Chronic Hepatitis C: Acute Exacerbation and Alanine Aminotransferase Flare
Abstract
1. Introduction
2. Acute Liver Failure (ALF) and Infection with Hepatitis C Virus (HCV)
3. Acute Exacerbation and Alanine Aminotransferase (ALT) Flare of Chronic Hepatitis C Virus (HCV) Infection
4. Acute Exacerbation and Alanine Aminotransferase (ALT) Flare among Chronic Hepatitis C Virus (HCV)-Infected Patients during Cancer Chemotherapy
5. Novel Anticancer Therapies and Acute Exacerbation and Alanine Aminotransferase (ALT) Flare of Chronic Hepatitis C Virus (HCV) Infection
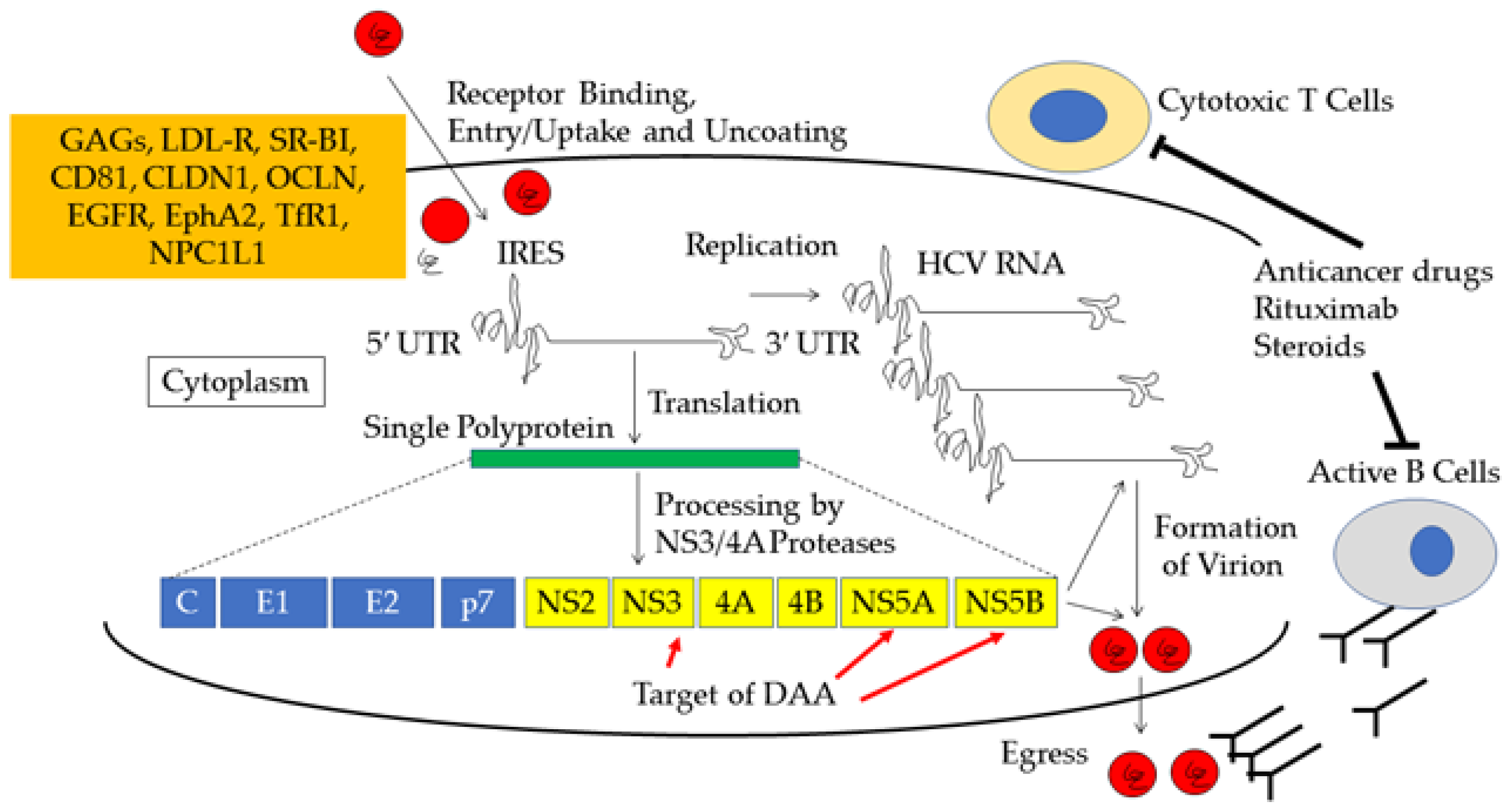
6. Mechanism of Acute Exacerbation and Alanine Aminotransferase (ALT) Flare of Chronic Hepatitis C Virus (HCV) Infection
7. Treatment for Acute Exacerbation and Alanine Aminotransferase (ALT) Flare among Chronically Hepatitis C Virus (HCV)-Infected Patients with Cancer
7.1. Treatment for Patients with Non-Hodgkin’s Lymphoma (NHL)
7.2. Treatment for Patients with Cancer
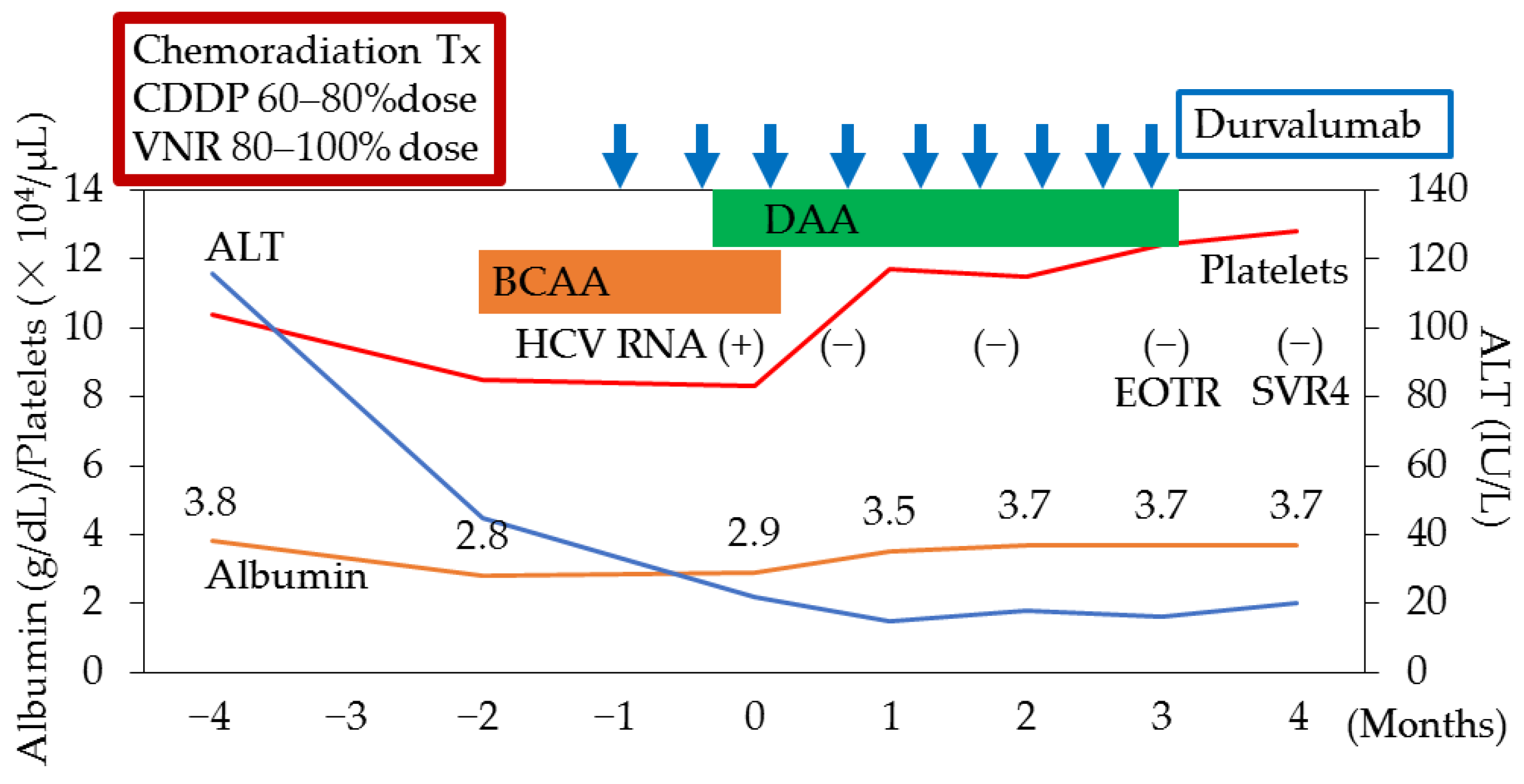
7.3. Direct-Acting Antivirals (DAAs) Could Support Sufficient Lung Cancer Chemotherapy in a Patient with Hepatitis C Virus (HCV) Infection and Decompensated Cirrhosis
8. Hepatitis B Virus (HBV) Reactivation in Patients Treated with Direct-Acting Antivirals (DAAs) for Hepatitis C Virus (HCV) Infection
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choo, Q.L.; Kuo, G.; Weiner, A.J.; Overby, L.R.; Bradley, D.W.; Houghton, M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989, 244, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Kuo, G.; Choo, Q.L.; Alter, H.J.; Gitnick, G.L.; Redeker, A.G.; Purcell, R.H.; Miyamura, T.; Dienstag, J.L.; Alter, M.J.; Stevens, C.E.; et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 1989, 244, 362–364. [Google Scholar] [CrossRef]
- Saito, I.; Miyamura, T.; Ohbayashi, A.; Harada, H.; Katayama, T.; Kikuchi, S.; Watanabe, Y.; Koi, S.; Onji, M.; Ohta, Y.; et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 1990, 87, 6547–6549. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, E.; Zuckerman, T.; Levine, A.M.; Douer, D.; Gutekunst, K.; Mizokami, M.; Qian, D.G.; Velankar, M.; Nathwani, B.N.; Fong, T.L. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann. Intern. Med. 1997, 127, 423–428. [Google Scholar] [CrossRef]
- Lingala, S.; Ghany, M.G. Natural History of Hepatitis C. Gastroenterol Clin. N. Am. 2015, 44, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M.; Shiffman, M.L.; Everson, G.T.; Lindsay, K.L.; Everhart, J.E.; Wright, E.C.; Lee, W.M.; Lok, A.S.; Bonkovsky, H.L.; Morgan, T.R.; et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N. Engl. J. Med. 2008, 359, 2429–2441. [Google Scholar] [CrossRef]
- Yokosuka, O.; Kojima, H.; Imazeki, F.; Tagawa, M.; Saisho, H.; Tamatsukuri, S.; Omata, M. Spontaneous negativation of serum hepatitis C virus RNA is a rare event in type C chronic liver diseases: Analysis of HCV RNA in 320 patients who were followed for more than 3 years. J. Hepatol. 1999, 31, 394–399. [Google Scholar] [CrossRef]
- Mizokami, M.; Yokosuka, O.; Takehara, T.; Sakamoto, N.; Korenaga, M.; Mochizuki, H.; Nakane, K.; Enomoto, H.; Ikeda, F.; Yanase, M.; et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: An open-label, randomised, phase 3 trial. Lancet Infect. Dis. 2015, 15, 645–653. [Google Scholar] [CrossRef]
- Saab, S.; Park, S.H.; Mizokami, M.; Omata, M.; Mangia, A.; Eggleton, E.; Zhu, Y.; Knox, S.J.; Pang, P.; Subramanian, M.; et al. Safety and efficacy of ledipasvir/sofosbuvir for the treatment of genotype 1 hepatitis C in subjects aged 65 years or older. Hepatology 2016, 63, 1112–1119. [Google Scholar] [CrossRef]
- Kanda, T.; Yasui, S.; Nakamura, M.; Suzuki, E.; Arai, M.; Ooka, Y.; Ogasawara, S.; Chiba, T.; Saito, T.; Haga, Y.; et al. Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors. Int. J. Mol. Sci. 2017, 18, 906. [Google Scholar] [CrossRef]
- Takehara, T.; Sakamoto, N.; Nishiguchi, S.; Ikeda, F.; Tatsumi, T.; Ueno, Y.; Yatsuhashi, H.; Takikawa, Y.; Kanda, T.; Sakamoto, M.; et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: An open-label phase 3 trial. J. Gastroenterol. 2019, 54, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yamana, Y.; Kanda, T.; Matsumoto, N.; Honda, M.; Kumagawa, M.; Sasaki, R.; Kanezawa, S.; Mizutani, T.; Yamagami, H.; Masuzaki, R.; et al. Efficacy of Glecaprevir/Pibrentasvir for Real-World HCV Infected Patients in the Northern Part of Tokyo, Japan. J. Clin. Med. 2021, 10, 5529. [Google Scholar] [CrossRef] [PubMed]
- Kolykhalov, A.A.; Agapov, E.V.; Blight, K.J.; Mihalik, K.; Feinstone, S.M.; Rice, C.M. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 1997, 277, 570–574. [Google Scholar] [CrossRef]
- Hayes, C.N.; Imamura, M.; Tanaka, J.; Chayama, K. Road to elimination of HCV: Clinical challenges in HCV management. Liver Int. 2022, 42, 1935–1944. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 29 October 2022).
- Frey, S.E.; Houghton, M.; Coates, S.; Abrignani, S.; Chien, D.; Rosa, D.; Pileri, P.; Ray, R.; Di Bisceglie, A.M.; Rinella, P.; et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367–6373. [Google Scholar] [CrossRef]
- Ray, R. Progress toward development of a hepatitis C vaccine with broad shoulders. Sci. Transl. Med. 2011, 3, 94ps33. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Saisho, H. Acute hepatitis C virus infection, 1986–2001: A rare cause of fulminant hepatitis in Chiba, Japan. Hepatogastroenterology 2004, 51, 556–558. [Google Scholar]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar] [CrossRef]
- Omata, M.; Kanda, T.; Wei, L.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.; Sollano, J.; Kumar, M.; Jindal, A.; et al. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol. Int. 2016, 10, 681–701. [Google Scholar] [CrossRef]
- Kato, N.; Yokosuka, O.; Hosoda, K.; Ito, Y.; Ohto, M.; Omata, M. Detection of hepatitis C virus RNA in acute non-A, non-B hepatitis as an early diagnostic tool. Biochem. Biophys. Res. Commun. 1993, 192, 800–807. [Google Scholar] [CrossRef]
- Takano, S.; Omata, M.; Ohto, M.; Satomura, Y. Posttransfusion hepatitis in Japan. Vox Sang. 1992, 62, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Omata, M.; Ohto, M.; Satomura, Y. Prospective assessment of incidence of fulminant hepatitis in post-transfusion hepatitis: A study of 504 cases. Dig. Dis. Sci. 1994, 39, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Tsai, Y.T.; Hwang, S.J.; Wu, J.C.; Yung, C.H.; Cheng, K.K.; Lo, K.J. A prospective study of post-transfusion non-A, non-B (type C) hepatitis following cardiovascular surgery in Taiwan. J. Med. Virol. 1991, 33, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.L.; Mamish, D.; Combs, C.; Kim, M.; Donegan, E.; Ferrell, L.; Lake, J.; Roberts, J.; Ascher, N.L. Hepatitis B virus and apparent fulminant non-A, non-B hepatitis. Lancet 1992, 339, 952–955. [Google Scholar] [CrossRef]
- Dasarathy, S.; Misra, S.C.; Acharya, S.K.; Irshad, M.; Joshi, Y.K.; Venugopal, P.; Tandon, B.N. Prospective controlled study of post-transfusion hepatitis after cardiac surgery in a large referral hospital in India. Liver 1992, 12, 116–120. [Google Scholar] [CrossRef]
- Theilmann, L.; Solbach, C.; Toex, U.; Müller, H.M.; Pfaff, E.; Otto, G.; Goeser, T. Role of hepatitis C virus infection in German patients with fulminant and subacute hepatic failure. Eur. J. Clin. Investig. 1992, 22, 569–571. [Google Scholar] [CrossRef]
- Kolho, E.; Ruutu, P.; Ruutu, T. Hepatitis C infection in BMT patients. Bone Marrow Transplant. 1993, 11, 119–123. [Google Scholar] [PubMed]
- Féray, C.; Gigou, M.; Samuel, D.; Reyes, G.; Bernuau, J.; Reynes, M.; Bismuth, H.; Bréchot, C. Hepatitis C virus RNA and hepatitis B virus DNA in serum and liver of patients with fulminant hepatitis. Gastroenterology 1993, 104, 549–555. [Google Scholar] [CrossRef]
- Liang, T.J.; Jeffers, L.; Reddy, R.K.; Silva, M.O.; Cheinquer, H.; Findor, A.; De Medina, M.; Yarbough, P.O.; Reyes, G.R.; Schiff, E.R. Fulminant or subfulminant non-A, non-B viral hepatitis: The role of hepatitis C and E viruses. Gastroenterology 1993, 104, 556–562. [Google Scholar] [CrossRef]
- Kuwada, S.K.; Patel, V.M.; Hollinger, F.B.; Lin, H.J.; Yarbough, P.O.; Wiesner, R.H.; Kaese, D.; Rakela, J. Non-A, non-B fulminant hepatitis is also non-E and non-C. Am. J. Gastroenterol. 1994, 89, 57–61. [Google Scholar]
- Sallie, R.; Silva, A.E.; Purdy, M.; Smith, H.; McCaustland, K.; Tibbs, C.; Portmann, B.; Eddleston, A.; Bradley, D.; Williams, R. Hepatitis C and E in non-A non-B fulminant hepatic failure: A polymerase chain reaction and serological study. J. Hepatol. 1994, 20, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.L.; Silva, A.E.; Macdonald, G.A.; Tsarev, S.A.; Di Biscelgie, A.M.; Lucey, M.R. Fulminant hepatitis in patients undergoing liver transplantation: Evidence for a non-A, non-B, non-C, non-D, and non-E syndrome. Liver Transpl. Surg. 1996, 2, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Budhiraja, S.; Narang, A.; Chakravarthy, A. Etiology of sporadic acute and fulminant non-A, non-B viral hepatitis in north India. Indian J. Gastroenterol. 1997, 16, 43–45. [Google Scholar] [PubMed]
- Fukai, K.; Yokosuka, O.; Fujiwara, K.; Tagawa, M.; Imazeki, F.; Saisho, H.; Omata, M. Etiologic considerations of fulminant non-A, non-B viral hepatitis in Japan: Analyses by nucleic acid amplification method. J. Infect. Dis. 1998, 178, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, E.; Zuckerman, T.; Douer, D.; Qian, D.; Levine, A.M. Liver dysfunction in patients infected with hepatitis C virus undergoing chemotherapy for hematologic malignancies. Cancer 1998, 83, 1224–1230. [Google Scholar] [CrossRef]
- Strasser, S.I.; Myerson, D.; Spurgeon, C.L.; Sullivan, K.M.; Storer, B.; Schoch, H.G.; Kim, S.; Flowers, M.E.; McDonald, G.B. Hepatitis C virus infection and bone marrow transplantation: A cohort study with 10-year follow-up. Hepatology 1999, 29, 1893–1899. [Google Scholar] [CrossRef]
- Jain, A.; Kar, P.; Madan, K.; Das, U.P.; Budhiraja, S.; Gopalkrishna, V.; Sharma, J.K.; Das, B.C. Hepatitis C virus infection in sporadic fulminant viral hepatitis in North India: Cause or co-factor? Eur. J. Gastroenterol. Hepatol. 1999, 11, 1231–1237. [Google Scholar] [CrossRef]
- Mahmoud, I.M.; Sobh, M.A.; Amer, G.M.; El-Chenawy, F.A.; Gazareen, S.H.; El-Sherif, A.; El-Sawy, E.; Ghoneim, M.A. A prospective study of hepatitis C viremia in renal allograft recipients. Am. J. Nephrol. 1999, 19, 576–585. [Google Scholar] [CrossRef]
- Wiese, M.; Berr, F.; Lafrenz, M.; Porst, H.; Oesen, U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in Germany: A 20-year multicenter study. Hepatology 2000, 32, 91–96. [Google Scholar] [CrossRef]
- El-Sayed, M.H.; Mohamed, M.M.; Karim, A.; Maina, A.M.; Oliveri, F.; Brunetto, M.R.; Bonino, F. Severe liver disease is caused by HBV rather than HCV in children with hematological malignancies. Hematol. J. 2003, 4, 321–327. [Google Scholar] [CrossRef]
- Beniwal, M.; Kumar, A.; Kar, P.; Jilani, N.; Sharma, J.B. Prevalence and severity of acute viral hepatitis and fulminant hepatitis during pregnancy: A prospective study from north India. Indian J. Med. Microbiol. 2003, 21, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Ray, S.; Thuluvath, P.J. Acute hepatitis C. Lancet 2008, 372, 321–332. [Google Scholar] [CrossRef]
- Dirchwolf, M.; Marciano, S.; Mauro, E.; Ruf, A.E.; Rezzonico, L.; Anders, M.; Chiodi, D.; Petta, N.G.; Borzi, S.; Tanno, F.; et al. Clinical epidemiology of acute hepatitis C in South America. J. Med. Virol. 2017, 89, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Rule, J.A.; Cerro-Chiang, G.; Stravitz, R.T.; McGuire, B.M.; Lee, G.; Fontana, R.J.; Lee, W.M. Role of Hepatitis C Infection in Acute Liver Injury/Acute Liver Failure in North America. Dig. Dis. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Date, T.; Miyamoto, M.; Furusaka, A.; Tokushige, K.; Mizokami, M.; Wakita, T. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology 2003, 125, 1808–1817. [Google Scholar] [CrossRef]
- Wakita, T.; Pietschmann, T.; Kato, T.; Date, T.; Miyamoto, M.; Zhao, Z.; Murthy, K.; Habermann, A.; Kräusslich, H.G.; Mizokami, M.; et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005, 11, 791–796. [Google Scholar] [CrossRef]
- Wong, N.Z.; Reddy, K.R.; Bittermann, T. Acute Liver Failure Etiology Is an Independent Predictor of Waitlist Outcome but Not Posttransplantation Survival in a National Cohort. Liver Transpl. 2022, 28, 39–50. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Sam, J.; Thuluvath, P.J. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: A nationwide analysis. Hepatology 2008, 48, 1336–1341. [Google Scholar] [CrossRef]
- Uehara, T.; Kosyk, O.; Jeannot, E.; Bradford, B.U.; Tech, K.; Macdonald, J.M.; Boorman, G.A.; Chatterjee, S.; Mason, R.P.; Melnyk, S.B.; et al. Acetaminophen-induced acute liver injury in HCV transgenic mice. Toxicol. Appl. Pharmacol. 2013, 266, 224–232. [Google Scholar] [CrossRef]
- Ramachandran, A.; Lebofsky, M.; Yan, H.M.; Weinman, S.A.; Jaeschke, H. Hepatitis C virus structural proteins can exacerbate or ameliorate acetaminophen-induced liver injury in mice. Arch. Toxicol. 2015, 89, 773–783. [Google Scholar] [CrossRef]
- Locasciulli, A.; Pontisso, P.; Cavalletto, D.; Fraschini, D.; Uderzo, C.; Masera, G.; Alberti, A. Evidence against the role of hepatitis C virus in severe liver damage occurring early in the course of acute leukemia in children. Leuk. Lymphoma 1994, 13, 119–122. [Google Scholar] [CrossRef]
- Omata, F.; Ueno, F.; Kushibiki, Y.; Takahashi, H. Fulminant hepatitis following bone marrow transplantation in hepatitis B virus carrier siblings. J. Gastroenterol. 1994, 29, 653–655. [Google Scholar] [CrossRef]
- Gordon, F.D.; Anastopoulos, H.; Khettry, U.; Loda, M.; Jenkins, R.L.; Lewis, W.D.; Trey, C. Hepatitis C infection: A rare cause of fulminant hepatic failure. Am. J. Gastroenterol. 1995, 90, 117–120. [Google Scholar]
- Villamil, F.G.; Hu, K.Q.; Yu, C.H.; Lee, C.H.; Rojter, S.E.; Podesta, L.G.; Makowka, L.; Geller, S.A.; Vierling, J.M. Detection of hepatitis C virus with RNA polymerase chain reaction in fulminant hepatic failure. Hepatology 1995, 22, 1379–1386. [Google Scholar] [PubMed]
- Vento, S.; Cainelli, F.; Mirandola, F.; Cosco, L.; Di Perri, G.; Solbiati, M.; Ferraro, T.; Concia, E. Fulminant hepatitis on withdrawal of chemotherapy in carriers of hepatitis C virus. Lancet 1996, 347, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Funaoka, M.; Kato, K.; Komatsu, M.; Ono, T.; Hoshino, T.; Kato, J.; Kuramitsu, T.; Ishii, T.; Toyoshima, I.; Masamune, O. Fulminant hepatitis caused by hepatitis C virus during treatment for multiple sclerosis. J. Gastroenterol. 1996, 31, 119–122. [Google Scholar] [CrossRef]
- Inokuchi, K.; Nakata, K.; Hamasaki, K.; Daikoku, M.; Nakao, K.; Kato, Y.; Yatsuhashi, H.; Koga, M.; Yano, M.; Nagataki, S. Prevalence of hepatitis B or C virus infection in patients with fulminant viral hepatitis. An analysis using polymerase chain reaction. J. Hepatol. 1996, 24, 258–264. [Google Scholar] [CrossRef]
- Kato, T.; Furusaka, A.; Miyamoto, M.; Date, T.; Yasui, K.; Hiramoto, J.; Nagayama, K.; Tanaka, T.; Wakita, T. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J. Med. Virol. 2001, 64, 334–339. [Google Scholar] [CrossRef]
- Devalle, S.; de Paula, V.S.; de Oliveira, J.M.; Niel, C.; Gaspar, A.M. Case fatality rate of acute viral hepatitis in Italy: 1995–2000. An update. J. Infect. 2003, 47, 125–128. [Google Scholar] [CrossRef]
- Kogure, T.; Ueno, Y.; Fukushima, K.; Nagasaki, F.; Inoue, J.; Kakazu, E.; Matsuda, Y.; Kido, O.; Nakagome, Y.; Kimura, O.; et al. Fulminant hepatic failure in a case of autoimmune hepatitis in hepatitis C during peg-interferon-alpha 2b plus ribavirin treatment. World J. Gastroenterol. 2007, 13, 4394–4397. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, L.; Pietrosi, G.; Gruttadauria, S.; Vizzini, G.; Spampinato, M.; Spada, M.; Burgio, G.; Arcadipane, A.; Ida Minervini, M.; Pagnucco, G.; et al. Successful liver transplant in an HCV-infected haemophiliac patient with fulminant hepatic failure. Haemophilia 2007, 13, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Takaki, A.; Yagi, T.; Ikeda, F.; Yasunaka, T.; Koike, K.; Miyake, Y.; Iwasaki, Y.; Nouso, K.; Sadamori, H.; et al. A case of fulminant liver failure associated with hepatitis C virus. Clin. J. Gastroenterol. 2014, 7, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Younis, B.B.; Arshad, R.; Khurhsid, S.; Masood, J.; Nazir, F.; Tahira, M. Fulminant hepatic failure (FHF) due to acute hepatitis C. Pak. J. Med. Sci. 2015, 31, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, N.; Suzuki, F.; Akuta, N.; Suzuki, Y.; Sezaki, H.; Hosaka, T.; Someya, T.; Kobayashi, M.; Saitoh, S.; Arase, Y.; et al. Clinical and virological characteristics of untreated patients with chronic hepatitis C who develop serum alanine aminotransferase flare-up. J. Med. Virol. 2005, 75, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Rumi, M.G.; De Filippi, F.; La Vecchia, C.; Donato, M.F.; Gallus, S.; Del Ninno, E.; Colombo, M. Hepatitis C reactivation in patients with chronic infection with genotypes 1b and 2c: A retrospective cohort study of 206 untreated patients. Gut 2005, 54, 402–406. [Google Scholar] [CrossRef]
- Sagnelli, E.; Sagnelli, C.; Pisaturo, M.; Coppola, N. Hepatic flares in chronic hepatitis C: Spontaneous exacerbation vs hepatotropic viruses superinfection. World J. Gastroenterol. 2014, 20, 6707–6715. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Chen, W.C.; Tsai, W.L.; Cheng, J.S.; Tsay, F.W.; Kao, S.S.; Chen, H.C.; Hsu, P.I. Severe acute exacerbation of HCV infection in cancer patients who undergo chemotherapy without antiviral prophylaxis. J. Viral Hepat. 2020, 27, 873–879. [Google Scholar] [CrossRef]
- Torres, H.A.; Hosry, J.; Mahale, P.; Economides, M.P.; Jiang, Y.; Lok, A.S. Hepatitis C virus reactivation in patients receiving cancer treatment: A prospective observational study. Hepatology 2018, 67, 36–47. [Google Scholar] [CrossRef]
- Mustafayev, K.; Torres, H. Hepatitis B virus and hepatitis C virus reactivation in cancer patients receiving novel anticancer therapies. Clin. Microbiol. Infect. 2022, 28, 1321–1327. [Google Scholar] [CrossRef]
- Gardiner, D.; Lalezari, J.; Lawitz, E.; DiMicco, M.; Ghalib, R.; Reddy, K.R.; Chang, K.M.; Sulkowski, M.; Marro, S.O.; Anderson, J.; et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE 2013, 8, e63818. [Google Scholar] [CrossRef]
- Davar, D.; Wilson, M.; Pruckner, C.; Kirkwood, J.M. PD-1 Blockade in Advanced Melanoma in Patients with Hepatitis C and/or HIV. Case Rep. Oncol. Med. 2015, 2015, 737389. [Google Scholar] [CrossRef]
- Kothapalli, A.; Khattak, M.A. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: A case series. Melanoma Res. 2018, 28, 155–158. [Google Scholar] [CrossRef]
- Takahashi, K.; Kanda, T.; Nakamura, M.; Yasui, S.; Arai, M.; Kato, N. Acutely exacerbated chronic hepatitis C after administration of nivolumab: A case report. Kanzo 2019, 60, 459–465, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Hiura, M.; Onizuka, R.; Narita, R.; Abe, S.; Tabaru, A.; Harada, M. A case of severe acute hepatitis C and delayed antibody production due to rituximab therapy. Clin. J. Gastroenterol. 2010, 3, 254–258. [Google Scholar] [CrossRef]
- Sasaki, R.; Meyer, K.; Moriyama, M.; Kato, N.; Yokosuka, O.; Ray, R.B.; Aurora, R.; Ray, R.; Kanda, T. Rapid hepatitis C virus clearance by antivirals correlates with immune status of infected patients. J. Med. Virol. 2019, 91, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mahale, P.; Kontoyiannis, D.P.; Chemaly, R.F.; Jiang, Y.; Hwang, J.P.; Davila, M.; Torres, H.A. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J. Hepatol. 2012, 57, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Huang, H.H.; Lin, C.Y.; Chung, L.W.; Liao, Y.M.; Bai, L.Y.; Chiu, C.F. Rituximab-induced hepatitis C virus reactivation after spontaneous remission in diffuse large B-cell lymphoma. J. Clin. Oncol. 2008, 26, 2584–2586. [Google Scholar] [CrossRef]
- Sagnelli, E.; Pisaturo, M.; Sagnelli, C.; Coppola, N. Rituximab-based treatment, HCV replication, and hepatic flares. Clin. Dev. Immunol. 2012, 2012, 945950. [Google Scholar] [CrossRef]
- Aksoy, S.; Abali, H.; Kilickap, S.; Erman, M.; Kars, A. Accelerated hepatitis C virus replication with rituximab treatment in a non-Hodgkin’s lymphoma patient. Clin. Lab. Haematol. 2006, 28, 211–214. [Google Scholar] [CrossRef]
- Ennishi, D.; Maeda, Y.; Niitsu, N.; Kojima, M.; Izutsu, K.; Takizawa, J.; Kusumoto, S.; Okamoto, M.; Yokoyama, M.; Takamatsu, Y.; et al. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: A Japanese multicenter analysis. Blood 2010, 116, 5119–5125. [Google Scholar] [CrossRef]
- Pitini, V.; Sturniolo, G.; Arrigo, C.; Leonardi, S.; Pino, S.; Altavilla, G. HCV genotype 2 as a risk factor for reactivation in patients with B-cell lymphoma undergoing rituximab combination chemotherapy. Br. J. Haematol. 2010, 150, 116–118. [Google Scholar] [CrossRef]
- Arcaini, L.; Merli, M.; Passamonti, F.; Bruno, R.; Brusamolino, E.; Sacchi, P.; Rattotti, S.; Orlandi, E.; Rumi, E.; Ferretti, V.; et al. Impact of treatment-related liver toxicity on the outcome of HCV-positive non-Hodgkin’s lymphomas. Am. J. Hematol. 2010, 85, 46–50. [Google Scholar] [CrossRef]
- Marignani, M.; Mangone, M.; Cox, M.C.; Angeletti, S.; Veggia, B.; Ferrari, A.; di Fonzo, M.; Begini, P.; Gigante, E.; Laverde, G.; et al. HCV-positive status and hepatitis flares in patients with B-cell non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Dig. Liver Dis. 2011, 43, 139–142. [Google Scholar] [CrossRef]
- Nooka, A.; Shenoy, P.J.; Sinha, R.; Lonial, S.; Flowers, C.R. Hepatitis C reactivation in patients who have diffuse large B-cell lymphoma treated with rituximab: A case report and review of literature. Clin. Lymphoma Myeloma Leuk. 2011, 11, 379–384. [Google Scholar] [CrossRef]
- Coppola, N.; Pisaturo, M.; Guastafierro, S.; Tonziello, G.; Sica, A.; Iodice, V.; Sagnelli, C.; Ferrara, M.G.; Sagnelli, E. Increased hepatitis C viral load and reactivation of liver disease in HCV RNA-positive patients with onco-haematological disease undergoing chemotherapy. Dig. Liver Dis. 2012, 44, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lake-Bakaar, G.; Dustin, L.; McKeating, J.; Newton, K.; Freeman, V.; Frost, S.D. Hepatitis C virus and alanine aminotransferase kinetics following B-lymphocyte depletion with rituximab: Evidence for a significant role of humoral immunity in the control of viremia in chronic HCV liver disease. Blood 2007, 109, 845–846. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Ichiki, K.; Shiratori, S.; Kawamura, T.; Tanaka, J.; Asaka, M.; Imamura, M.; Masauzi, N. Changes in hepatitis C virus antibody titer and viral RNA load in non-Hodgkin’s lymphoma patients after rituximab chemotherapy. Int. J. Lab. Hematol. 2009, 31, 468–470. [Google Scholar] [CrossRef]
- Mori, N.; Imamura, M.; Takaki, S.; Araki, T.; Hayes, N.C.; Aisaka, Y.; Chayama, K. Hepatitis C virus (HCV) reactivation caused by steroid therapy for dermatomyositis. Intern. Med. 2014, 53, 2689–2693. [Google Scholar] [CrossRef] [PubMed]
- Vijayamahantesh, V.; Patra, T.; Meyer, K.; Alameh, M.G.; Reagan, E.K.; Weissman, D.; Ray, R. Modified E2 Glycoprotein of Hepatitis C Virus Enhances Proinflammatory Cytokines and Protective Immune Response. J. Virol. 2022, 96, e0052322. [Google Scholar] [CrossRef] [PubMed]
- Ait-Goughoulte, M.; Kanda, T.; Meyer, K.; Ryerse, J.S.; Ray, R.B.; Ray, R. Hepatitis C virus genotype 1a growth and induction of autophagy. J. Virol. 2008, 82, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Inhibition of intrahepatic gamma interferon production by hepatitis C virus nonstructural protein 5A in transgenic mice. J. Virol. 2009, 83, 8463–8469. [Google Scholar] [CrossRef]
- Raychoudhuri, A.; Shrivastava, S.; Steele, R.; Dash, S.; Kanda, T.; Ray, R.; Ray, R.B. Hepatitis C virus infection impairs IRF-7 translocation and Alpha interferon synthesis in immortalized human hepatocytes. J. Virol. 2010, 84, 10991–10998. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.A.; Economides, M.P.; Angelidakis, G.; Hosry, J.; Kyvernitakis, A.; Mahale, P.; Jiang, Y.; Miller, E.; Blechacz, B.; Naing, A.; et al. Sofosbuvir-Based Therapy in Hepatitis C Virus-Infected Cancer Patients: A Prospective Observational Study. Am. J. Gastroenterol. 2019, 114, 250–257. [Google Scholar] [CrossRef]
- Levine, A.M.; Shimodaira, S.; Lai, M.M. Treatment of HCV-related mantle-cell lymphoma with ribavirin and pegylated interferon Alfa. N. Engl. J. Med. 2003, 349, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Kusano, A.; Sugumar, A.; Tajima, K.; Mueller, N.; Nakamura, S. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: A meta-analysis of epidemiological studies. Cancer Sci. 2004, 95, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Schollkopf, C.; Smedby, K.E.; Hjalgrin, H.; Rostgaard, K.; Panum, I.; Vinner, L.; Chang, E.T.; Glimelius, B.; Porwit, A.; Sundstrom, C.; et al. Hepatitis C infection and risk of malignant lymphoma. Int. J. Cancer. 2008, 122, 1885–1890. [Google Scholar] [CrossRef]
- Arcaini, L.; Vallisa, D.; Rattotti, S.; Ferretti, V.V.; Ferreri, A.J.M.; Bernuzzi, P.; Merli, M.; Varettoni, M.; Chiappella, A.; Ambrosetti, A.; et al. Antiviral treatment in patients with indolent B-cell lymphomas associated with HCV infection: A study of the Fondazione Italiana Linfomi. Ann. Oncol. 2014, 25, 1404–1410. [Google Scholar] [CrossRef]
- Merli, M.; Carli, G.; Arcaini, L.; Visco, C. Antiviral therapy of hepatitis C as curative treatment of indolent B-cell lymphoma. World J. Gastroenterol. 2016, 22, 8447–8458. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Nakayama, C.; Kamada, K.; Kikuchi, R.; Kudo, D.; Ito, S.; Matsuoka, S.; Shiratori, S.; Yamamoto, Y.; Naruse, H.; et al. Efficacy and prognosis of antiviral therapy on hepatitis C following treatment of lymphoma in HCV-positive diffuse large-cell lymphoma. Ann. Hematol. 2017, 96, 2057–2061. [Google Scholar] [CrossRef]
- Siba, Y.; Obiokoye, K.; Ferstenberg, R.; Robilotti, J.; Culpepper-Morgan, J. Case report of acute-on-chronic liver failure secondary to diffuse large B-cell lymphoma. World J. Gastroenterol. 2014, 20, 16774–16778. [Google Scholar] [CrossRef]
- Torres, H.A.; Chong, P.P.; De Lima, M.; Friedman, M.S.; Giralt, S.; Hammond, S.P.; Kiel, P.J.; Masur, H.; McDonald, G.B.; Wingard, J.R.; et al. Hepatitis C Virus Infection among Hematopoietic Cell Transplant Donors and Recipients: American Society for Blood and Marrow Transplantation Task Force Recommendations. Biol. Blood Marrow Transplant. 2015, 21, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Kanda, T.; Nakaseko, C.; Sakaida, E.; Ohwada, C.; Takeuchi, M.; Takeda, Y.; Mimura, N.; Iseki, T.; Wu, S.; et al. Reactivation of Hepatitis B Virus in Hematopoietic Stem Cell Transplant Recipients in Japan: Efficacy of Nucleos(t)ide Analogues for Prevention and Treatment. Int. J. Mol. Sci. 2014, 15, 21455–21467. [Google Scholar] [CrossRef]
- Oliver, N.T.; Nieto, Y.L.; Blechacz, B.; Anderlini, P.; Ariza-Heredia, E.; Torres, H.A. Severe hepatitis C reactivation as an early complication of hematopoietic cell transplantation. Bone Marrow Transplant. 2017, 52, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.A.; McDonald, G.B. How I treat hepatitis C virus infection in patients with hematologic malignancies. Blood 2016, 128, 1449–1457. [Google Scholar] [CrossRef]
- Ikegami, C.; Kanda, T.; Ishii, T.; Honda, M.; Yamana, Y.; Tanaka, R.S.; Kumagawa, M.; Kanezawa, S.; Mizutani, T.; Yamagami, H.; et al. COVID-19 After Treatment with Direct-acting Antivirals for HCV Infection and Decompensated Cirrhosis: A Case Report. In Vivo 2022, 36, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Abutaleb, A.; Almario, J.A.; Alghsoon, S.; Yoon, J.A.; Gheysens, K.; Kottilil, S.; Wilson, E. Higher Levels of Fibrosis in a Cohort of Veterans with Chronic Viral Hepatitis are Associated with Extrahepatic Cancers. J. Clin. Exp. Hepatol. 2021, 11, 195–200. [Google Scholar] [CrossRef]
- Asatani, S.; Kanda, T.; Honda, M.; Ishii, T.; Yamana, Y.; Kaneko, T.; Mizutani, T.; Takahashi, H.; Kumagawa, M.; Sasaki, R.; et al. Occurrence of hepatitis in an elderly woman during the treatment of pembrolizumab for right advanced renal pelvis, ureteral cancer, and bladder cancer. JGH Open 2021, 5, 722–724. [Google Scholar] [CrossRef]
- Kanda, T.; Yasui, S.; Nakamura, M.; Nakamoto, S.; Takahashi, K.; Wu, S.; Sasaki, R.; Haga, Y.; Ogasawara, S.; Saito, T.; et al. Interferon-free treatment for patients with chronic hepatitis C and autoimmune liver disease: Higher SVR rates with special precautions for deterioration of autoimmune hepatitis. Oncotarget 2018, 9, 11631–11637. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ogasawara, S.; Takahashi, A.; Seko, Y.; Unozawa, H.; Sato, R.; Watanabe, S.; Moriguchi, M.; Morimoto, N.; Tsuchiya, S.; et al. Evolution of Survival Impact of Molecular Target Agents in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer. 2021, 11, 48–60. [Google Scholar] [CrossRef]
- Boutin, C.A.; Adam, J.P.; Martel, D.; Doucet, S.; Martel-Laferrière, V. Risks of hepatitis C virus reactivation in a real-life population of oncology patients treated in an academic center. J. Oncol. Pharm. Pract. 2021, 27, 1815–1820. [Google Scholar] [CrossRef]
- Albain, K.S.; Crowley, J.J.; LeBlanc, M.; Livingston, R.B. Survival determinants in extensive-stage non-small-cell lung cancer: The Southwest Oncology Group experience. J. Clin. Oncol. 1991, 9, 1618–1626. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Imazeki, F.; Yokosuka, O.; Fukai, K.; Kawai, S.; Kanda, T.; Kojima, H.; Saisho, H. Lower incidence of hepatic failure than hepatocellular carcinoma in Japanese patients with chronic hepatitis C. Liver Int. 2005, 25, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Toka, B.; Köksal, A.Ş.; Dertli, R.; Şirin, G.; Fidan, S.; Ülger, Y.; Harmandar, F.; Yıldırım, A.E.; Eminler, A.T.; Asil, M.; et al. Hepatitis B Reactivation in Patients Treated with Direct-Acting Antivirals for Hepatitis C. Dig. Dis. 2022, 40, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.L.; Huang, C.I.; Huang, C.F.; Hsieh, M.H.; Liu, T.W.; Lin, Y.H.; Liang, P.C.; Hsieh, M.Y.; Lin, Z.Y.; Chen, S.C.; et al. Pretreatment Hepatitis B Viral Load Predicts Long-Term Hepatitis B Response After Anti-Hepatitis C Therapy in Hepatitis B/C Dual-Infected Patients. J. Infect. Dis. 2019, 219, 1224–1233. [Google Scholar] [CrossRef]
- Huang, S.C.; Cheng, P.N.; Liu, C.H.; Yang, H.C.; Su, T.H.; Tseng, T.C.; Chen, P.J.; Kao, J.H.; Liu, C.J. Serum cytokine/chemokine profiles predict hepatitis B reactivation in HBV/HCV co-infected subjects receiving direct-acting antiviral agents. J. Formos Med. Assoc. 2022, 121, 920–929. [Google Scholar] [CrossRef]
- Kawagishi, N.; Suda, G.; Sakamori, R.; Matsui, T.; Onozawa, M.; Yang, Z.; Yoshida, S.; Ohara, M.; Kimura, M.; Kubo, A.; et al. Serum IL-1β predicts de novo hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C, not during anti-cancer/immunosuppressive therapy. Sci. Rep. 2022, 12, 16800. [Google Scholar] [CrossRef]
- Tseng, C.W.; Liu, W.C.; Ko, P.H.; Chen, Y.C.; Tseng, K.C.; Chang, T.T. The Predictive Role of Hepatitis B Biomarkers on HBV Reactivation following Direct-Acting Antiviral Therapy in HBV/HCV Coinfected Patients. Viruses 2022, 14, 1812. [Google Scholar] [CrossRef]
- Su, Y.T.; Chang, M.L.; Chien, R.N.; Liaw, Y.F. Hepatitis C Virus Reactivation in Anti-HCV Antibody-Positive Patients with Chronic Hepatitis B Following Anti-HBV Therapies. Viruses 2022, 14, 1858. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Chien, R.N.; Lin, S.M.; Yeh, C.T.; Tsai, S.L.; Sheen, I.S.; Chu, C.M. Response of patients with dual hepatitis B virus and C virus infection to interferon therapy. J. Interferon Cytokine Res. 1997, 17, 449–452. [Google Scholar] [CrossRef]
- Sato, K.; Inoue, J.; Kakazu, E.; Ninomiya, M.; Iwata, T.; Sano, A.; Tsuruoka, M.; Masamune, A. Reactivation of hepatitis C virus with severe hepatitis flare during steroid administration for interstitial pneumonia. Clin. J. Gastroenterol. 2021, 14, 1221–1226. [Google Scholar] [CrossRef]
- Hatanaka, T.; Naganuma, A.; Tateyama, Y.; Yoshinari, F.; Hoshino, T.; Sato, K.; Hmwe, S.S.; Aizaki, H.; Wakita, T.; Kakizaki, S.; et al. Ledipasvir and Sofosbuvir for Acute Hepatitis C Virus Monoinfection Associated with a High Risk of Acute Liver Failure. Intern. Med. 2019, 58, 2969–2975. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kwak, J.Y.; Lee, S.S.; Kim, H.G.; Son, H.J.; Jeon, H.; Kim, H.J.; Cha, R.R.; Lee, J.M.; Kim, H.J. Clinical Features of Hepatitis C Virus-related Acute-on-chronic Liver Failure in a Korean Population. Korean J. Gastroenterol. 2022, 80, 169–176. [Google Scholar] [CrossRef]
- El Ray, A.; Fouad, R.; El Makhzangy, H.; El Beshlawy, M.; Moreau, R.; Sherbiny, M. Characterizing a cohort of Egyptian patients with acute-on-chronic liver failure. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Metawea, M.I.; Moteleub, H.N.A.E. Diagnostic role of simple indices in HCV-related liver cirrhosis outcomes: A prospective cross-sectional study. Clin. Exp. Hepatol. 2022, 8, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Cerbu, B.; Grigoras, M.L.; Bratosin, F.; Bogdan, I.; Citu, C.; Bota, A.V.; Timircan, M.; Bratu, M.L.; Levai, M.C.; Marincu, I. Laboratory Profile of COVID-19 Patients with Hepatitis C-Related Liver Cirrhosis. J. Clin. Med. 2022, 11, 652. [Google Scholar] [CrossRef]
- Elhence, A.; Vaishnav, M.; Biswas, S.; Anand, A.; Gunjan, D.; Kedia, S.; Mahapatra, S.J.; Nayak, B.; Sheikh, S.; Soni, K.D.; et al. Predictors of in-hospital Outcomes in Patients with Cirrhosis and Coronavirus Disease-2019. J. Clin. Exp. Hepatol. 2022, 12, 876–886. [Google Scholar] [CrossRef] [PubMed]
| Case No. | Age (years) /Sex | HCV RNA (LIU/mL) /HCV Genotypes (GTs) | Max ALT (IU/L) | HIV | Type of Cancer | Drugs /Continued | DAAs for HCV | Refs |
|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | 4.55/GT 1a | 700–800 | Unknown | Unknown | Nivolumab/ Unknown | None | [71] |
| 2 | 59/Female | 6.36/GT 1b | 100–120 | Unknown | Metastatic melanoma | Pembrolizumab /Yes | LDV/SOF | [72] |
| 3 | 49/Male | 5.94/GT 1c | Elevation (CTCAE grade 1) | Positive | Metastatic melanoma | Pembrolizumab /No due to PD | None | [72] |
| 4 | 54/Male | Positive/Unknown | Elevation (CTCAE grade 2) | Unknown | Melanoma Stage IV | Pembrolizumab /Yes | LDV/SOF | [73] |
| 5 | 48/Male | 6.3/GT 2a | 559 | Negative | Squamous cell lung cancer | Nivolumab/No | SOF/RBV | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Matsumoto, N.; Ishii, T.; Arima, S.; Shibuya, S.; Honda, M.; Sasaki-Tanaka, R.; Masuzaki, R.; Kanezawa, S.; Nishizawa, T.; et al. Chronic Hepatitis C: Acute Exacerbation and Alanine Aminotransferase Flare. Viruses 2023, 15, 183. https://doi.org/10.3390/v15010183
Kanda T, Matsumoto N, Ishii T, Arima S, Shibuya S, Honda M, Sasaki-Tanaka R, Masuzaki R, Kanezawa S, Nishizawa T, et al. Chronic Hepatitis C: Acute Exacerbation and Alanine Aminotransferase Flare. Viruses. 2023; 15(1):183. https://doi.org/10.3390/v15010183
Chicago/Turabian StyleKanda, Tatsuo, Naoki Matsumoto, Tomotaka Ishii, Shuhei Arima, Shinji Shibuya, Masayuki Honda, Reina Sasaki-Tanaka, Ryota Masuzaki, Shini Kanezawa, Tsukasa Nishizawa, and et al. 2023. "Chronic Hepatitis C: Acute Exacerbation and Alanine Aminotransferase Flare" Viruses 15, no. 1: 183. https://doi.org/10.3390/v15010183
APA StyleKanda, T., Matsumoto, N., Ishii, T., Arima, S., Shibuya, S., Honda, M., Sasaki-Tanaka, R., Masuzaki, R., Kanezawa, S., Nishizawa, T., Gon, Y., Ogawa, M., & Kogure, H. (2023). Chronic Hepatitis C: Acute Exacerbation and Alanine Aminotransferase Flare. Viruses, 15(1), 183. https://doi.org/10.3390/v15010183









