the upper respiratory tract may have provided differential results when comparing vaccine groups. Lastly, within the pre-immune ferret model, the pre-existing antibodies to the HA protein of Sing/86 virus may interfere with the measurement of the functional NA-specific antibodies and prohibit comparison between the elicited NA antibodies in both naïve and pre-immune models.
Overall, N1-I COBRA NA is a promising candidate for a broadly protective influenza vaccine. Inclusion of the N1-I COBRA NA can enhance current split-inactivated vaccines or be included in new influenza vaccine formulations. Split-inactivated vaccines elicit mostly an HA-specific antibody response with minimal NA response. Inclusion of the broadly protective NA antigens, such as the N1-I COBRA NA, opens the door to eliciting a more balanced response after vaccination. New influenza vaccine candidates, whether subunit or microparticles, can also be designed to include the N1-I COBRA NA. The N1-I COBRA NA can help us achieve the future of the influenza vaccines eliciting a broadly protective response to multiple antigens and increasing the potential protection that individuals receive.