Tumor-Infiltrating T Cells in EBV-Associated Gastric Carcinomas Exhibit High Levels of Multiple Markers of Activation, Effector Gene Expression, and Exhaustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Ethics
2.2. Analysis of Cellular mRNA
2.3. Analysis of Immune Landscape Features
2.4. Pathology-Based Analysis of TIL Infiltration and Spatial Organization
2.5. Correlation of EBV miR-BARTs with Cellular Gene Expression and Pathology Scores
2.6. Survival Analysis
3. Results
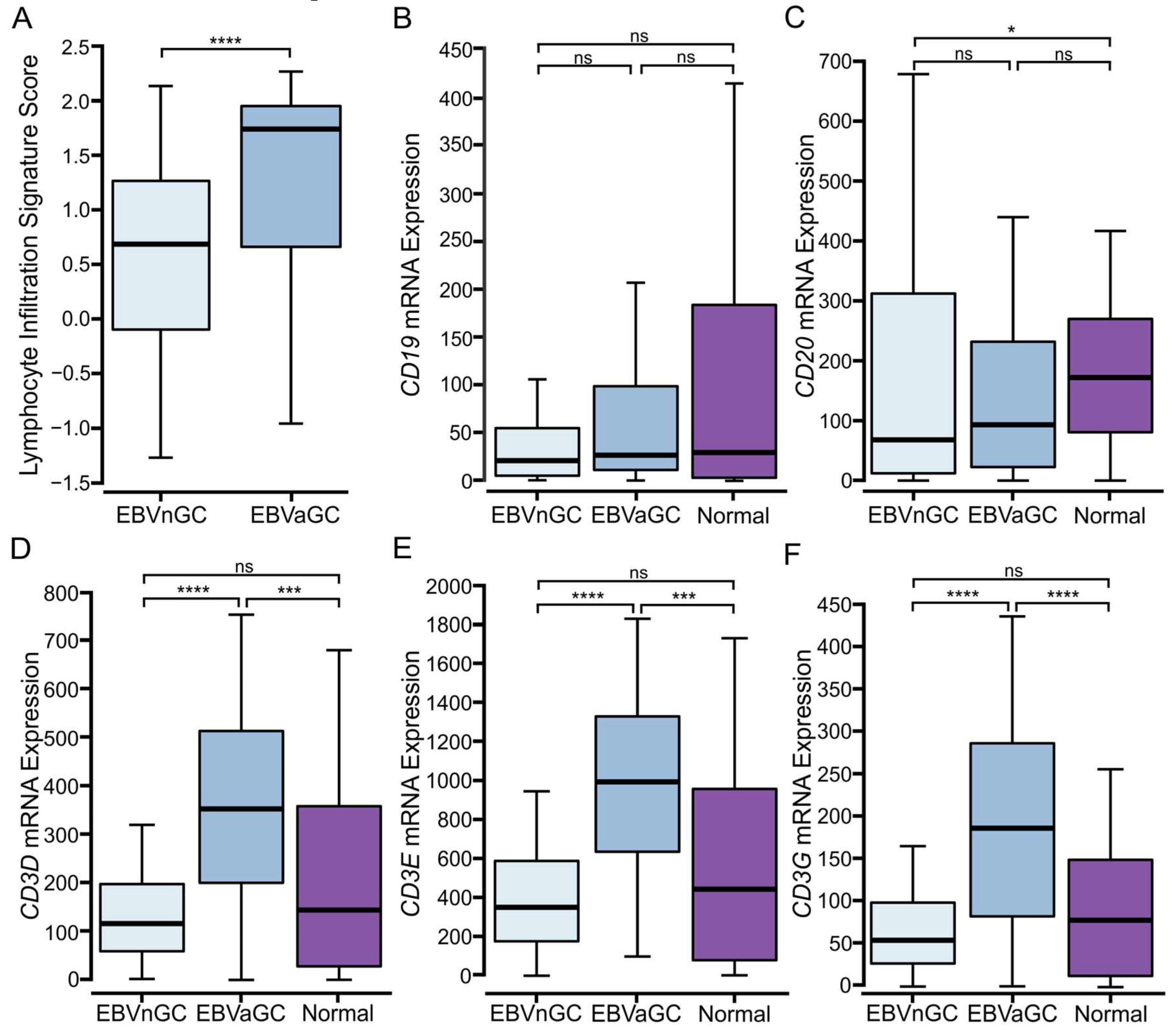
3.1. EBVaGCs Exhibit Greater Tumor Lymphocyte Infiltration as Compared to EBVnGCs
3.2. Higher Levels of T and NK, but Not B Lymphocytes Are Present in EBVaGCs
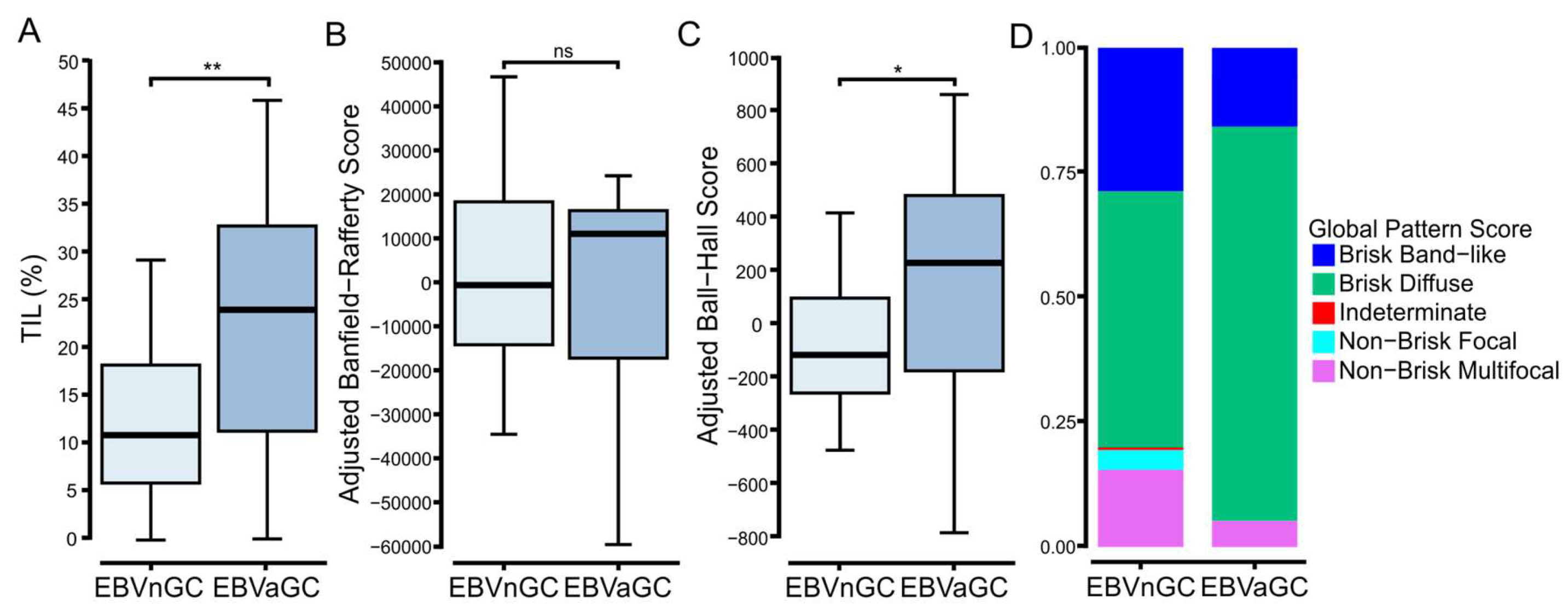
3.3. Digital Pathology-Based Analysis Reveals Increased TIL Infiltration and Altered Spatial Organization of Lymphocytes in EBVaGCs Compared to EBVnGCs
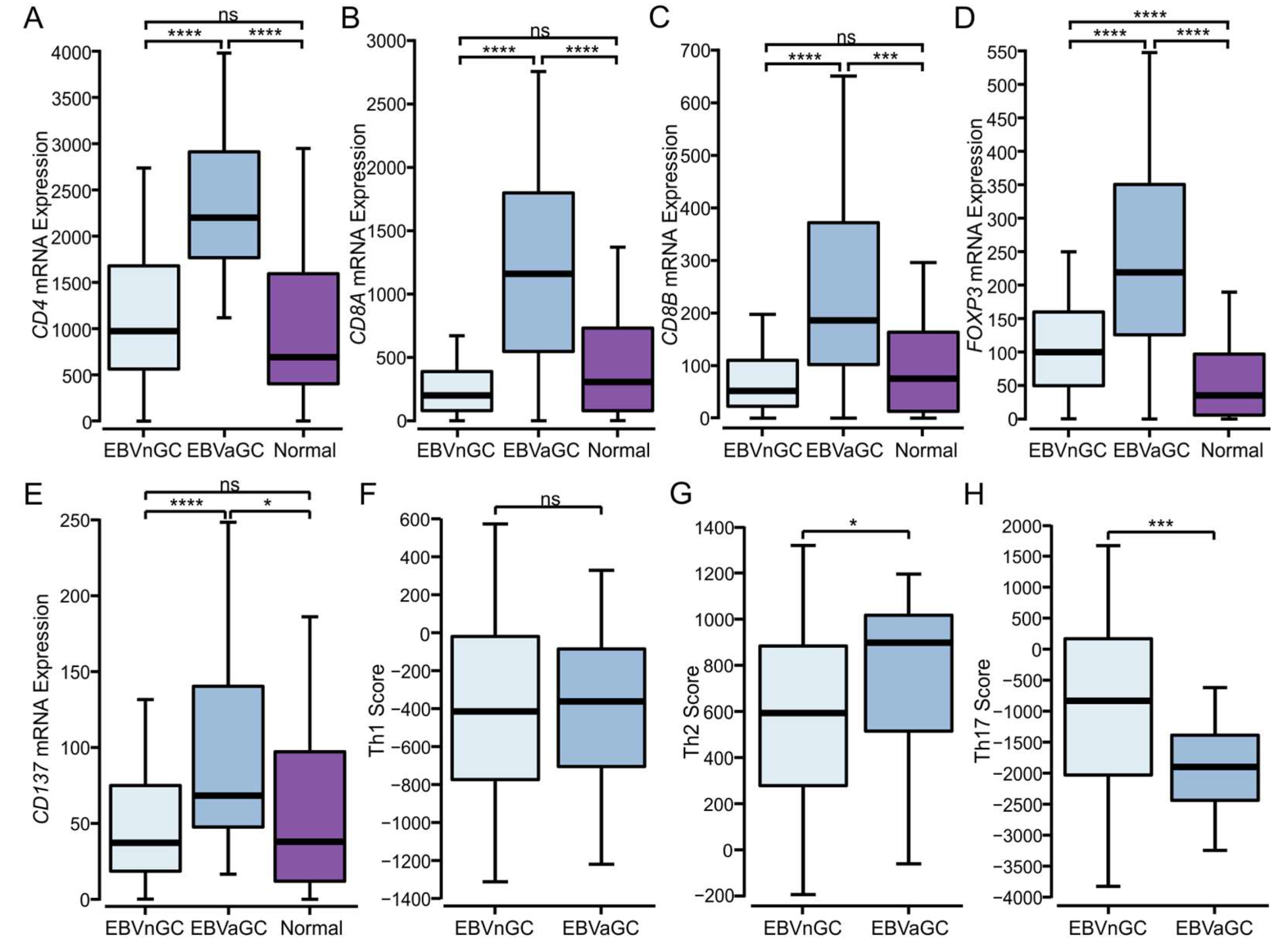
3.4. Higher Levels of CD4+, CD8+ T Cells, and T Regulatory Cells Are Present in EBVaGCs
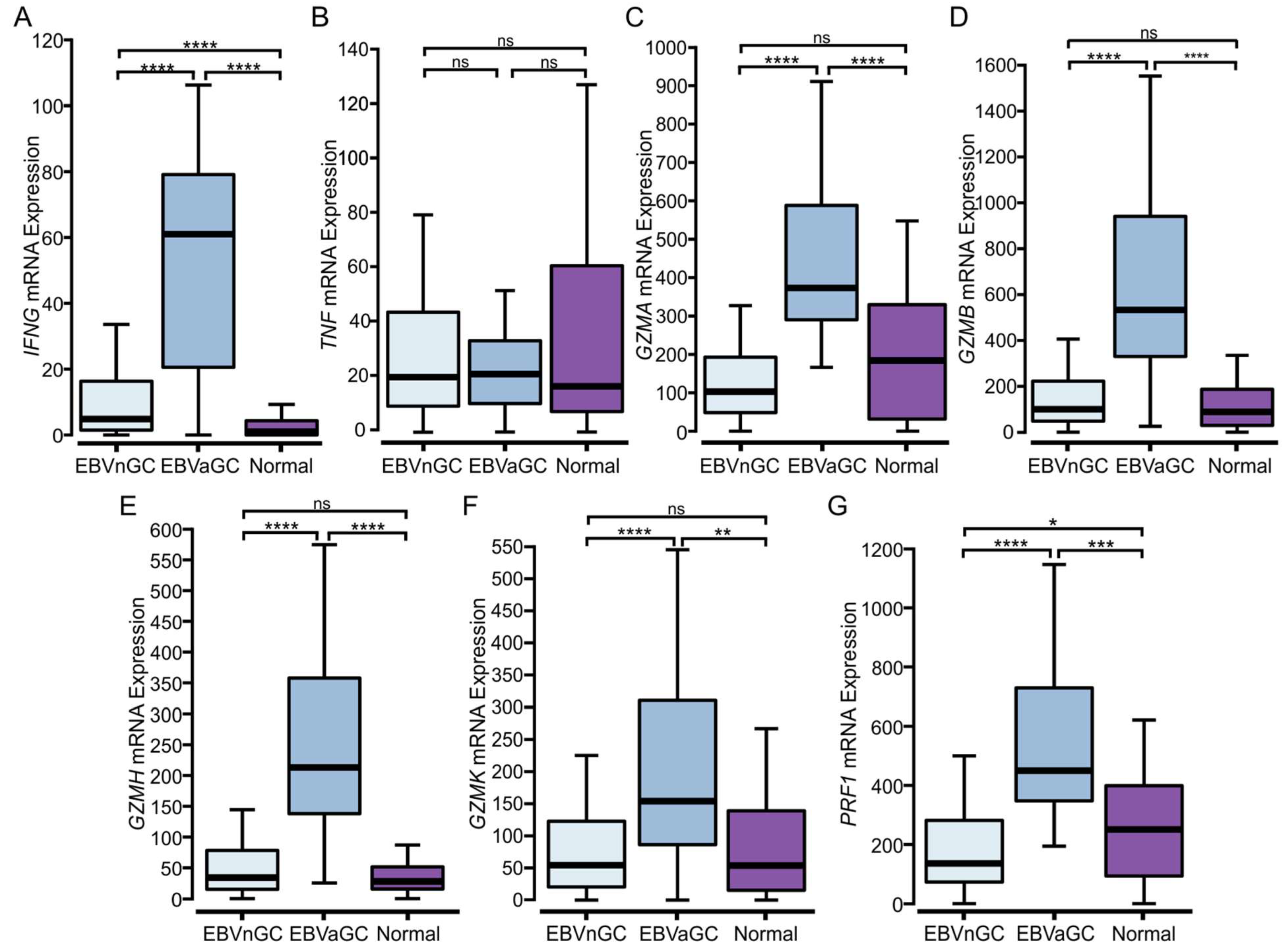
3.5. EBVaGCs Express Higher Levels of T Cell Effector Molecules as Compared to EBVnGCs with Characteristics of a T Cell-Inflamed Phenotype
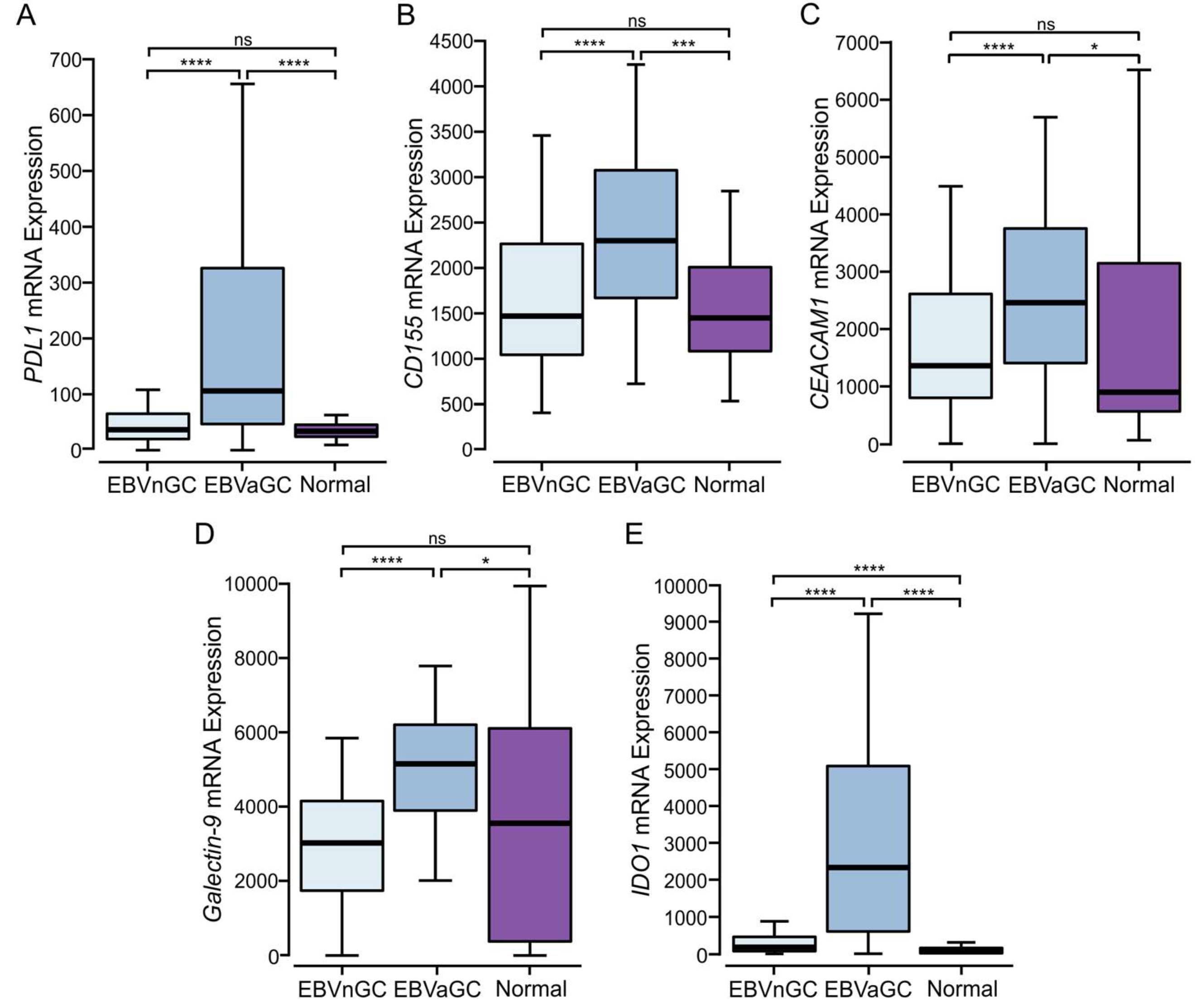
3.6. EBVaGCs Express Higher Levels of Immune Checkpoint Markers
3.7. Comparison of the T Cell Receptor Repertoire between EBVaGC and EBVnGC
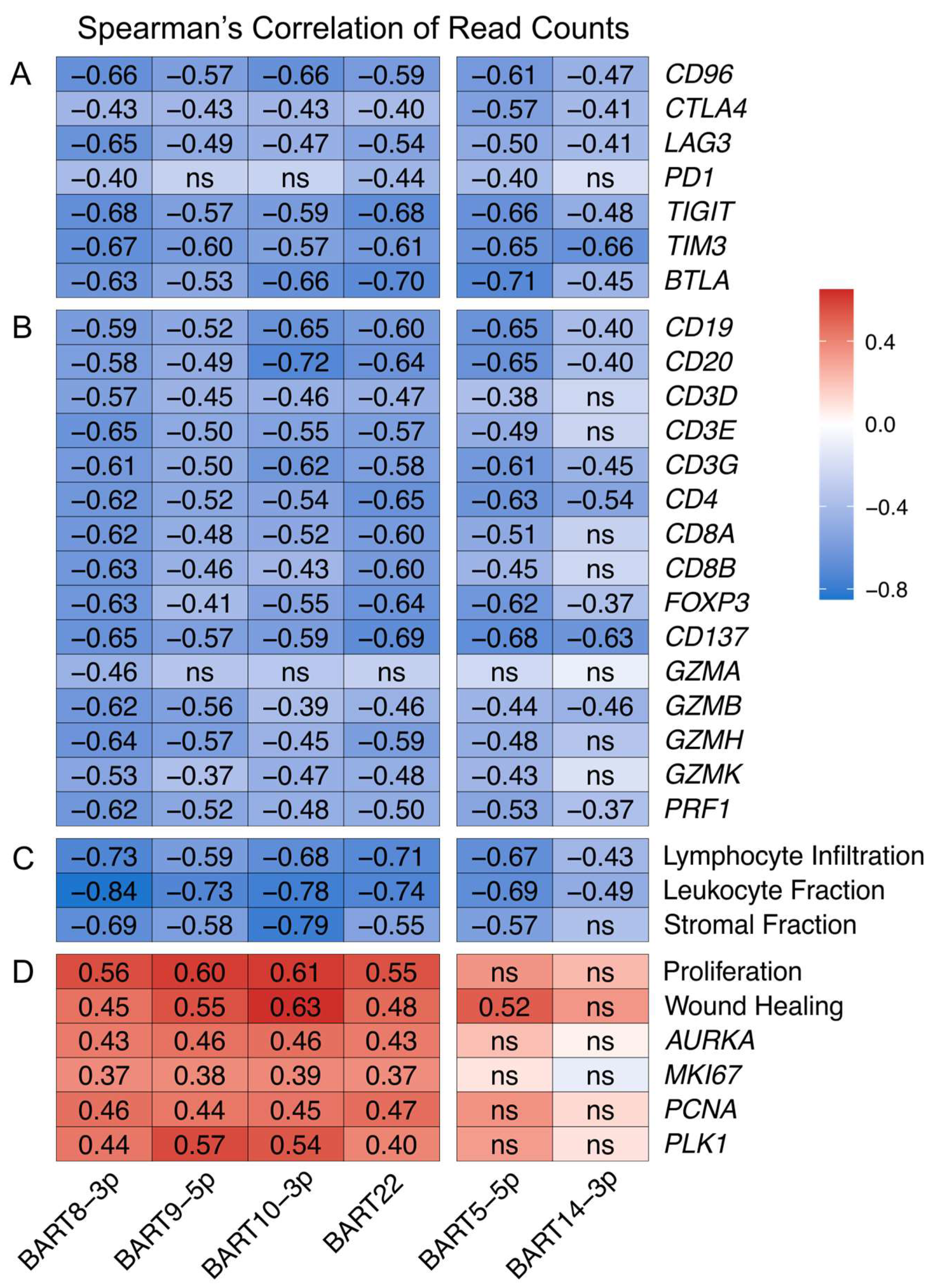
3.8. EBV-Encoded miRNAs Strongly Impact the Tumor Immune Microenvironment
3.9. Clinical Impact of the Tumor Immune Microenvironment and miR-BART Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, L.S.; Rickinson, A.B. Epstein-Barr Virus: 40 Years On. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Eichelberg, M.R.; Welch, R.; Guidry, J.T.; Ali, A.; Ohashi, M.; Makielski, K.R.; McChesney, K.; Van Sciver, N.; Lambert, P.F.; Keleș, S.; et al. Epstein-Barr Virus Infection Promotes Epithelial Cell Growth by Attenuating Differentiation-Dependent Exit from the Cell Cycle. mBio 2019, 10, e01332-19. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, J.; Imai, S.; Oda, T.; Kojima, T.; Okita, K.; Takada, K. Epstein-Barr Virus Promotes Epithelial Cell Growth in the Absence of EBNA2 and LMP1 Expression. J. Virol. 1999, 73, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Tagawa, T.; Hammerschmidt, W. Strategies of Epstein-Barr Virus to Evade Innate Antiviral Immunity of Its Human Host. Front. Microbiol. 2022, 13, 955603. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.; Zuo, J. Immune Responses to Epstein–Barr Virus: Molecular Interactions in the Virus Evasion of CD8+ T Cell Immunity. Microbes Infect. 2010, 12, 173–181. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef]
- Han, S.; Tay, J.K.; Loh, C.J.L.; Chu, A.J.M.; Yeong, J.P.S.; Lim, C.M.; Toh, H.C. Epstein-Barr Virus Epithelial Cancers-A Comprehensive Understanding to Drive Novel Therapies. Front. Immunol. 2021, 12, 734293. [Google Scholar] [CrossRef]
- Burke, A.P.; Yen, T.S.; Shekitka, K.M.; Sobin, L.H. Lymphoepithelial Carcinoma of the Stomach with Epstein-Barr Virus Demonstrated by Polymerase Chain Reaction. Mod. Pathol. 1990, 3, 377–380. [Google Scholar]
- Shibata, D.; Weiss, L.M. Epstein-Barr Virus-Associated Gastric Adenocarcinoma. Am. J. Pathol. 1992, 140, 769–774. [Google Scholar]
- Akiba, S.; Koriyama, C.; Herrera-Goepfert, R.; Eizuru, Y. Epstein-Barr Virus Associated Gastric Carcinoma: Epidemiological and Clinicopathological Features. Cancer Sci. 2008, 9, 195–201. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [CrossRef]
- Minamoto, T.; Mai, M.; Watanabe, K.; Ooi, A.; Kitamura, T.; Takahashi, Y.; Ueda, H.; Ogino, T.; Nakanishi, I. Medullary Carcinoma with Lymphocytic Infiltration of the Stomach. Clinicopathologic Study of 27 Cases and Immunohistochemical Analysis of the Subpopulations of Infiltrating Lymphocytes in the Tumor. Cancer 1990, 66, 945–952. [Google Scholar] [CrossRef]
- Kang, G.H.; Lee, S.; Kim, W.H.; Lee, H.W.; Kim, J.C.; Rhyu, M.-G.; Ro, J.Y. Epstein-Barr Virus-Positive Gastric Carcinoma Demonstrates Frequent Aberrant Methylation of Multiple Genes and Constitutes CpG Island Methylator Phenotype-Positive Gastric Carcinoma. Am. J. Pathol. 2002, 160, 787–794. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Hao, Y.; Nie, Y.; Li, Z.; Qian, M.; Liang, Q.; Yu, J.; Zeng, M.; Wu, K. Differentiated Tumor Immune Microenvironment of Epstein–Barr Virus-Associated and Negative Gastric Cancer: Implication in Prognosis and Immunotherapy. Oncotarget 2017, 8, 67094–67103. [Google Scholar] [CrossRef]
- Ghasemi, F.; Gameiro, S.F.; Tessier, T.M.; Maciver, A.H.; Mymryk, J.S. High Levels of Class I Major Histocompatibility Complex MRNA Are Present in Epstein-Barr Virus-Associated Gastric Adenocarcinomas. Cells 2020, 9, 499. [Google Scholar] [CrossRef]
- Ghasemi, F.; Tessier, T.M.; Gameiro, S.F.; Maciver, A.H.; Cecchini, M.J.; Mymryk, J.S. High MHC-II Expression in Epstein-Barr Virus-Associated Gastric Cancers Suggests That Tumor Cells Serve an Important Role in Antigen Presentation. Sci. Rep. 2020, 10, 14786. [Google Scholar] [CrossRef]
- Cho, J.; Kang, M.-S.; Kim, K.-M. Epstein-Barr Virus-Associated Gastric Carcinoma and Specific Features of the Accompanying Immune Response. J. Gastric Cancer. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Deng, S.-Z.; Wang, X.-X.; Zhao, X.-Y.; Bai, Y.-M.; Zhang, H.-M. Exploration of the Tumor Immune Landscape and Identification of Two Novel Immunotherapy-Related Genes for Epstein-Barr Virus-Associated Gastric Carcinoma via Integrated Bioinformatics Analysis. Front. Surg. 2022, 9, 898733. [Google Scholar] [CrossRef]
- Kang, B.W.; Seo, A.N.; Yoon, S.; Bae, H.I.; Jeon, S.W.; Kwon, O.K.; Chung, H.Y.; Yu, W.; Kang, H.; Kim, J.G. Prognostic Value of Tumor-Infiltrating Lymphocytes in Epstein–Barr Virus-Associated Gastric Cancer. Ann. Oncol. 2016, 27, 494–501. [Google Scholar] [CrossRef]
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Corrales, L.; Williams, J.; Horton, B.; Sivan, A.; Spranger, S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv. Exp. Med. Biol. 2017, 1036, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.A.; Sweis, R.F.; Bao, R.; Luke, J.J. T Cell-Inflamed versus Non-T Cell-Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol. Res. 2018, 6, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 2018, 23, 181–193.e7. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Ungerleider, N.; Bullard, W.; Kara, M.; Wang, X.; Roberts, C.; Renne, R.; Tibbetts, S.; Flemington, E.K. EBV MiRNAs Are Potent Effectors of Tumor Cell Transcriptome Remodeling in Promoting Immune Escape. PLoS Pathog. 2021, 17, e1009217. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef]
- Evans, A.M.; Salnikov, M.; Gameiro, S.F.; Maleki Vareki, S.; Mymryk, J.S. HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct. J. Clin. Med. 2022, 11, 4825. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Donkor, M.; Scholar, E. Inflammatory Cell Infiltration of Tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007, 26, 373–400. [Google Scholar] [CrossRef]
- Kleinschek, M.A.; Owyang, A.M.; Joyce-Shaikh, B.; Langrish, C.L.; Chen, Y.; Gorman, D.M.; Blumenschein, W.M.; McClanahan, T.; Brombacher, F.; Hurst, S.D.; et al. IL-25 Regulates Th17 Function in Autoimmune Inflammation. J. Exp. Med. 2007, 204, 161–170. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT Co-Inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Kidman, J.; Principe, N.; Watson, M.; Lassmann, T.; Holt, R.A.; Nowak, A.K.; Lesterhuis, W.J.; Lake, R.A.; Chee, J. Characteristics of TCR Repertoire Associated With Successful Immune Checkpoint Therapy Responses. Front. Immunol. 2020, 11, 587014. [Google Scholar] [CrossRef]
- Edwards, R.H.; Marquitz, A.R.; Raab-Traub, N. Epstein-Barr Virus BART MicroRNAs Are Produced from a Large Intron Prior to Splicing. J. Virol. 2008, 82, 9094–9106. [Google Scholar] [CrossRef] [PubMed]
- Hooykaas, M.J.G.; Kruse, E.; Wiertz, E.J.H.J.; Lebbink, R.J. Comprehensive Profiling of Functional Epstein-Barr Virus MiRNA Expression in Human Cell Lines. BMC Genom. 2016, 17, 644. [Google Scholar] [CrossRef]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Fanotto, V.; Miolo, G.; Puglisi, F.; Cannizzaro, R.; Canzonieri, V.; Steffan, A.; Farruggia, P.; et al. Epstein-Barr Virus BART MicroRNAs in EBV- Associated Hodgkin Lymphoma and Gastric Cancer. Infect. Agent Cancer 2020, 15, 42. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Whitfield, M.L.; George, L.K.; Grant, G.D.; Perou, C.M. Common Markers of Proliferation. Nat. Rev. Cancer. 2006, 6, 99–106. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sneddon, J.B.; Alizadeh, A.A.; Sood, R.; West, R.B.; Montgomery, K.; Chi, J.-T.; van de Rijn, M.; Botstein, D.; Brown, P.O. Gene Expression Signature of Fibroblast Serum Response Predicts Human Cancer Progression: Similarities between Tumors and Wounds. PLoS Biol. 2004, 2, E7. [Google Scholar] [CrossRef]
- Huang, S.-C.; Ng, K.-F.; Chen, K.-H.; Hsu, J.-T.; Liu, K.-H.; Yeh, T.-S.; Chen, T.-C. Prognostic Factors in Epstein-Barr Virus-Associated Stage I-III Gastric Carcinoma: Implications for a Unique Type of Carcinogenesis. Oncol. Rep. 2014, 32, 530–538. [Google Scholar] [CrossRef]
- Koriyama, C.; Akiba, S.; Shimaoka, S.; Itoh, T.; Akiyama, S.; Eizuru, Y. Frequent Expression of Thymidine Phosphorylase in Epstein-Barr Virus-Associated Gastric Carcinoma of Diffuse Type. Anticancer Res. 2010, 30, 2431–2437. [Google Scholar]
- Camargo, M.C.; Kim, W.-H.; Chiaravalli, A.M.; Kim, K.-M.; Corvalan, A.H.; Matsuo, K.; Yu, J.; Sung, J.J.Y.; Herrera-Goepfert, R.; Meneses-Gonzalez, F.; et al. Improved Survival of Gastric Cancer with Tumour Epstein-Barr Virus Positivity: An International Pooled Analysis. Gut 2014, 63, 236–243. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Qiu, H.; Kong, P.; Chen, S.; Li, W.; Zhan, Y.; Li, Y.; Chen, Y.; Zhou, Z.; et al. Prognostic Significance of Epstein-Barr Virus Infection in Gastric Cancer: A Meta-Analysis. BMC Cancer 2015, 15, 782. [Google Scholar] [CrossRef]
- Gasenko, E.; Isajevs, S.; Camargo, M.C.; Offerhaus, G.J.A.; Polaka, I.; Gulley, M.L.; Skapars, R.; Sivins, A.; Kojalo, I.; Kirsners, A.; et al. Clinicopathological Characteristics of Epstein-Barr Virus-Positive Gastric Cancer in Latvia. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1328–1333. [Google Scholar] [CrossRef]
- Gullo, I.; Carvalho, J.; Martins, D.; Lemos, D.; Monteiro, A.R.; Ferreira, M.; Das, K.; Tan, P.; Oliveira, C.; Carneiro, F.; et al. The Transcriptomic Landscape of Gastric Cancer: Insights into Epstein-Barr Virus Infected and Microsatellite Unstable Tumors. Int. J. Mol. Sci. 2018, 19, 2079. [Google Scholar] [CrossRef]
- Kim, T.S.; da Silva, E.; Coit, D.G.; Tang, L.H. Intratumoral Immune Response to Gastric Cancer Varies by Molecular and Histologic Subtype. Am. J. Surg. Pathol. 2019, 43, 851–860. [Google Scholar] [CrossRef]
- Ma, C.; Patel, K.; Singhi, A.D.; Ren, B.; Zhu, B.; Shaikh, F.; Sun, W. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am. J. Surg. Pathol. 2016, 40, 1496–1506. [Google Scholar] [CrossRef]
- Wong, L.M.; Li, W.T.; Shende, N.; Tsai, J.C.; Ma, J.; Chakladar, J.; Gnanasekar, A.; Qu, Y.; Dereschuk, K.; Wang-Rodriguez, J.; et al. Analysis of the Immune Landscape in Virus-Induced Cancers Using a Novel Integrative Mechanism Discovery Approach. Comput. Struct. Biotechnol. J. 2021, 19, 6240–6254. [Google Scholar] [CrossRef]
- Jia, X.; Guo, T.; Li, Z.; Zhang, M.; Feng, Y.; Dong, B.; Li, Z.; Hu, Y.; Li, Z.; Xing, X.; et al. Clinicopathological and Immunomicroenvironment Characteristics of Epstein-Barr Virus-Associated Gastric Cancer in a Chinese Population. Front. Oncol. 2020, 10, 586752. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global Signatures of Protein and MRNA Expression Levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, D.; Yang, P.; Guo, R.; Kong, M.; Gao, Y.; Yu, X.; Lu, X.; Fan, X. Revealing the Transcriptional Heterogeneity of Organ-Specific Metastasis in Human Gastric Cancer Using Single-Cell RNA Sequencing. Clin. Transl. Med. 2022, 12, e730. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Shooshtari, P.; Haeryfar, S.M.M. Leveraging Public Single-Cell and Bulk Transcriptomic Datasets to Delineate MAIT Cell Roles and Phenotypic Characteristics in Human Malignancies. Front. Immunol. 2020, 11, 1691. [Google Scholar] [CrossRef] [PubMed]
- Sathe, A.; Grimes, S.M.; Lau, B.T.; Chen, J.; Suarez, C.; Huang, R.J.; Poultsides, G.; Ji, H.P. Single Cell Genomic Characterization Reveals the Cellular Reprogramming of the Gastric Tumor Microenvironment. Clin. Cancer Res. 2020, 26, 2640–2653. [Google Scholar] [CrossRef]
- van Beek, J.; zur Hausen, A.; Snel, S.N.; Berkhof, J.; Kranenbarg, E.K.; van de Velde, C.J.H.; van den Brule, A.J.C.; Middeldorp, J.M.; Meijer, C.J.L.M.; Bloemena, E. Morphological Evidence of an Activated Cytotoxic T-Cell Infiltrate in EBV-Positive Gastric Carcinoma Preventing Lymph Node Metastases. Am. J. Surg. Pathol. 2006, 30, 59–65. [Google Scholar] [CrossRef]
- Kuzushima, K.; Nakamura, S.; Nakamura, T.; Yamamura, Y.; Yokoyama, N.; Fujita, M.; Kiyono, T.; Tsurumi, T. Increased Frequency of Antigen-Specific CD8(+) Cytotoxic T Lymphocytes Infiltrating an Epstein-Barr Virus-Associated Gastric Carcinoma. J. Clin. Invest. 1999, 104, 163–171. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Xiao, L.; Tang, F.; Zhang, Z.; Zhang, Y.; Feng, Z.; Jiang, Y.; Shao, C. Accumulation Mechanisms of CD4(+)CD25(+)FOXP3(+) Regulatory T Cells in EBV-Associated Gastric Carcinoma. Sci. Rep. 2015, 5, 18057. [Google Scholar] [CrossRef]
- Kijima, Y.; Ishigami, S.; Hokita, S.; Koriyama, C.; Akiba, S.; Eizuru, Y.; Aikou, T. The Comparison of the Prognosis between Epstein-Barr Virus (EBV)-Positive Gastric Carcinomas and EBV-Negative Ones. Cancer Lett. 2003, 200, 33–40. [Google Scholar] [CrossRef]
- Gabitass, R.F.; Annels, N.E.; Stocken, D.D.; Pandha, H.A.; Middleton, G.W. Elevated Myeloid-Derived Suppressor Cells in Pancreatic, Esophageal and Gastric Cancer Are an Independent Prognostic Factor and Are Associated with Significant Elevation of the Th2 Cytokine Interleukin-13. Cancer Immunol. Immunother. 2011, 60, 1419–1430. [Google Scholar] [CrossRef]
- Yang, P.; Qiu, G.; Wang, S.; Su, Z.; Chen, J.; Wang, S.; Kong, F.; Lu, L.; Ezaki, T.; Xu, H. The Mutations of Th1 Cell-Specific T-Box Transcription Factor May Be Associated with a Predominant Th2 Phenotype in Gastric Cancers. Int. J. Immunogenet. 2010, 37, 111–115. [Google Scholar] [CrossRef]
- Ellyard, J.I.; Simson, L.; Parish, C.R. Th2-Mediated Anti-Tumour Immunity: Friend or Foe? Tissue Antigens 2007, 70, 1–11. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Peng, Y.; Fang, J.; Fang, T.; Wu, J.; Zhou, J. Identification of Immune Cells and MRNA Associated with Prognosis of Gastric Cancer. BMC Cancer 2020, 20, 206. [Google Scholar] [CrossRef]
- Chiaravalli, A.M.; Feltri, M.; Bertolini, V.; Bagnoli, E.; Furlan, D.; Cerutti, R.; Novario, R.; Capella, C. Intratumour T Cells, Their Activation Status and Survival in Gastric Carcinomas Characterised for Microsatellite Instability and Epstein-Barr Virus Infection. Virchows. Arch. 2006, 448, 344–353. [Google Scholar] [CrossRef]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-Regulation of PD-L1, IDO, and T(Regs) in the Melanoma Tumor Microenvironment Is Driven by CD8(+) T Cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, Y.; Chen, X.; Li, L.; Wu, H.; Liu, Y.; Xu, H.; Zhou, G.; Fan, X.; Xia, H. Prognostic Perspectives of STING and PD-L1 Expression and Correlation with the Prognosis of Epstein-Barr Virus-Associated Gastric Cancers. Gut Liver 2022, 16, 875–891. [Google Scholar] [CrossRef]
- Dislich, B.; Mertz, K.D.; Gloor, B.; Langer, R. Interspatial Distribution of Tumor and Immune Cells in Correlation with PD-L1 in Molecular Subtypes of Gastric Cancers. Cancers 2022, 14, 1736. [Google Scholar] [CrossRef]
- Kawazoe, A.; Kuwata, T.; Kuboki, Y.; Shitara, K.; Nagatsuma, A.K.; Aizawa, M.; Yoshino, T.; Doi, T.; Ohtsu, A.; Ochiai, A. Clinicopathological Features of Programmed Death Ligand 1 Expression with Tumor-Infiltrating Lymphocyte, Mismatch Repair, and Epstein-Barr Virus Status in a Large Cohort of Gastric Cancer Patients. Gastric. Cancer 2017, 20, 407–415. [Google Scholar] [CrossRef]
- Derks, S.; Liao, X.; Chiaravalli, A.M.; Xu, X.; Camargo, M.C.; Solcia, E.; Sessa, F.; Fleitas, T.; Freeman, G.J.; Rodig, S.J.; et al. Abundant PD-L1 Expression in Epstein-Barr Virus-Infected Gastric Cancers. Oncotarget 2016, 7, 32925–32932. [Google Scholar] [CrossRef]
- Kim, H.; Heo, Y.J.; Cho, Y.A.; Kang, S.Y.; Ahn, S.; Kim, K.-M. Tumor Immune Microenvironment Is Influenced by Frameshift Mutations and Tumor Mutational Burden in Gastric Cancer. Clin. Transl. Oncol. 2022, 24, 556–567. [Google Scholar] [CrossRef]
- Sasaki, S.; Nishikawa, J.; Sakai, K.; Iizasa, H.; Yoshiyama, H.; Yanagihara, M.; Shuto, T.; Shimokuri, K.; Kanda, T.; Suehiro, Y.; et al. EBV-Associated Gastric Cancer Evades T-Cell Immunity by PD-1/PD-L1 Interactions. Gastric. Cancer 2019, 22, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Seo, A.N.; Koh, J.; Nam, S.K.; Kwak, Y.; Ahn, S.-H.; Park, D.J.; Kim, H.-H.; Lee, H.S. Expression of the Immune Checkpoint Receptors PD-1, LAG3, and TIM3 in the Immune Context of Stage II and III Gastric Cancer by Using Single and Chromogenic Multiplex Immunohistochemistry. Oncoimmunology 2021, 10, 1954761. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fang, H.; Gu, Y.; Yu, K.; Wang, J.; Lin, C.; Zhang, H.; Li, H.; He, H.; Liu, H.; et al. Impact of Intratumoural CD96 Expression on Clinical Outcome and Therapeutic Benefit in Gastric Cancer. Cancer Sci. 2022, 1132, 4070–7081. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Arrazubi, V.; Diez, M.; Tabernero, J. Current Developments in Gastric Cancer: From Molecular Profiling to Treatment Strategy. Nat. Rev. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next Generation of Immune Checkpoint Inhibitors and Beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Song, X.; Qi, W.; Guo, J.; Sun, L.; Ding, A.; Zhao, G.; Li, H.; Qiu, W.; Lv, J. Immune Checkpoint Inhibitor Combination Therapy for Gastric Cancer: Research Progress. Oncol. Lett. 2020, 20, 46. [Google Scholar] [CrossRef]
- Paik, J. Nivolumab Plus Relatlimab: First Approval. Drugs 2022, 82, 925–931. [Google Scholar] [CrossRef]
- Wang, J.; Ge, J.; Wang, Y.; Xiong, F.; Guo, J.; Jiang, X.; Zhang, L.; Deng, X.; Gong, Z.; Zhang, S.; et al. EBV MiRNAs BART11 and BART17-3p Promote Immune Escape through the Enhancer-Mediated Transcription of PD-L1. Nat. Commun. 2022, 13, 866. [Google Scholar] [CrossRef]
- Min, K.; Lee, S.K. EBV MiR-BART10-3p Promotes Cell Proliferation and Migration by Targeting DKK1. Int. J. Biol. Sci. 2019, 15, 657–667. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Donahue, H.; Garcia, D.; Tan, J.; Shimizu, N.; Rice, A.P.; Ling, P.D. Epstein-Barr Virus BART9 MiRNA Modulates LMP1 Levels and Affects Growth Rate of Nasal NK T Cell Lymphomas. PLoS ONE 2011, 6, e27271. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, J.; Tang, Y.; Lin, Y.; Wang, L.; Li, Y.; Liu, C.; Wu, D.; Cai, L. EBV Encoded MiRNA BART8-3p Promotes Radioresistance in Nasopharyngeal Carcinoma by Regulating ATM/ATR Signaling Pathway. Biosci. Rep. 2019, 39, BSR20190415. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Q.; Liu, X.; Lin, X.; Tang, Z.; Liu, C.; Zhou, J.; Zhao, M.; Li, X.; Cheng, Z.; et al. Cinobufotalin Powerfully Reversed EBV-MiR-BART22-Induced Cisplatin Resistance via Stimulating MAP2K4 to Antagonize Non-Muscle Myosin Heavy Chain IIA/Glycogen Synthase 3β/β-Catenin Signaling Pathway. EBioMedicine 2019, 48, 386–404. [Google Scholar] [CrossRef]
- Luo, W.-J.; He, S.-W.; Zou, W.-Q.; Zhao, Y.; He, Q.-M.; Yang, X.-J.; Guo, R.; Mao, Y.-P. Epstein-Barr Virus MicroRNA BART10-3p Promotes Dedifferentiation and Proliferation of Nasopharyngeal Carcinoma by Targeting ALK7. Exp. Biol. Med. 2021, 246, 2618–2629. [Google Scholar] [CrossRef]
- Gong, L.-P.; Chen, J.-N.; Xiao, L.; He, Q.; Feng, Z.-Y.; Zhang, Z.-G.; Liu, J.-P.; Wei, H.-B.; Shao, C.-K. The Implication of Tumor-Infiltrating Lymphocytes in Epstein-Barr Virus-Associated Gastric Carcinoma. Hum. Pathol. 2019, 85, 82–91. [Google Scholar] [CrossRef]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK Cell-Cancer Cycle: Advances and New Challenges in NK Cell-Based Immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef]
- Imai, C.; Iwamoto, S.; Campana, D. Genetic Modification of Primary Natural Killer Cells Overcomes Inhibitory Signals and Induces Specific Killing of Leukemic Cells. Blood 2005, 106, 376–383. [Google Scholar] [CrossRef]
- Wu, X.; Huang, S. HER2-Specific Chimeric Antigen Receptor-Engineered Natural Killer Cells Combined with Apatinib for the Treatment of Gastric Cancer. Bull Cancer 2019, 106, 946–958. [Google Scholar] [CrossRef]
- Cao, B.; Liu, M.; Huang, J.; Zhou, J.; Li, J.; Lian, H.; Huang, W.; Guo, Y.; Yang, S.; Lin, L.; et al. Development of Mesothelin-Specific CAR NK-92 Cells for the Treatment of Gastric Cancer. Int. J. Biol. Sci. 2021, 17, 3850–3861. [Google Scholar] [CrossRef]
- van Hall, T.; André, P.; Horowitz, A.; Ruan, D.F.; Borst, L.; Zerbib, R.; Narni-Mancinelli, E.; van der Burg, S.H.; Vivier, E. Monalizumab: Inhibiting the Novel Immune Checkpoint NKG2A. J. Immunother. Cancer 2019, 7, 263. [Google Scholar] [CrossRef]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A MAb Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salnikov, M.; Prusinkiewicz, M.A.; Lin, S.; Ghasemi, F.; Cecchini, M.J.; Mymryk, J.S. Tumor-Infiltrating T Cells in EBV-Associated Gastric Carcinomas Exhibit High Levels of Multiple Markers of Activation, Effector Gene Expression, and Exhaustion. Viruses 2023, 15, 176. https://doi.org/10.3390/v15010176
Salnikov M, Prusinkiewicz MA, Lin S, Ghasemi F, Cecchini MJ, Mymryk JS. Tumor-Infiltrating T Cells in EBV-Associated Gastric Carcinomas Exhibit High Levels of Multiple Markers of Activation, Effector Gene Expression, and Exhaustion. Viruses. 2023; 15(1):176. https://doi.org/10.3390/v15010176
Chicago/Turabian StyleSalnikov, Mikhail, Martin A. Prusinkiewicz, Sherman Lin, Farhad Ghasemi, Matthew J. Cecchini, and Joe S. Mymryk. 2023. "Tumor-Infiltrating T Cells in EBV-Associated Gastric Carcinomas Exhibit High Levels of Multiple Markers of Activation, Effector Gene Expression, and Exhaustion" Viruses 15, no. 1: 176. https://doi.org/10.3390/v15010176
APA StyleSalnikov, M., Prusinkiewicz, M. A., Lin, S., Ghasemi, F., Cecchini, M. J., & Mymryk, J. S. (2023). Tumor-Infiltrating T Cells in EBV-Associated Gastric Carcinomas Exhibit High Levels of Multiple Markers of Activation, Effector Gene Expression, and Exhaustion. Viruses, 15(1), 176. https://doi.org/10.3390/v15010176








