Cytopathic and Genomic Characteristics of a Human-Originated Pseudorabies Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. PRV Strains, Cells, Culture Conditions, and Whole-Genome Sequences
2.2. Genomic DNA Extraction and Pacific Biosciences (PacBio) Sequencing
2.3. Bioinformatic Analysis
2.4. Cells Infection Tests
3. Results
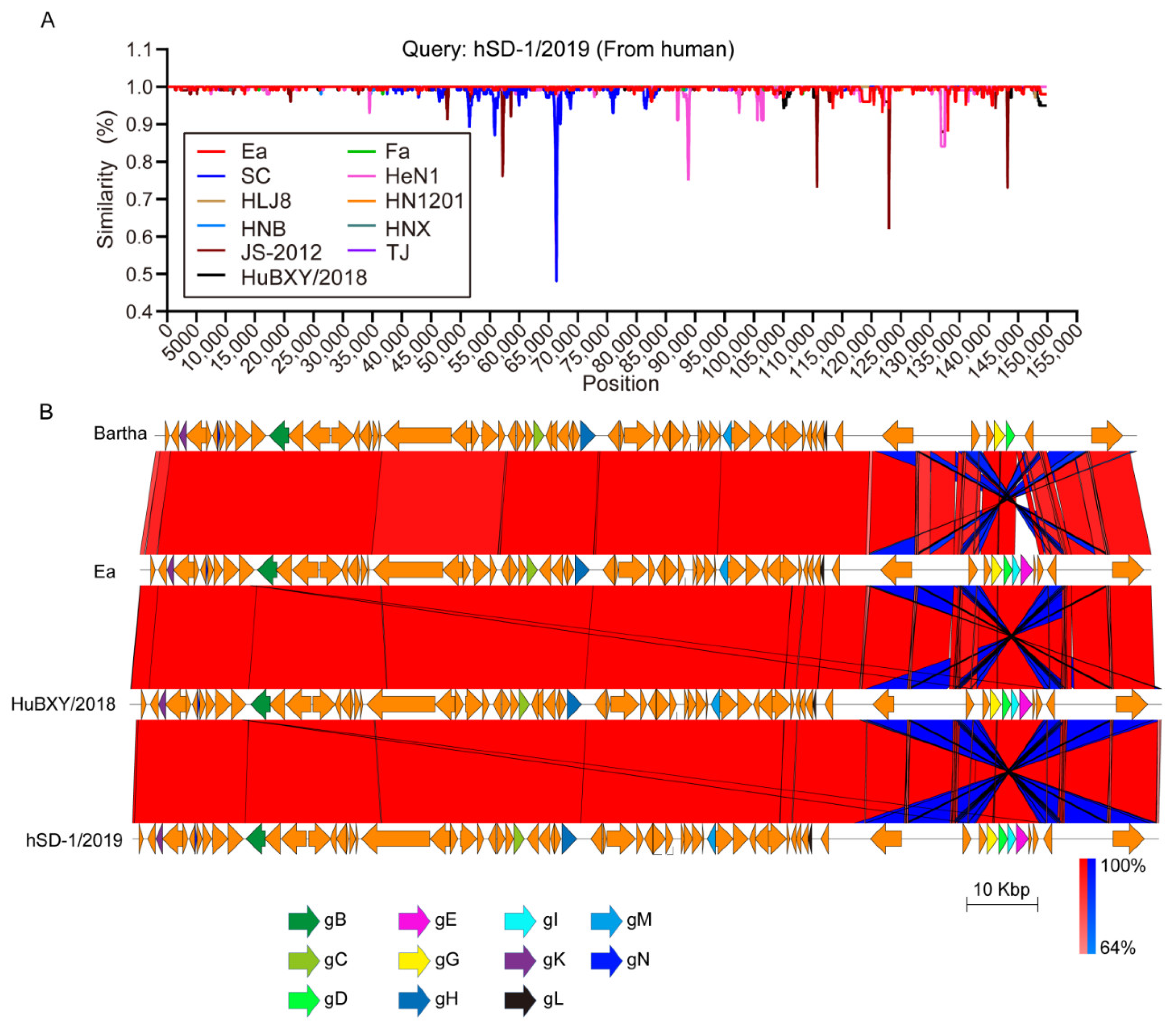
3.1. Overview of the Human-Originated PRV Genome
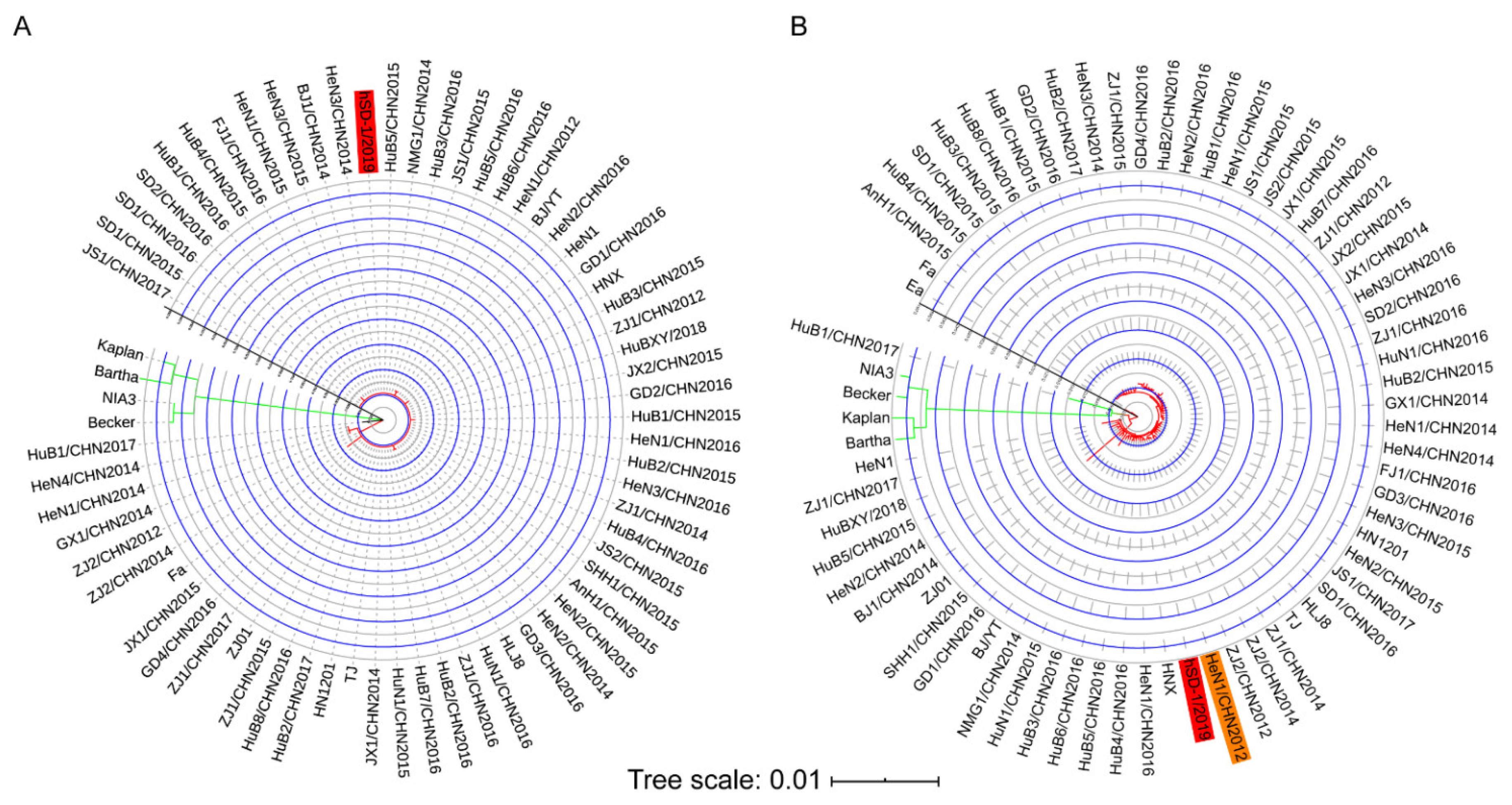
3.2. Phylogenetic Relationship of the Human and Pig-Originated PRV Strains
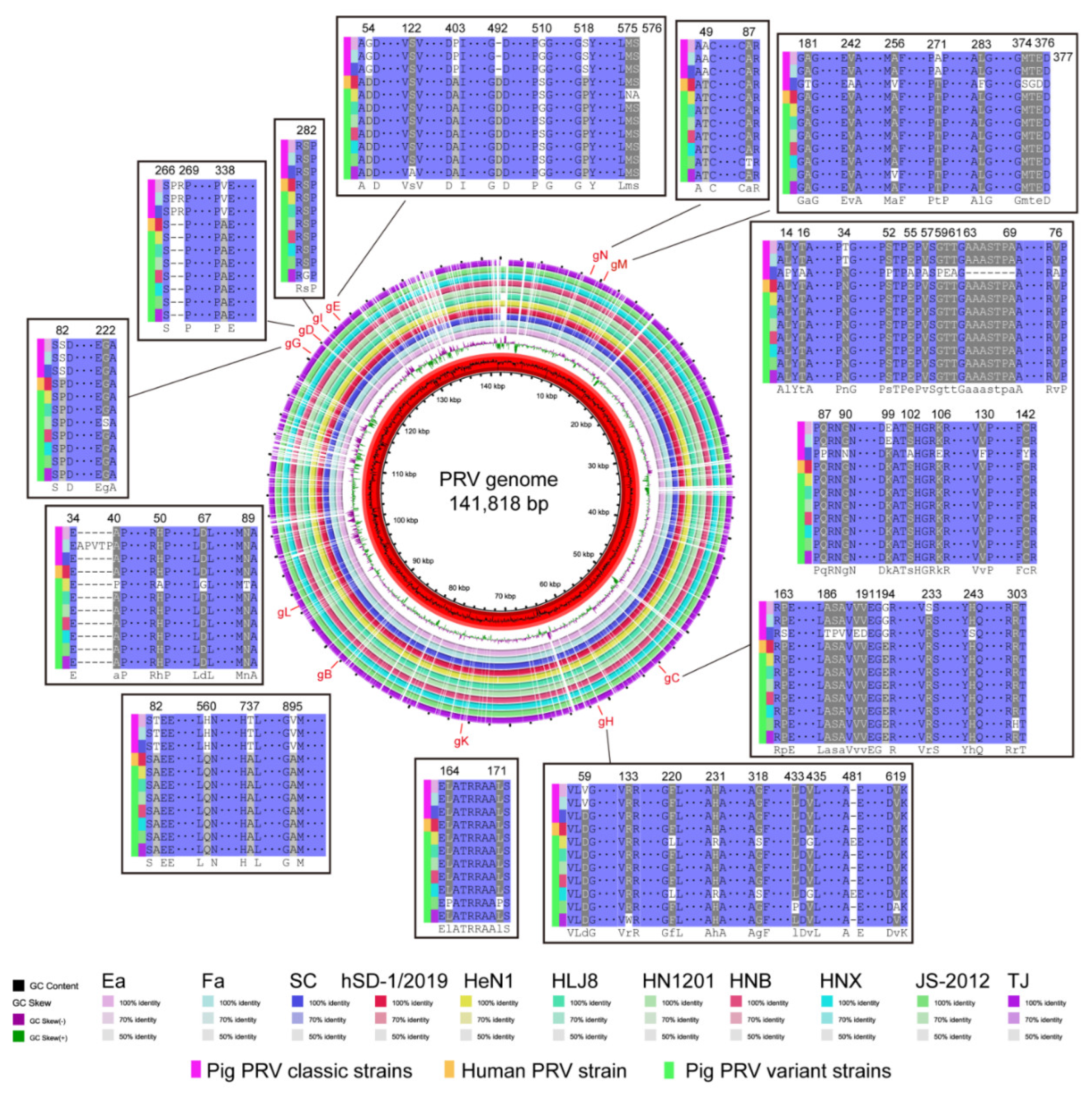
3.3. Glycoproteins of the Human and Pig-Originated PRV Strains
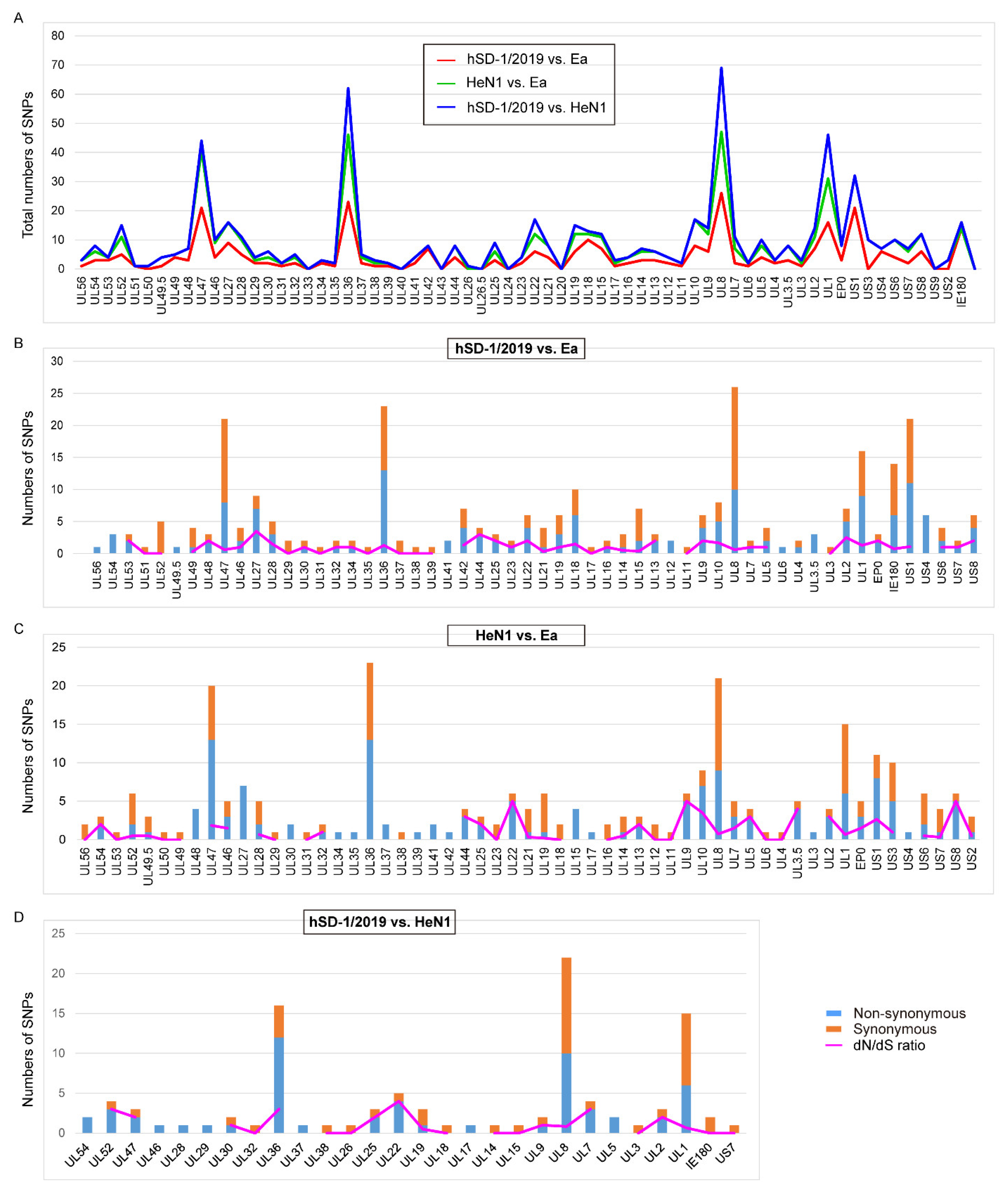
3.4. Diversifying Selection Analyses of the Human and Pig-Originated PRV Strains
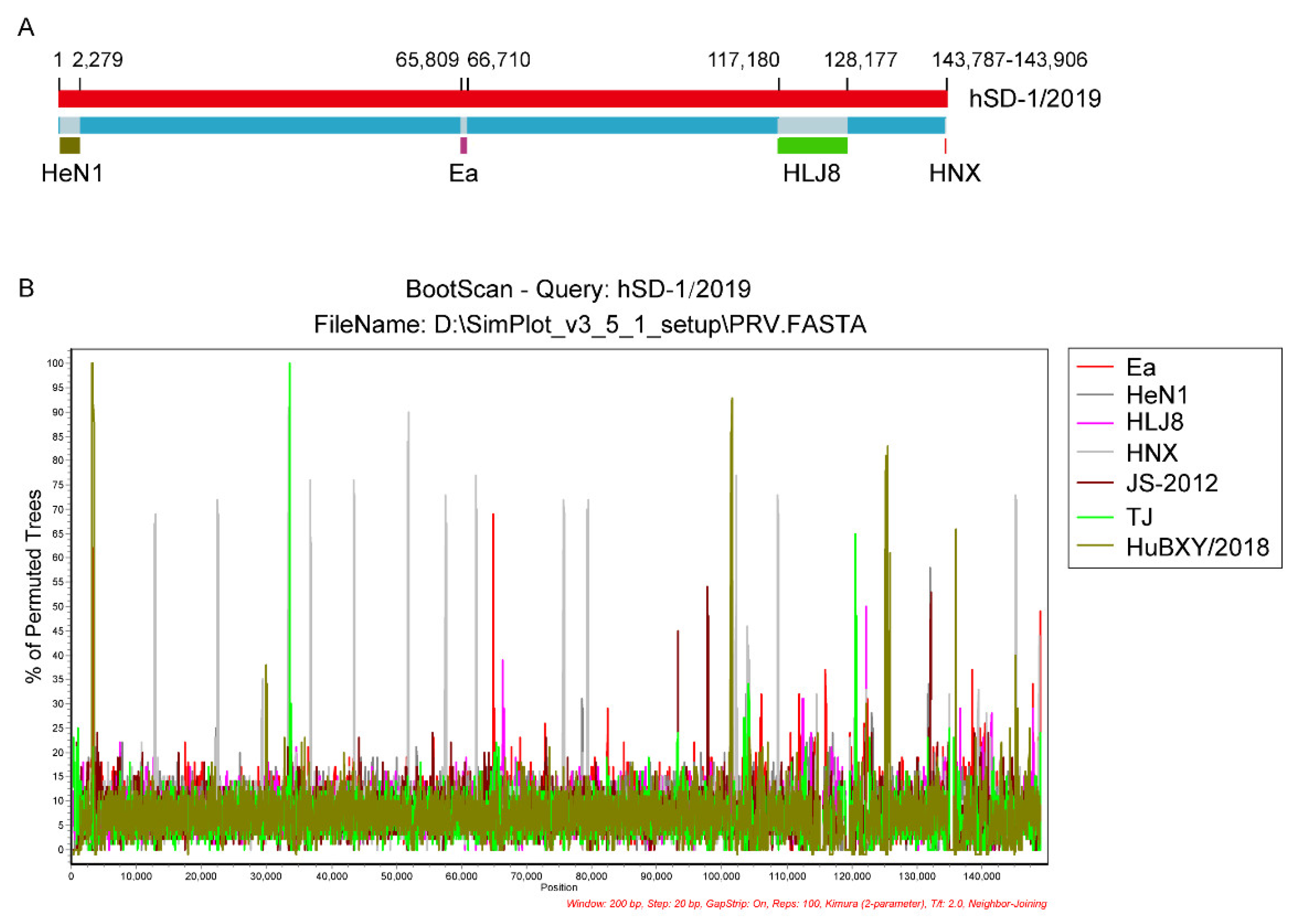
3.5. Genomic Recombinant Analyses of the Human and Pig-Originated PRV Strains
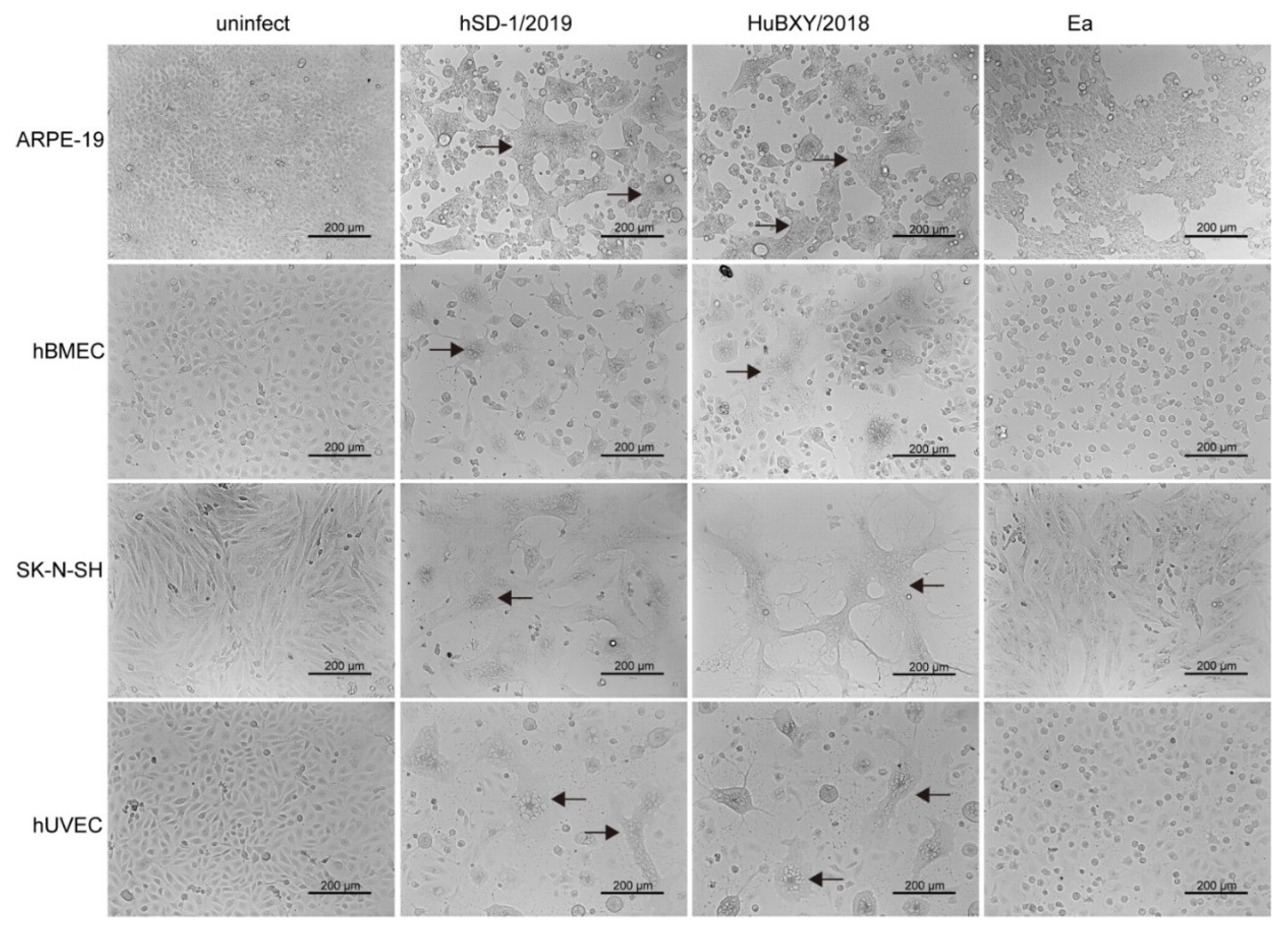
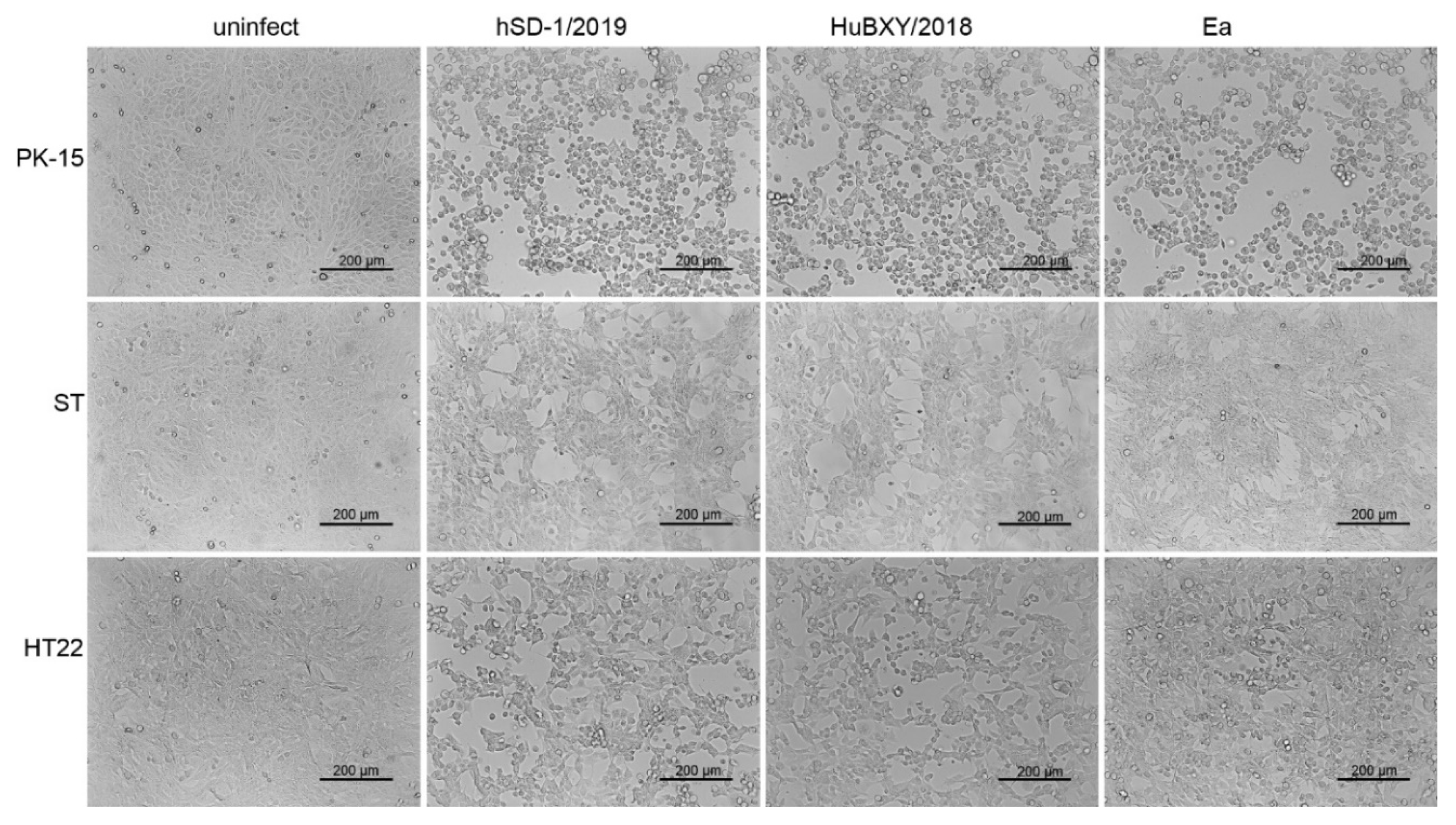
3.6. Cytopathic Effects Induced by Human and Pig-Originated PRV Strains in Different Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, S.; Kim, B.I.; Lim, J.S.; Tan, C.S.; Chun, B.C. One Health Perspectives on Emerging Public Health Threats. J. Prev. Med. Public Health 2017, 50, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettenleiter, T.C. Aujeszky’s disease (pseudorabies) virus: The virus and molecular pathogenesis-state of the art, June 1999. Vet. Res. 2000, 31, 99–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.; Zhang, Q.Z.; Tian, Z.J.; Zheng, H.; Zhao, K.; Liu, F.; Guo, J.C.; Tong, W.; Jiang, C.G.; Wang, S.J.; et al. Genomic characterization of emergent pseudorabies virus in China reveals marked sequence divergence: Evidence for the existence of two major genotypes. Virology 2015, 483, 32–43. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, W.; Liu, Q.; Zhao, T.; Zhu, H.; Hua, L.; Peng, Z.; Tang, X.; Stratton, C.W.; Zhou, D.; et al. Epidemiological and genetic characteristics of swine pseudorabies virus in mainland China between 2012 and 2017. PeerJ 2018, 6, e5785. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.; Lu, J.; Zhang, W.; Gao, G.F. Pseudorabies virus: A neglected zoonotic pathogen in humans? Emerg. Microbes Infect. 2019, 8, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Cai, S.; Zheng, F.; Lu, G.; Zhang, G. Human pseudorabies virus infection: A new threat in China. J. Infect. 2020, 80, 578–606. [Google Scholar] [CrossRef]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef]
- Luo, Y.; Li, N.; Cong, X.; Wang, C.H.; Du, M.; Li, L.; Zhao, B.; Yuan, J.; Liu, D.D.; Li, S.; et al. Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet. Microbiol. 2014, 174, 107–115. [Google Scholar] [CrossRef]
- Ai, J.W.; Weng, S.S.; Cheng, Q.; Cui, P.; Li, Y.J.; Wu, H.L.; Zhu, Y.M.; Xu, B.; Zhang, W.H. Human Endophthalmitis Caused By Pseudorabies Virus Infection, China, 2017. Emerg. Infect. Dis. 2018, 24, 1087–1090. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Tao, X.; Fei, M.; Chen, J.; Guo, W.; Li, P.; Wang, J. Human encephalitis caused by pseudorabies virus infection: A case report. J. Neurovirol 2020, 26, 442–448. [Google Scholar] [CrossRef]
- Wang, Y.; Nian, H.; Li, Z.; Wang, W.; Wang, X.; Cui, Y. Human encephalitis complicated with bilateral acute retinal necrosis associated with pseudorabies virus infection: A case report. Int. J. Infect. Dis. 2019, 89, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Han, H.; Wang, H.; Cui, Y.; Liu, H.; Ding, S. A Case of Human Viral Encephalitis Caused by Pseudorabies Virus Infection in China. Front. Neurol. 2019, 10, 534. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Guan, H.; Li, C.; Li, Y.; Wang, S.; Zhao, X.; Zhao, Y.; Liu, Y. Characteristics of human encephalitis caused by pseudorabies virus: A case series study. Int. J. Infect. Dis. 2019, 87, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.L.; Wu, Y.H.; Li, H.F.; Li, S.Y.; Fan, S.Y.; Wu, H.L.; Li, Y.J.; Lü, Y.L.; Han, J.; Zhang, W.C.; et al. Clinical experience and next-generation sequencing analysis of encephalitis caused by pseudorabies virus. Zhonghua Yi Xue Za Zhi 2018, 98, 1152–1157. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, X.; Yuan, D.; Li, R.; Lu, J.; Li, X.; Tian, K.; Dai, E. Dynamic cerebrospinal fluid analyses of severe pseudorabies encephalitis. Transbound. Emerg. Dis. 2019, 66, 2562–2565. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Xie, C.; Ding, S.; Yang, H.; Guo, S.; Li, J.; Qin, L.; Ban, F.; Wang, D.; et al. A novel human acute encephalitis caused by pseudorabies virus variant strain. Clin. Infect. Dis. 2021, 73, 3690–3700. [Google Scholar] [CrossRef]
- Szpara, M.L.; Tafuri, Y.R.; Enquist, L.W. Preparation of viral DNA from nucleocapsids. J. Vis. Exp. 2011, 10, 3151. [Google Scholar] [CrossRef]
- Lindgreen, S. AdapterRemoval: Easy cleaning of next-generation sequencing reads. BMC Res. Notes 2012, 5, 337. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coil, D.; Jospin, G.; Darling, A.E. A5-miseq: An updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 2015, 31, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [Green Version]
- Rozewicki, J.; Li, S.; Amada, K.M.; Standley, D.M.; Katoh, K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 2019, 47, 5–10. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [Green Version]
- Gatto, L.; Catanzaro, D.; Milinkovitch, M.C. Assessing the applicability of the GTR nucleotide substitution model through simulations. Evol. Bioinform. 2007, 2, 145–155. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and open software for comparing large genomes. Genome Biol. 2004, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Liang, W.; Liu, W.; Wu, B.; Tang, B.; Tan, C.; Zhou, R.; Chen, H. Genomic characterization of Pasteurella multocida HB01, a serotype A bovine isolate from China. Gene 2016, 581, 85–93. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, 003. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhao, S.; Rao, H. Whole genomic analysis of a potential recombinant human adenovirus type 1 in Qinghai plateau, China. Virol. J. 2020, 17, 111. [Google Scholar] [CrossRef]
- Mravak, S.; Bienzle, U.; Feldmeier, H.; Hampl, H.; Habermehl, K.O. Pseudorabies in man. Lancet 1987, 1, 501–502. [Google Scholar] [CrossRef]
- Zhai, X.; Zhao, W.; Li, K.; Zhang, C.; Wang, C.; Su, S.; Zhou, J.; Lei, J.; Xing, G.; Sun, H.; et al. Genome Characteristics and Evolution of Pseudorabies Virus Strains in Eastern China from 2017 to 2019. Virol. Sin. 2019, 34, 601–609. [Google Scholar] [CrossRef]
- Hu, R.M.; Zhou, Q.; Song, W.B.; Sun, E.C.; Zhang, M.M.; He, Q.G.; Chen, H.C.; Wu, B.; Liu, Z.F. Novel pseudorabies virus variant with defects in TK, gE and gI protects growing pigs against lethal challenge. Vaccine 2015, 33, 5733–5740. [Google Scholar] [CrossRef]
- Li, A.; Lu, G.; Qi, J.; Wu, L.; Tian, K.; Luo, T.; Shi, Y.; Yan, J.; Gao, G.F. Structural basis of nectin-1 recognition by pseudorabies virus glycoprotein D. PLoS Pathog. 2017, 13, e1006314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Yan, J.; Lu, G.; Guo, Z.; Fan, Z.; Wang, J.; Shi, Y.; Qi, J.; Gao, G.F. Binding of herpes simplex virus glycoprotein D to nectin-1 exploits host cell adhesion. Nat. Commun. 2011, 2, 577. [Google Scholar] [CrossRef] [Green Version]
- Rocha, E.P.; Smith, J.M.; Hurst, L.D.; Holden, M.T.; Cooper, J.E.; Smith, N.H.; Feil, E.J. Comparisons of dN/dS are time dependent for closely related bacterial genomes. J. Theor. Biol. 2006, 239, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Guo, J.C.; Gao, J.C.; Wang, T.Y.; Zhao, K.; Chang, X.B.; Wang, Q.; Peng, J.M.; Tian, Z.J.; Cai, X.H.; et al. Genomic analyses reveal that partial sequence of an earlier pseudorabies virus in China is originated from a Bartha-vaccine-like strain. J. Virol. 2016, 491, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Nauwynck, H.; Glorieux, S.; Favoreel, H.; Pensaert, M. Cell biological and molecular characteristics of pseudorabies virus infections in cell cultures and in pigs with emphasis on the respiratory tract. Vet. Res. 2007, 38, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, A.; Nasser, H.; Uriu, K.; Kosugi, Y.; Irie, T.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; Ito, J.; Wu, J. SARS-CoV-2 spike P681R mutation enhances and accelerates viral fusion. bioRxiv 2021. [Google Scholar] [CrossRef]
- Klupp, B.G.; Nixdorf, R.; Mettenleiter, T.C. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 2000, 74, 6760–6768. [Google Scholar] [CrossRef] [Green Version]
- Favoreel, H.W.; Van Minnebruggen, G.; Nauwynck, H.J.; Enquist, L.W.; Pensaert, M.B. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 2002, 76, 6845–6851. [Google Scholar] [CrossRef] [Green Version]
- Zsak, L.; Zuckermann, F.; Sugg, N.; Ben-Porat, T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J. Virol. 1992, 66, 2316–2325. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, T.X.; Wang, T.Y.; Tang, Y.D.; Wei, P. Isobavachalcone inhibits Pseudorabies virus by impairing virus-induced cell-to-cell fusion. Virol. J. 2020, 17, 39. [Google Scholar] [CrossRef]
| Average Nucleotide Identity (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hSD-1/2019 (Human PRV; GenBank Accession No. MT468550) | ||||||||||||
| Type | Pig PRV Classic Strain | Pig PRV Variant Strain | Vaccine Strain | |||||||||
| Strain | Ea | Fa | SC | HeN1 | HLJ8 | HN1201 | HNB | HNX | JS-2012 | TJ | HuBXY/2018 | Bartha |
| GenBank accession | KX423960 | KM189913 | KT809429 | KP098534 | KT824771 | KP722022 | KM189914 | KM189912 | KP257591 | KJ789182 | MT468549 | JF797217 |
| Year of isolation | 1990 | 1990 | 1990 | 2012 | 2014 | 2012 | 2012 | 2012 | 2012 | 2012 | 2018 | 1950s |
| Place of isolation | China | China | China | China | China | China | China | China | China | China | China | Hungry |
| Complete genome | 99.36% | 99.44% | 99.03% | 99.45% | 99.83% | 99.82% | 99.83% | 99.90% | 99.65% | 99.80% | 99.62% | 96.73% |
| UL27 | 99.82% | 99.82% | 99.82% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 99.96% | 98.14% |
| UL44 | 99.66% | 99.73% | 95.83% | 100% | 100% | 100% | 100% | 100% | 99.93% | 100% | 100% | 95.11% |
| US6 | 99.18% | 99.18% | 99.18% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 98.43% |
| US8 | 99.41% | 99.48% | 99.48% | 99.89% | 99.94% | 100.00% | 100.00% | 100.00% | 100.00% | 99.83% | 100.00% | Deletion |
| US4 | 99.93% | 99.93% | 99.93% | 100.00% | 100.00% | 99.87% | 100.00% | 100.00% | 99.93% | 100.00% | 100.00% | 99.13% |
| UL22 | 99.95% | 99.95% | 99.95% | 99.61% | 100.00% | 100.00% | 100.00% | 100.00% | 99.90% | 99.95% | 99.95% | 99.85% |
| US7 | 99.82% | 99.82% | 99.82% | 99.91% | 99.91% | 100.00% | 99.91% | 100.00% | 100.00% | 99.91% | 100.00% | Deletion |
| US3 | 99.90% | 99.90% | 99.90% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 99.90% | 100.00% | 100.00% | 98.71% |
| UL1 | 100.00% | 96.91% | 100.00% | 96.82% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 95.97% |
| UL10 | 99.92% | 99.92% | 99.07% | 100% | 100% | 100% | 100% | 100% | 99.92% | 100% | 99.92% | 98.73% |
| UL49.5 | 99.33% | 99.33% | 99.33% | 100% | 100% | 100% | 100% | 100% | 99.67% | 99.67% | 100% | 93.81% |
| Comparisons | Non-Synonymous | Synonymous | dN/dS Ratio |
|---|---|---|---|
| hSD-1/2019 vs. Ea | 153 | 139 | 1.10 |
| HeN1 vs. Ea | 145 | 112 | 1.29 |
| hSD-1/2019 vs. HeN1 | 56 | 45 | 1.24 |
| Recombinant Strain | Parent Major/Minor | Recombinant Region in Alignment | Model (Average p-Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RDP | GENECONV | BootScan | MaxChi | Chimaera | SiScan | Phylpro | |||
| hSD-1/2019 | HuBXY/HeN1 | 1–2279 | 1.73 × 10−2 | - | - | 4.35 × 10−2 | 2.4 × 10−2 | - | 3.54 × 10−2 |
| HuBXY/Ea | 65,809–66,710 | 1.98 × 10−8 | 2.99 × 10−8 | 1.96 × 10−9 | 1.22 × 10−2 | 1.20 × 10−2 | 9.60 × 10−9 | 9.47 × 10−6 | |
| TJ/HLJ8 | 117,180–128,177 | 6.74 × 10−12 | 3.21 × 10−14 | 7.85 × 10−8 | 1.62 × 10−8 | 1.18 × 10−4 | 4.28 × 10−30 | 4.02 × 10−10 | |
| HNX/JS | 143,787–143,906 | 1.69 × 10−19 | 4.29 × 10−22 | 1.23 × 10−20 | 5.84 × 10−7 | - | 3.35 × 10−10 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Liu, Q.; Zhang, Y.; Wu, B.; Chen, H.; Wang, X. Cytopathic and Genomic Characteristics of a Human-Originated Pseudorabies Virus. Viruses 2023, 15, 170. https://doi.org/10.3390/v15010170
Peng Z, Liu Q, Zhang Y, Wu B, Chen H, Wang X. Cytopathic and Genomic Characteristics of a Human-Originated Pseudorabies Virus. Viruses. 2023; 15(1):170. https://doi.org/10.3390/v15010170
Chicago/Turabian StylePeng, Zhong, Qingyun Liu, Yibo Zhang, Bin Wu, Huanchun Chen, and Xiangru Wang. 2023. "Cytopathic and Genomic Characteristics of a Human-Originated Pseudorabies Virus" Viruses 15, no. 1: 170. https://doi.org/10.3390/v15010170
APA StylePeng, Z., Liu, Q., Zhang, Y., Wu, B., Chen, H., & Wang, X. (2023). Cytopathic and Genomic Characteristics of a Human-Originated Pseudorabies Virus. Viruses, 15(1), 170. https://doi.org/10.3390/v15010170







